电解液添加剂稳定水系电池锌负极界面的研究进展
Research progress and prospect on electrolyte additives for stabilizing the zinc anode interface in aqueous batteries
电解液添加剂稳定水系电池锌负极界面的研究进展 |
| 时文超, 刘宇, 张博冕, 李琪, 韩春华, 麦立强 |
|
Research progress and prospect on electrolyte additives for stabilizing the zinc anode interface in aqueous batteries |
| Wenchao SHI, Yu LIU, Bomian ZHANG, Qi LI, Chunhua HAN, Liqiang MAI |
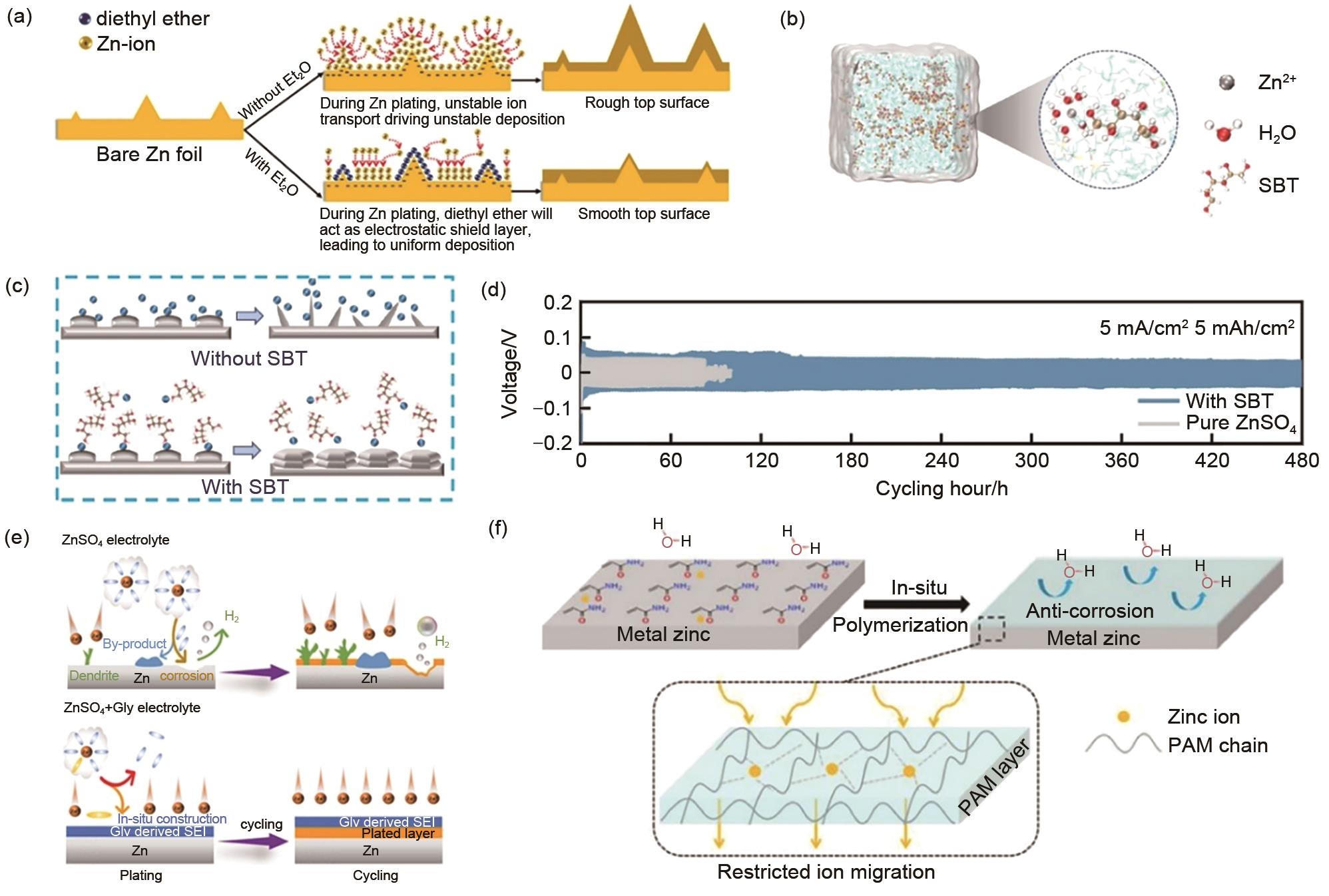
| 图5 (a) 含和不含Et2O添加剂的温和水系电解液中锌负极沉积/溶解循环的形貌演变示意图[ |
| Fig. 5 (a) Schematics of morphology evolution for Zn anodes in mild aqueous electrolyte with and without Et2O additive during Zn deposition/dissolution cycling[ |

|