Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (4): 1264-1277.doi: 10.19799/j.cnki.2095-4239.2022.0108
• Special issue of International Outstanding Young Scientists for Energy Storage • Previous Articles Next Articles
Yezhou HU1( ), Shuang WANG2, Tao SHEN2, Ye ZHU1(
), Shuang WANG2, Tao SHEN2, Ye ZHU1( ), Deli WANG2(
), Deli WANG2( )
)
Received:2022-03-01
Revised:2022-03-23
Online:2022-04-05
Published:2022-04-11
Contact:
Ye ZHU,Deli WANG
E-mail:huyezhou@163.com;yezhu@polyu.edu.hk;wangdl81125@ hust.edu.cn
CLC Number:
Yezhou HU, Shuang WANG, Tao SHEN, Ye ZHU, Deli WANG. Recent progress in confined noble-metal electrocatalysts for oxygen reduction reaction[J]. Energy Storage Science and Technology, 2022, 11(4): 1264-1277.
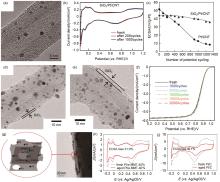
Fig. 3
Inorganic oxide confinement. (a) TEM image of SiO2/Pt/CNT using TEOS as precursor; (b) CV curves and (c)ECSA change of SiO2/Pt/CNT at different potential cycles, (d)—(e) TEM images of SiO2/Pt/CNT(MTEOS) using MTEOS as precursor; (f) ORR stability test of SiO2/Pt/CNT(MTEOS); (g) TEM image of Pt/e-MMT; The CV curves of (h) Pt/e-MMT and (i) Pt/C before and after stability tests"
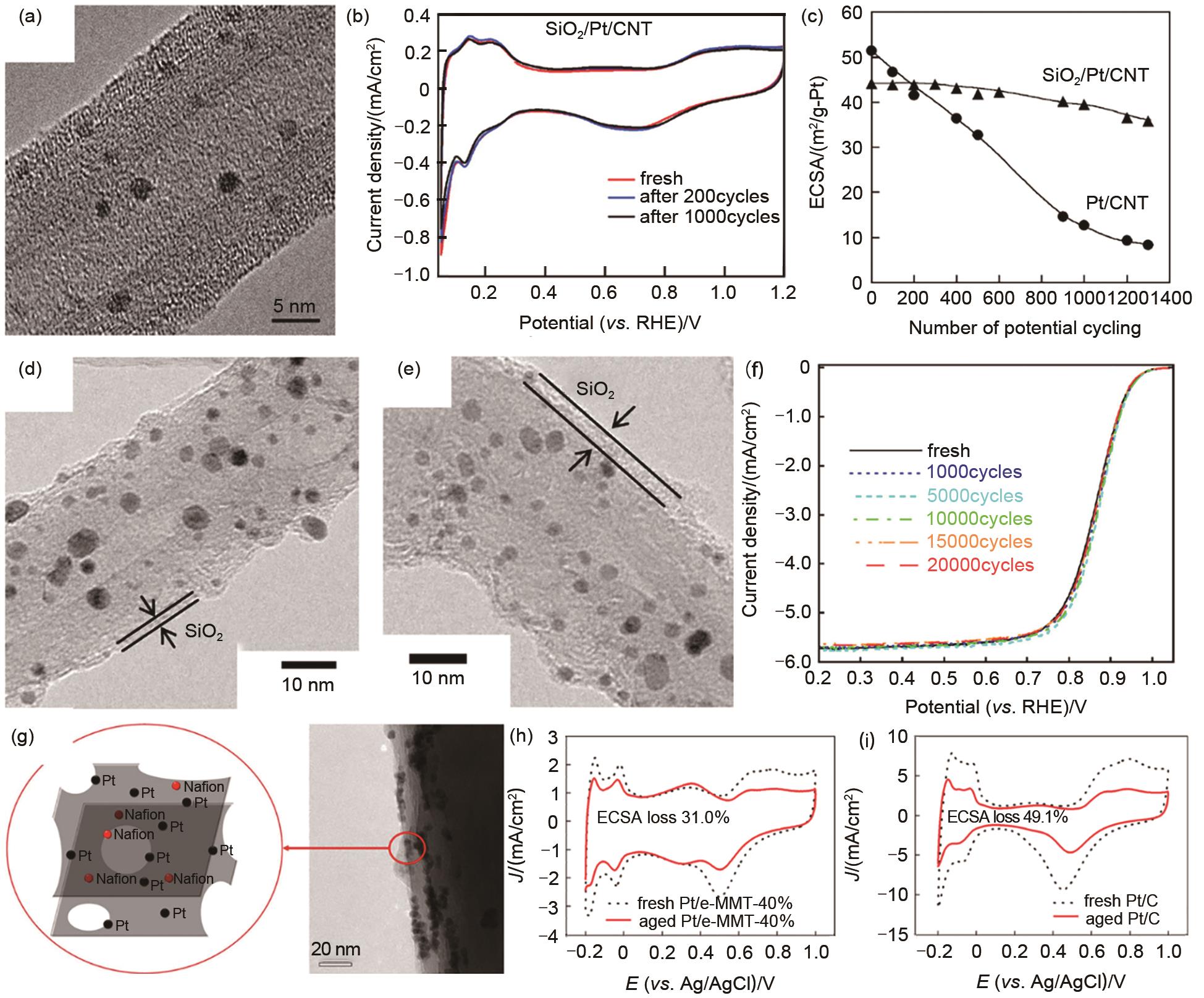
Fig. 4
Metal oxides confinement. (a) Schematic diagram of ZrO2 confined Pt nanoparticles; (b) The normalized ECSA of ALDPt/NCNT, ALD50ZrO2-Pt/NCNT600 ℃ and E-TEK Pt/CPMTT at different cycling stages; (c) The STEM image of ALD50ZrO2-Pt/NCNT after stability test; (d) MgO confined fcc-FePt nanoparticles; (e) fct-FePt nanoparticles after removal of MgO; (f) The Fe composition in fcc-FePt/C and fct-FePt/C at different times; (g)—(h) STEM images of Fe2O3 confined Pd2FeCo nanoparticles; (i) The stability test of Pd2FeCo/C and Pd/C"
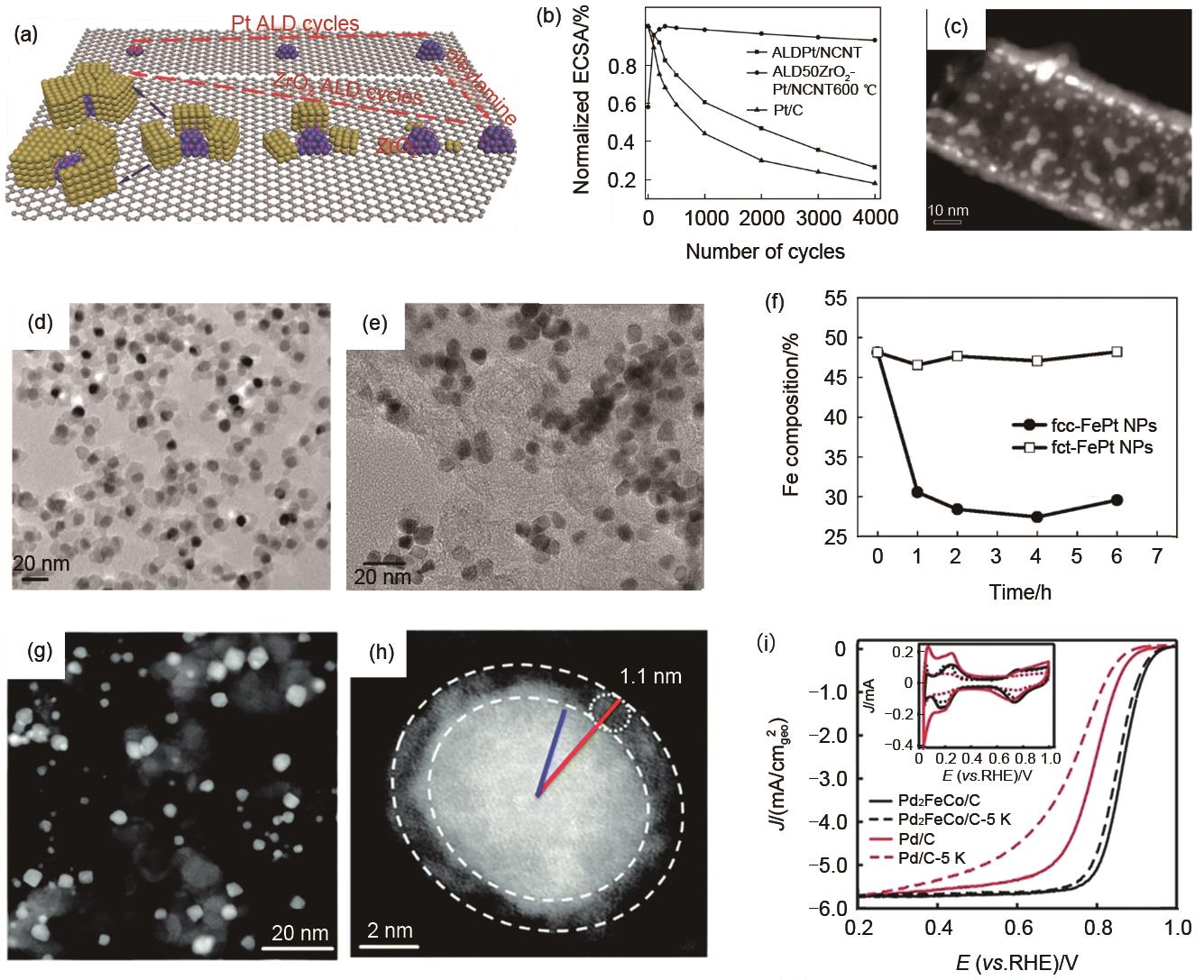

Fig. 5
