Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (1): 240-251.doi: 10.19799/j.cnki.2095-4239.2023.0737
Previous Articles Next Articles
Zeping FANG1,2( ), Bao QIU1,2(
), Bao QIU1,2( ), Zhaoping LIU1,2(
), Zhaoping LIU1,2( )
)
Received:2023-10-08
Revised:2023-11-13
Online:2024-01-05
Published:2024-01-22
Contact:
Bao QIU, Zhaoping LIU
E-mail:fangzeping@nimte.ac.cn;qiubao@nimte.ac.cn;liuzp@nimte.ac.cn
CLC Number:
Zeping FANG, Bao QIU, Zhaoping LIU. Progress of "reversible high-oxygen activity" of lithium-rich layered oxide anode materials[J]. Energy Storage Science and Technology, 2024, 13(1): 240-251.
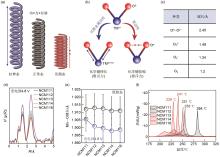
Fig. 2
(a) Schematic diagram of energy conversion; (b) Bond length changes of different redox activities due to different forms of electrical energy converted to chemical energy during charging; (c) Oxygen-oxygen bond lengths in different oxygen states; (d) K-edge extended X-ray absorption fine structure (EXAFS) spectra of Mn for NCM11X (X = 1, 2, 3, 4, 6) samples when charged to 4.8 V; (e) EXAFS spectra based on the fitted Mn—O bond lengths; (f) Differential scanning calorimetry (DSC) curves of different compositions of cathode materials in the charged state (charged to 4.8 V) [5]"

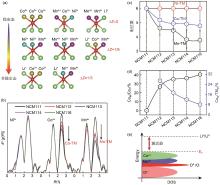
Fig. 3
(a) Schematic of the atomic coordination structure centered on the oxygen atom; (b) K-edge EXAFS of Ni2+, Co3+, and Mn4+ for NCM11X (X = 1, 2, 3, 4, 6) samples; (c) Fitted coordination numbers of Ni2+, Co3+, and Mn4+ in the second coordination shell; (d) Calculated ratio of Co3+ content in M phase to total Co3+ content (black), and the ratio of Co3+ content in M phase to total transition metal cations in M phase (blue); (e) Energy band structure diagram of the structural domains of the cobalt-containing Li-rich phase [6]"
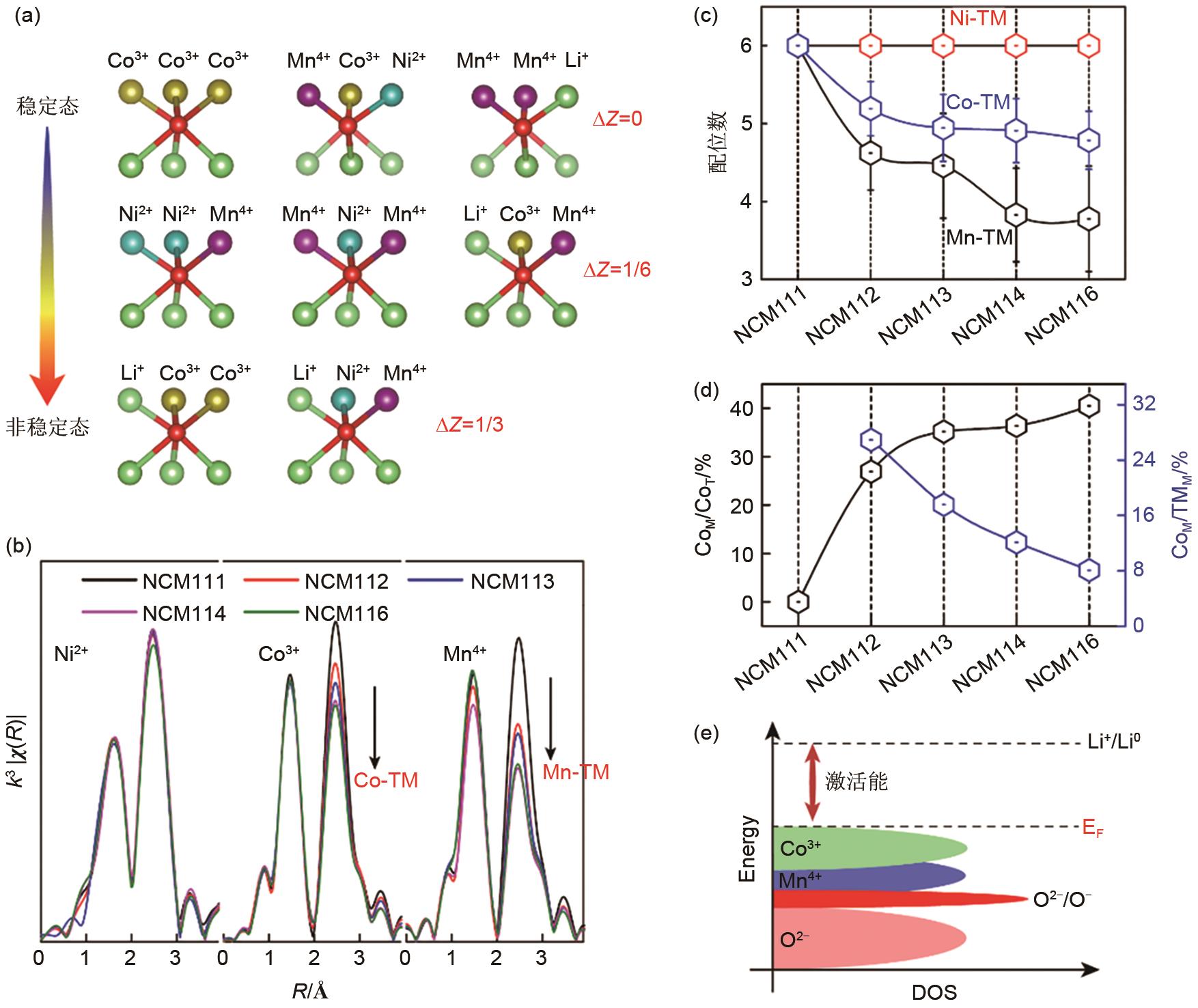
Fig. 4
