电解液添加剂稳定水系电池锌负极界面的研究进展
|
|
时文超, 刘宇, 张博冕, 李琪, 韩春华, 麦立强
|
Research progress and prospect on electrolyte additives for stabilizing the zinc anode interface in aqueous batteries
|
|
Wenchao SHI, Yu LIU, Bomian ZHANG, Qi LI, Chunhua HAN, Liqiang MAI
|
|
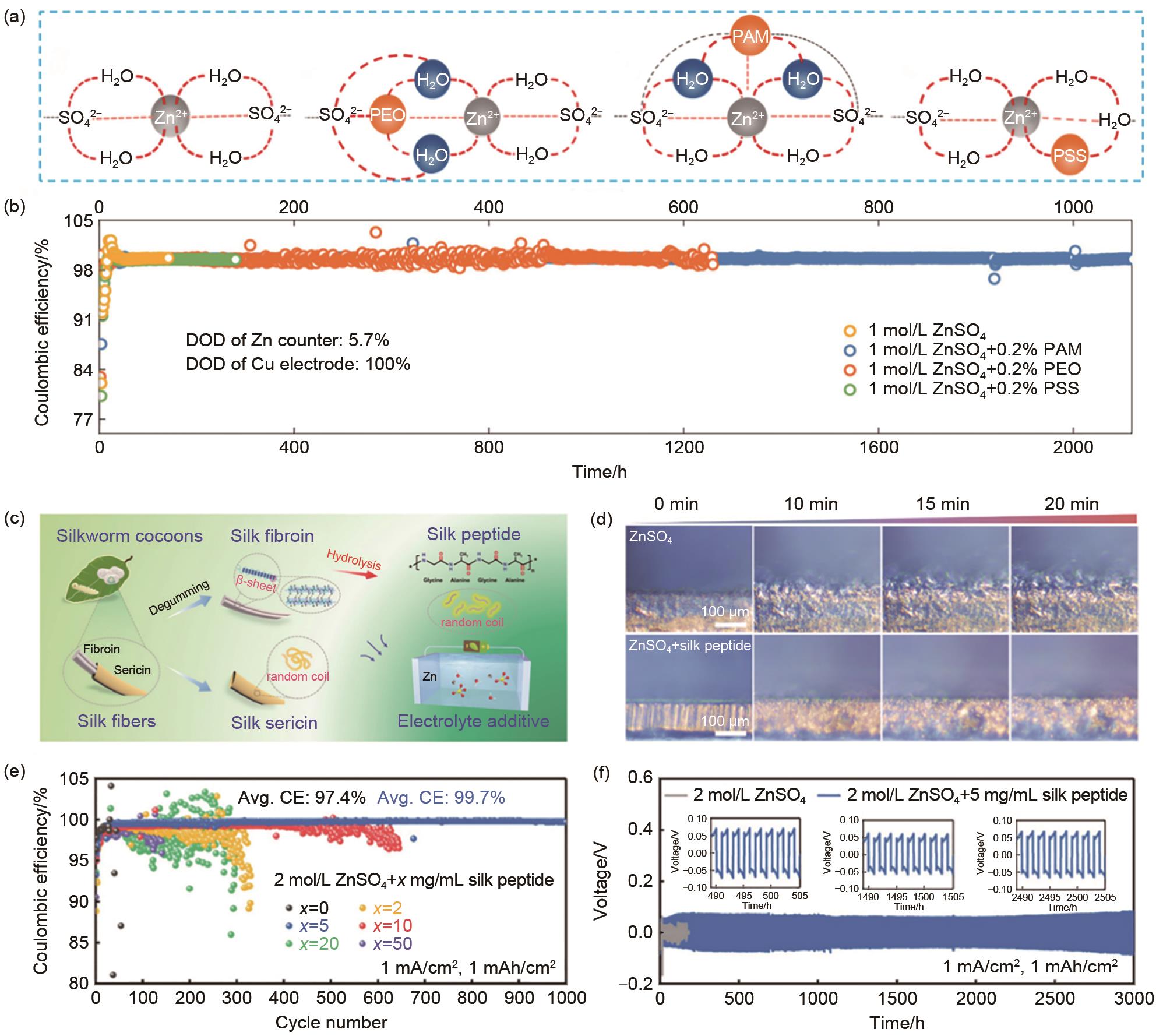
图6 (a) 加入和不加入聚合物添加剂的不同水电解质中的键合网络示意图[87];(b) 在不同聚合物添加剂的1 mol/L ZnSO4 水溶液中,在1 mA/cm2 、1 mAh/cm2 的测试条件下,Zn-Cu电池的库仑效率和循环性能[87];(c) 丝胶蛋白、丝素蛋白和具有不同构象和极性基团的肽分子之间的关系示意图,以及它们在AZMBs中作为电解液添加剂的应用[89];(d) 在电流密度为10 mA/cm2 时,含/不含丝肽的ZnSO4 电解质中Zn沉积形貌的原位光学观察[89];(e) 在含不同浓度的丝肽添加剂的ZnSO4 电解液中Zn-Cu电池的库仑效率[89];(f) 在含/不含丝肽的ZnSO4 电解液中Zn-Zn对称电池的循环性能[89]
|
Fig. 6 (a) Schematic diagrams of the bonding networks in different aqueous electrolytes with and without polymer additives[87]; (b) Coulombic efficiency and cycling performance of Zn anodes in 1 mol/L ZnSO4 aqueous electrolytes with different polymer additives using the Zn-Cu cells under 1 mA/cm2, 1 mAh/cm2[87]; (c) Schematic illustration of the relationship among silk sericin, fibroin, and peptide molecules with diverse conformations and polar groups, and their applications as electrolyte additives in AZMBs[89]; (d) In situ optical observations of Zn deposition morphologies in the ZnSO4 electrolytes with/without silk peptide at a current density of 10 mA/cm2[89]; (e) Coulomb efficiency of Zn-Cu cells in ZnSO4 electrolyte containing different concentrations of silk peptide additive[89]; (f) Cycling performance of Zn-Zn symmetric cells in the ZnSO4 aqueous electrolytes with/without silk peptide[89]
|
|
|
|
|