Carbon confinement base on ‘deposition-conversion’ strategy. (a) Schematic diagram of Pt@C/MC; (b) Schematic diagram of Pt/C@NGC; (c) The TEM image of Pt/C@NGC; (d) Schematic diagram of L10-PtFe nanoparticles; (e) The TEM images of L10-PtFe nanoparticles with different thickness of carbon layers; (f) The stability test of carbon confined L10-PtFe nanoparticles in MEA; (g) Schematic diagram of PtCo/Co@NHPCC; (h) The TEM image of PtCo/Co@NHPCC"
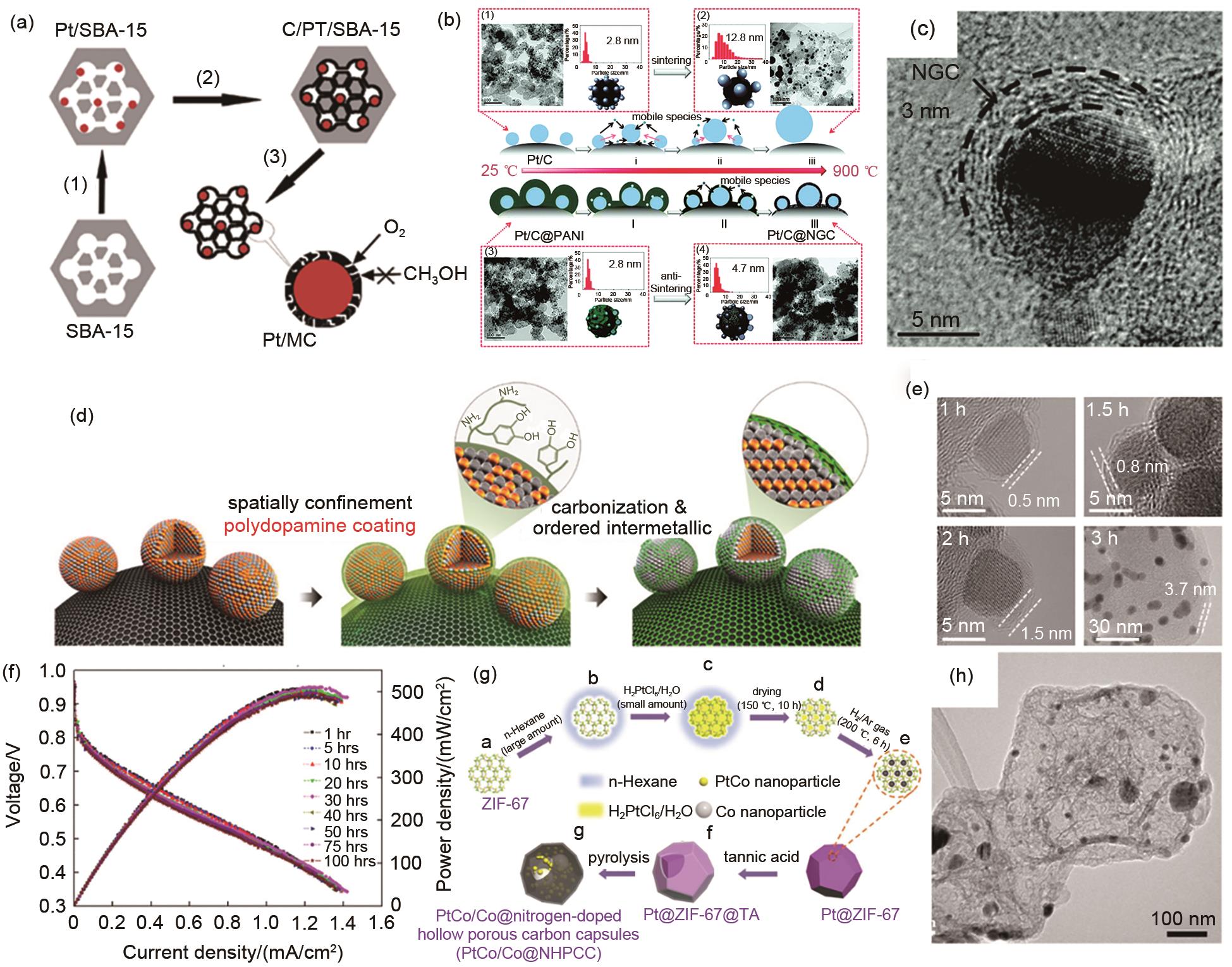
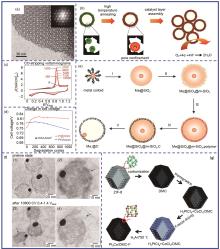
Fig. 6
Carbon confinement base on ‘insertion-conversion’ strategy. (a) The TEM image of ordered nanoporous arrays of carbon; (b) The schematic diagram of Pt@HGS; (c) The CO stripping curves of Pt@HSG at different potential cycling; (d) Comparison of cell voltage change of Pt@HGS and Pt/Vulcan during stability test; (e) Schematic diagram of encapsulated metal nanoparticles in porous carbon shells; (f) IL-TEM images of AuPt@C before and after stability test; (g) Schematic diagram of Pt3Co/DMF-F"

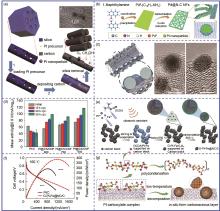
Fig. 7
Carbon confinement base on ‘one-step pyrolysis’ strategy. (a) The schematic diagram of Pt@GC; (b) The schematic diagram of Pd@N-C NFs; (c) The schematic diagram of Pt@CN x /CNT; (d) The stability tests of Pt@CS/CNF; (e) The schematic diagram of O-Pt-Fe@NC/C; (f) The high-temperature fuel cell polarization curves of O-Pt-Fe@NC/C and Pt/C; (g) The synthesis of Pt@C/C trough one-step low temperature pyrolysis"

| 1 | HAN A L, ZHANG Z D, YANG J R, et al. Carbon-supported single-atom catalysts for formic acid oxidation and oxygen reduction reactions[J]. Small, 2021, 17(16): doi: 10.1002/smll.202004500. |
| 2 | QIAO Z, HWANG S, LI X, et al. 3D porous graphitic nanocarbon for enhancing the performance and durability of Pt catalysts: A balance between graphitization and hierarchical porosity[J]. Energy & Environmental Science, 2019, 12(9): 2830-2841. |
| 3 | HAIDER R, WEN Y C, MA Z F, et al. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies[J]. Chemical Society Reviews, 2021, 50(2): 1138-1187. |
| 4 | GAO W B, LIU T T, ZHANG Z P, et al. Stabilization of Pt nanoparticles at the Ta2O5-TaC binary junction: An effective strategy to achieve high durability for oxygen reduction[J]. Journal of Materials Chemistry A, 2020, 8(11): 5525-5534. |