(a) Schematic of size effect; (b) Scanning transmission electron microscopy-high angle annular dark field (STEM-HAADF) of micrometer-sized samples after 200 cycles; (c) Results of Li concentration distribution in charged and discharged states of particles with different particle size sizes obtained by finite element analysis (FEA) [9]; Structural evolutions of Li-rich manganese-based cathode materials with aggregation of Li2MnO3 domain during cycling in (d) and (e); (f) Cycling performance at 0.2C[10]; (g) Schematic of cation arrangement of Li-rich layered oxides in the presence of Mn3+[11]"
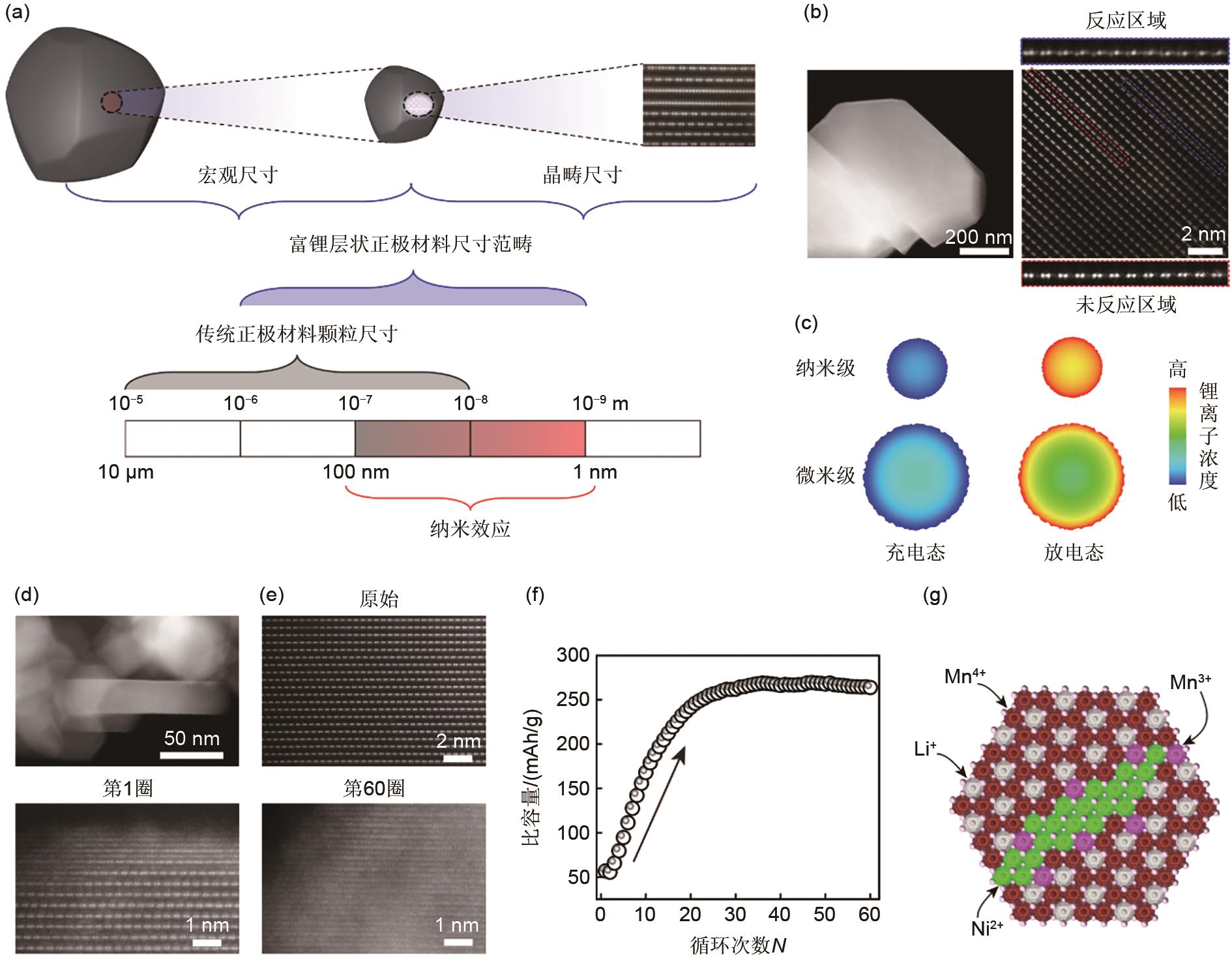
Fig. 5
(a) Schematic illustration of bulk and surface structure with atomic-layer-deposition technique after heat and the phase delocalization in Li-rich layered cathodes[18]; (b) Oxygen profiles in in-situ differential electrochemical mass spectrometry (DEMS) of pristine and gas-solid interface-modified Li-rich layered oxides with initial charging and discharging[17]; (c) Oxygen profiles in DEMS of Li-rich layered oxides initially charged and discharged after optimization of bulk-phase crystal domain distribution[18]"


Fig. 6
(a) Normalized charge-discharge curves of cycled electrodes of Li-rich layered oxides after annealing at different temperatures; (b) Enlarged in situ temperature-dependent synchrotron X-ray diffraction (TD-SXRD) and schematic diagrams of the metastability state of cycled electrodes of Li-rich layered oxides after annealing [20]; (c) Schematic diagrams of the pristine Li-rich layered oxides and Li-rich complex oxides with a high concentration of defects after deep chemical delithiation; (d) Aberration-corrected high-angle annular dark-field (HAADF)-scanning transmission electron microscopy (STEM) image of high concentration of defective material; (e) Average discharge voltage versus number of cycles curves of pristine and high-concentration defective materials [23]; (f) high Li/O ratio of Li-rich layered oxide materials with highly disordered schematic; (g) Cycling capacity and average discharge voltage of highly disordered Li-rich layered oxides [24]"
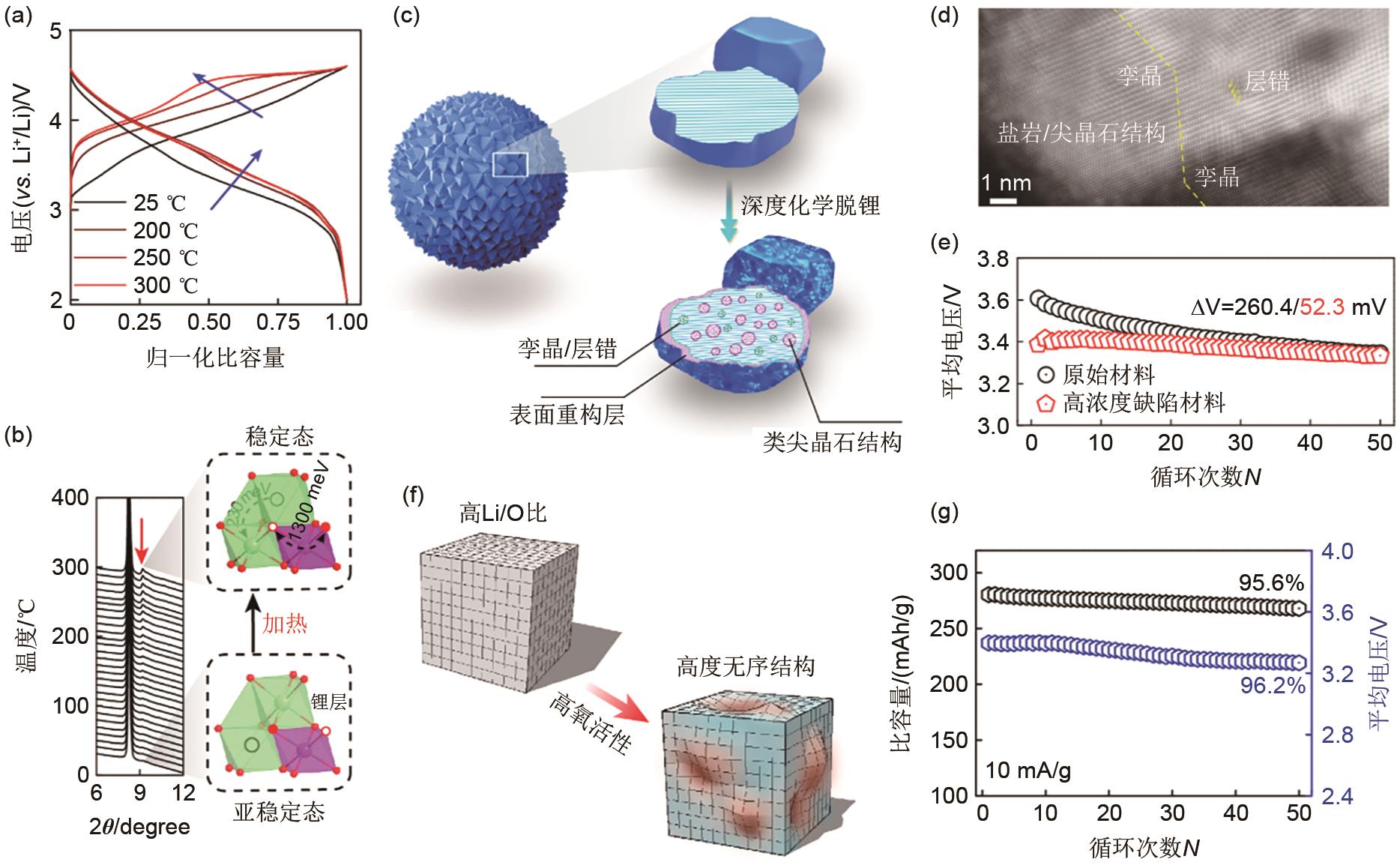

Fig. 7