| 5 | SHI Q R, ZHU C Z, DU D, et al. Robust noble metal-based electrocatalysts for oxygen evolution reaction[J]. Chemical Society Reviews, 2019, 48(12): 3181-3192. |
| 6 | HU Y Z, LU Y, ZHAO X R, et al. Highly active N-doped carbon encapsulated Pd-Fe intermetallic nanoparticles for the oxygen reduction reaction[J]. Nano Research, 2020, 13(9): 2365-2370. |
| 7 | TIAN N, ZHOU Z Y, SUN S G, et al. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity[J]. Science, 2007, 316(5825): 732-735. |
| 8 | WANG G Z, YANG Z Z, DU Y G, et al. Programmable exposure of Pt active facets for efficient oxygen reduction[J]. Angewandte Chemie International Edition, 2019, 58(44): 15848-15854. |
| 9 | YANG Y, XIAO W P, FENG X R, et al. Golden palladium zinc ordered intermetallics as oxygen reduction electrocatalysts[J]. ACS Nano, 2019, 13(5): 5968-5974. |
| 10 | KONG Z J, MASWADEH Y, VARGAS J A, et al. Origin of high activity and durability of twisty nanowire alloy catalysts under oxygen reduction and fuel cell operating conditions[J]. Journal of the American Chemical Society, 2020, 142(3): 1287-1299. |
| 11 | GONG M X, DENG Z P, XIAO D D, et al. One-nanometer-thick Pt3Ni bimetallic alloy nanowires advanced oxygen reduction reaction: Integrating multiple advantages into one catalyst[J]. ACS Catalysis, 2019, 9(5): 4488-4494. |
| 12 | BU L Z, ZHANG N, GUO S J, et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis[J]. Science, 2016, 354(6318): 1410-1414. |
| 13 | GONG M X, XIAO D D, DENG Z P, et al. Structure evolution of PtCu nanoframes from disordered to ordered for the oxygen reduction reaction[J]. Applied Catalysis B: Environmental, 2021, 282: doi: 10.1016/j.apcatb.2020.119617. |
| 14 | ZANA A, SPEDER J, REELER N E A, et al. Investigating the corrosion of high surface area carbons during start/stop fuel cell conditions: A Raman study[J]. Electrochimica Acta, 2013, 114: 455-461. |
| 15 | YANG Z, BALL S, CONDIT D, et al. Systematic study on the impact of Pt particle size and operating conditions on PEMFC cathode catalyst durability[J]. Journal of the Electrochemical Society, 2011, 158(11): B1439. |
| 16 | ZHOU Q Q, SHI G Q. Conducting polymer-based catalysts[J]. Journal of the American Chemical Society, 2016, 138(9): 2868-2876. |
| 17 | LIU Y, LU N, POYRAZ S, et al. One-pot formation of multifunctional Pt-conducting polymer intercalated nanostructures[J]. Nanoscale, 2013, 5(9): 3872-3879. |
| 18 | MIZUHATA M, OGA M, DEKI S. Preparation of Pt/polypyrrole loaded carbon composite in order to improve electrode durability for fuel cells[J]. ECS Transactions, 2007, 2(8): 63-72. |
| 19 | CHEN S G, WEI Z D, QI X Q, et al. Nanostructured polyaniline-decorated Pt/C@PANI core-shell catalyst with enhanced durability and activity[J]. Journal of the American Chemical Society, 2012, 134(32): 13252-13255. |
| 20 | TAKENAKA S, MATSUMORI H, NAKAGAWA K, et al. Improvement in the durability of Pt electrocatalysts by coverage with silica layers[J]. The Journal of Physical Chemistry C, 2007, 111(42): 15133-15136. |
| 21 | TAKENAKA S, MIYAMOTO H, UTSUNOMIYA Y, et al. Catalytic activity of highly durable Pt/CNT catalysts covered with hydrophobic silica layers for the oxygen reduction reaction in PEFCs[J]. The Journal of Physical Chemistry C, 2014, 118(2): 774-783. |
| 22 | AOKI N, INOUE H, KAWASAKI H, et al. Durability improvement of Pd core-Pt shell structured catalyst by porous SiO2 coating[J]. Journal of the Electrochemical Society, 2018, 165(10): F737-F747. |
| 23 | LI W, DING W, NIE Y, et al. Enhancing the stability and activity by anchoring Pt nanoparticles between the layers of etched montmorillonite for oxygen reduction reaction[J]. Science Bulletin, 2016, 61(18): 1435-1439. |
| 24 | ANDO F, GUNJI T K, TANABE T, et al. Enhancement of the oxygen reduction reaction activity of Pt by tuning its d-band center via transition metal oxide support interactions[J]. ACS Catalysis, 2021, 11(15): 9317-9332. |