(a) Photographs of a 20 Ah full battery with a Li-rich cathode ||silicon based anode system; (b) First charge/discharge curves of a 20 Ah full battery with a Li-rich cathode||silicon based anode system; (c) Cycling performance of a 20 Ah full battery with a Li-rich cathode||silicon based anode system [25]; (d) Photographs of a 10 Ah full battery with a Li-rich cathode||metal Li negative system; (e) First charge/discharge curves of a 10 Ah full battery with a Li-rich anode||metal Li negative system; (f) Cycling performance of a 10 Ah full battery with a Li-rich layered cathode || Li metal anode system [26]"

| 1 | QIU B, ZHANG M H, XIA Y G, et al. Understanding and controlling anionic electrochemical activity in high-capacity oxides for next generation Li-ion batteries[J]. Chemistry of Materials, 2017, 29(3): 908-915. |
| 2 | THACKERAY M M, KANG S H, JOHNSON C S, et al. Li2MnO3-stabilized LiMO2 (M=Mn, Ni, Co) electrodes for lithium-ion batteries[J]. Journal of Materials Chemistry, 2007, 17(30): 3112-3125. |
| 3 | YU H J, ISHIKAWA R, SO Y G, et al. Direct atomic-resolution observation of two phases in the Li1.2Mn0.567Ni0.166Co0.067O2 cathode material for lithium-ion batteries[J]. Angewandte Chemie International Edition, 2013, 52(23): 5969-5973. |
| 4 | LEE G H, LAU V W H, YANG W L, et al. Utilizing oxygen redox in layered cathode materials from multiscale perspective[J]. Advanced Energy Materials, 2021, 11(27): 2003227. |
| 5 | LI X, GU Q W, QIU B, et al. Rational design of thermally stable polymorphic layered cathode materials for next generation lithium rechargeable batteries[J]. Materials Today, 2022, 61: 91-103. |
| 6 | YIN C, WEI Z N, ZHANG M H, et al. Structural insights into composition design of Li-rich layered cathode materials for high-energy rechargeable battery[J]. Materials Today, 2021, 51: 15-26. |
| 7 | YIN C, WAN L Y, QIU B, et al. Boosting energy efficiency of Li-rich layered oxide cathodes by tuning oxygen redox kinetics and reversibility[J]. Energy Storage Materials, 2021, 35: 388-399. |