| 25 | CHENG N C, BANIS M N, LIU J, et al. Extremely stable platinum nanoparticles encapsulated in a zirconia nanocage by area-selective atomic layer deposition for the oxygen reduction reaction[J]. Advanced Materials, 2015, 27(2): 277-281. |
| 26 | KANG S, XIA F, HU Z F, et al. Platinum nanoparticles with TiO2-skin as a durable catalyst for photoelectrochemical methanol oxidation and electrochemical oxygen reduction reactions[J]. Electrochimica Acta, 2020, 343: doi: 10.1016/j.electacta.2020.136119. |
| 27 | KIM J, LEE Y, SUN S H. Structurally ordered FePt nanoparticles and their enhanced catalysis for oxygen reduction reaction[J]. Journal of the American Chemical Society, 2010, 132(14): 4996-4997. |
| 28 | XIAO W P, LIUTHEVICIENE CORDEIRO M A, GONG M X, et al. Optimizing the ORR activity of Pd based nanocatalysts by tuning their strain and particle size[J]. Journal of Materials Chemistry A, 2017, 5(20): 9867-9872. |
| 29 | WEN Z, LIU J, LI J. Core/shell Pt/C nanoparticles embedded in mesoporous carbon as a methanol-tolerant cathode catalyst in direct methanol fuel cells[J]. Advanced Materials, 2008, 20(4): 743-747. |
| 30 | NIE Y, CHEN S G, DING W, et al. Pt/C trapped in activated graphitic carbon layers as a highly durable electrocatalyst for the oxygen reduction reaction[J]. Chemical Communications (Cambridge, England), 2014, 50(97): 15431-15434. |
| 31 | TAN J L, XIE Z, ZHANG Z, et al. Dopamine modified polyaniline with improved adhesion, dispersibility, and biocompatibility[J]. Journal of Materials Science, 2018, 53(1): 447-455. |
| 32 | CHUNG D Y, JUN S W, YOON G, et al. Highly durable and active PtFe nanocatalyst for electrochemical oxygen reduction reaction[J]. Journal of the American Chemical Society, 2015, 137(49): 15478-15485. |
| 33 | SUN K, LI J, WANG F, et al. Highly enhanced durability of a graphitic carbon layer decorated PtNi3 alloy electrocatalyst toward the oxygen reduction reaction[J]. Chemical Communications (Cambridge, England), 2019, 55(40): 5693-5696. |
| 34 | YING J, LI J, JIANG G P, et al. Metal-organic frameworks derived platinum-cobalt bimetallic nanoparticles in nitrogen-doped hollow porous carbon capsules as a highly active and durable catalyst for oxygen reduction reaction[J]. Applied Catalysis B: Environmental, 2018, 225: 496-503. |
| 35 | JOO S H, CHOI S J, OH I, et al. Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles[J]. Nature, 2001, 412 (6843): 169-172. |
| 36 | GALEANO C, MEIER J C, PEINECKE V, et al. Toward highly stable electrocatalysts via nanoparticle pore confinement[J]. Journal of the American Chemical Society, 2012, 134(50): 20457-20465. |
| 37 | GALEANO C, BALDIZZONE C, BONGARD H, et al. Carbon-based yolk-shell materials for fuel cell applications[J]. Advanced Functional Materials, 2014, 24(2): 220-232. |
| 38 | ZHAO W Y, YE Y K, JIANG W J, et al. Mesoporous carbon confined intermetallic nanoparticles as highly durable electrocatalysts for the oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2020, 8(31): 15822-15828. |
| 39 | WU Z X, LV Y Y, XIA Y Y, et al. Ordered mesoporous platinum@graphitic carbon embedded nanophase as a highly active, stable, and methanol-tolerant oxygen reduction electrocatalyst[J]. Journal of the American Chemical Society, 2012, 134(4): 2236-2245. |
| 40 | JIANG X, WANG J X, HUANG T, et al. Sub-5 nm palladium nanoparticles in situ embedded in N-doped carbon nanoframes: Facile synthesis, excellent sinter resistance and electrocatalytic properties[J]. Journal of Materials Chemistry A, 2019, 7(46): 26243-26249. |
| 41 | GUO L, JIANG W J, ZHANG Y, et al. Embedding Pt nanocrystals in N-doped porous carbon/carbon nanotubes toward highly stable electrocatalysts for the oxygen reduction reaction[J]. ACS Catalysis, 2015, 5(5): 2903-2909. |
| 42 | KARUPPANNAN M, KIM Y, GOK S, et al. A highly durable carbon-nanofiber-supported Pt-C core-shell cathode catalyst for ultra-low Pt loading proton exchange membrane fuel cells: Facile carbon encapsulation[J]. Energy & Environmental Science, 2019, 12(9): 2820-2829. |