| 8 | GONG Y, LI X A, ZENG L C, et al. Tuning local structural configurations to improve oxygen-redox reversibility of Li-rich layered oxides[J]. The Journal of Physical Chemistry Letters, 2023, 14(19): 4575-4582. |
| 9 | ZHANG Y B, YIN C, QIU B, et al. Revealing Li-ion diffusion kinetic limitations in micron-sized Li-rich layered oxides[J]. Energy Storage Materials, 2022, 53: 763-773. |
| 10 | ZHANG Y B, WEN X H, SHI Z P, et al. Oxygen-defects evolution to stimulate continuous capacity increase in Co-free Li-rich layered oxides[J]. Journal of Energy Chemistry, 2023, 82: 259-267. |
| 11 | WEN X H, YIN C, QIU B, et al. Controls of oxygen-partial pressure to accelerate the electrochemical activation in Co-free Li-rich layered oxide cathodes[J]. Journal of Power Sources, 2022, 523: 231022. |
| 12 | WEN X H, QIU B, GAO H, et al. Synergistic effects of Ni2+ and Mn3+ on the electrochemical activation of Li2MnO3 in co-free and Ni-poor Li-rich layered cathodes[J]. ACS Applied Energy Materials, 2022, 5(7): 9079-9089. |
| 13 | LI T T, SHI Z P, LI L, et al. Non-eutectic-salt reaction route towards morphological and structural rearrangement of Li-rich layered oxides for high-volumetric Li-ion batteries[J]. Chemical Engineering Journal, 2023, 474: 145728. |
| 14 | LIU S Y, ZHOU Y H, ZHANG Y B, et al. Surface yttrium-doping induced by element segregation to suppress oxygen release in Li-rich layered oxide cathodes[J]. Tungsten, 2022, 4(4): 336-345. |
| 15 | YIN C, WEN X H, WAN L Y, et al. Surface reinforcement doping to suppress oxygen release of Li-rich layered oxides[J]. Journal of Power Sources, 2021, 503: 230048. |
| 16 | QIU B, ZHANG M H, WU L J, et al. Gas-solid interfacial modification of oxygen activity in layered oxide cathodes for lithium-ion batteries[J]. Nature Communications, 2016, 7: 12108. |
| 17 | SHI Z P, GU Q W, YUN L, et al. A composite surface configuration towards improving cycling stability of Li-rich layered oxide materials[J]. Journal of Materials Chemistry A, 2021, 9(43): 24426-24437. |
| 18 | LI Y, SHI Z P, QIU B, et al. Optimizing both bulk and surface structure of Li-rich layered cathodes for long-life and safe Li-ion batteries[J]. Advanced Functional Materials, 2023, 33(41): 2302236. |
| 19 | ZENG L C, LIANG H Y, QIU B, et al. Voltage decay of Li-rich layered oxides: Mechanism, modification strategies, and perspectives[J]. Advanced Functional Materials, 2023, 33(25): 2213260. |
| 20 | QIU B, ZHANG M H, LEE S Y, et al. Metastability and reversibility of anionic redox-based cathode for high-energy rechargeable batteries[J]. Cell Reports Physical Science, 2020, 1(3): 100028. |
| 21 | ZHANG M H, QIU B, GALLARDO-AMORES J M, et al. High pressure effect on structural and electrochemical properties of anionic redox-based lithium transition metal oxides[J]. Matter, 2021, 4(1): 164-181. |
| 22 | JIANG W, YIN C, XIA Y G, et al. Understanding the discrepancy of defect kinetics on anionic redox in lithium-rich cathode oxides[J]. ACS Applied Materials & Interfaces, 2019, 11(15): 14023-14034. |
| 23 | GUO H C, WEI Z, JIA K, et al. Abundant nanoscale defects to eliminate voltage decay in Li-rich cathode materials[J]. Energy Storage Materials, 2019, 16: 220-227. |
| 24 | ZHOU Y H, CUI H F, QIU B, et al. Sufficient oxygen redox activation against voltage decay in Li-rich layered oxide cathode materials[J]. ACS Materials Letters, 2021, 3(4): 433-441. |
| 25 | ZHAO J L, SHI Z, HE Z, et al. Optimizing the potential of intercalation on anode for long-cycle 420 Wh/kg Li-ion batteries[J]. Journal of Power Sources 2023, 580: 233393. |
| 26 | WEI Z N, SHI Z P, WEN X H, et al. Eliminating oxygen releasing of Li-rich layered cathodes by tuning the distribution of superlattice domain[J]. Materials Today Energy, 2022, 27: 101039. |
| 27 | MCCOLL K, HOUSE R A, REES G J, et al. Transition metal migration and O2 formation underpin voltage hysteresis in oxygen-redox disordered rocksalt cathodes[J]. Nature Communications, 2022, 13: 5275. |
| 28 | LI M, LU J. Cobalt in lithium-ion batteries[J]. Science, 2020, 367(6481): 979-980. |
| 29 | PAULING L. The principles determining the structure of complex ionic crystals[J]. Journal of the American Chemical Society, 1929, 51(4): 1010-1026. |