| 43 | HU Y Z, SHEN T, ZHAO X R, et al. Combining structurally ordered intermetallics with N-doped carbon confinement for efficient and anti-poisoning electrocatalysis[J]. Applied Catalysis B: Environmental, 2020, 279: doi: 10.1016/j.apcatb.2020.119370. |
| 44 | HU Y Z, ZHANG J J, SHEN T, et al. A low-temperature carbon encapsulation strategy for stable and poisoning-tolerant electrocatalysts[J]. Small Methods, 2021, 5(11): doi: 10.1002/smtd.202100937. |
| [1] | Xiaohua DENG, Zhu JANG, Chao CHEN, Dai DANG. Recent advances in zeolitic imidazolium-based metal-organic frameworks (ZIFs) and their derivatives as efficient cathode catalysts for zinc-air batteries [J]. Energy Storage Science and Technology, 2022, 11(3): 964-981. |
| [2] | Shenzhi ZHANG, Likai WANG, Yinggang SUN, Heng LÜ, Ziyin YANG, Leilei LI, Zhongfang LI. Construction of two dimensional carbon-supported Au4Pd2 catalysts and their electrocatalytic performances [J]. Energy Storage Science and Technology, 2021, 10(6): 2028-2038. |
| [3] | Wenwu ZOU, Guoxing JIANG, Li DU. Recent advances in covalent organic frameworks (COFs) for electrocatalysis of oxygen electrodes [J]. Energy Storage Science and Technology, 2021, 10(6): 1891-1905. |
| [4] | Yuexia LI, Quanbing LIU. Application of MXene-based nanomaterials in electrocatalysis for oxygen reduction reaction [J]. Energy Storage Science and Technology, 2021, 10(6): 1918-1930. |
| [5] | Feng HE, Jingjing ZHANG, Yijun CHEN, Jian ZHANG, Deli WANG. Recent progress on carbon-based catalysts for electrochemical synthesis of H2O2 via oxygen reduction reaction [J]. Energy Storage Science and Technology, 2021, 10(6): 1963-1976. |
| [6] | Ziyue ZHU, Dongju FU, Jianjun CHEN, Bianrong ZENG. Research progress of non-precious metal bifunctional cathode electrocatalysts for zinc-air batteries [J]. Energy Storage Science and Technology, 2020, 9(5): 1489-1496. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