| 30 | QIU B, QIAO Y, LI B, et al. Next-generation cathode materials for ultrahigh-energy batteries[J]. Next Materials, 2023, 1(3): 100034. |
| [1] | Qiang LI, Junnan WANG, Hong SUN. Graphite felt electrode modified with MWCNTs-COOH-NS for vanadium flow battery [J]. Energy Storage Science and Technology, 2021, 10(6): 2097-2105. |
| [2] | WANG Qiushi, SUN Miaomiao, LIU Qinghua, YANG Hong CHEN Jingyun, LIU Junqing, LIANG Wenbin. Surface modification of carbon fiber paper for vanadium redox flow battery [J]. Energy Storage Science and Technology, 2020, 9(3): 714-719. |
| [3] | ZHANG Jianyu, LU Liping, YU Zhihui, SONG Jin, XIA Dingguo. Synthesis and performance of P2-O3 composite-phase Li-rich Mn-based cathode materials [J]. Energy Storage Science and Technology, 2020, 9(2): 346-352. |
| [4] | REN Ya, WANG Ying, XU Zhiyu, YAN Xiao, HUANG Bixiong. Graphite modified LiNi1/3Co1/3Mn1/3O2 cathodes with improved performance for lithium-ion battery [J]. Energy Storage Science and Technology, 2019, 8(5): 935-940. |
| [5] | LI Yu, ZHAO Huichun, BAI Ying, WU Feng, WU Chuan. Progress in the modification of lithium-rich manganese-based layered cathode material [J]. Energy Storage Science and Technology, 2018, 7(3): 394-403. |
| [6] | WANG Hao, BEN Liubin, LIN Mingxiang, CHEN Yuyang, HUANG Xuejie. Research progress on high voltage cathode material LiNi0.5Mn1.5O4 for lithium-ion batteries [J]. Energy Storage Science and Technology, 2017, 6(5): 841-854. |
| [7] | YUAN Huadong, LUO Jianmin, JIN Chengbin, SHENG Ouwei, HUANG Hui, ZHANG Wenkui, TAO Xinyong . Carbon materials with modified surfaces and interfaces for the high performance cathode of lithium sulfur batteries [J]. Energy Storage Science and Technology, 2017, 6(3): 380-410. |
| [8] | XIA Yonggao, LIU Zhaoping. Research progress on the Li-excess Mn-based cathode materials with high capacity for lithium-ion battery [J]. Energy Storage Science and Technology, 2016, 5(3): 384-387. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
