Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (9): 2884-2906.doi: 10.19799/j.cnki.2095-4239.2024.0699
Previous Articles Next Articles
Bin DENG1( ), Haiming HUA1, Yuzhi ZHANG1, Xiaoxu WANG1(
), Haiming HUA1, Yuzhi ZHANG1, Xiaoxu WANG1( ), Linfeng ZHANG1,2
), Linfeng ZHANG1,2
Received:2024-07-29
Revised:2024-08-26
Online:2024-09-28
Published:2024-09-20
Contact:
Xiaoxu WANG
E-mail:dengb@dp.tech;wangxx@dp.tech
CLC Number:
Bin DENG, Haiming HUA, Yuzhi ZHANG, Xiaoxu WANG, Linfeng ZHANG. Deep potential model: Applications and insights for electrochemical energy storage materials[J]. Energy Storage Science and Technology, 2024, 13(9): 2884-2906.
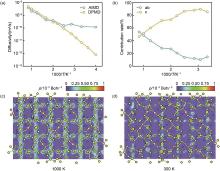
Fig. 9
Li+ diffusion behavior in the Li10GeP2S12 lattice. (a) Arrhenius plot of diffusion coefficients simulated with AIMD and DPMD. (b) Dimensional contribution of Li-ion diffusion as a function of temperature inverse. (c) Color-filled plots of Li-ion probability density in Li10GeP2S12 at 1000 K and (d) 300 K[37]"
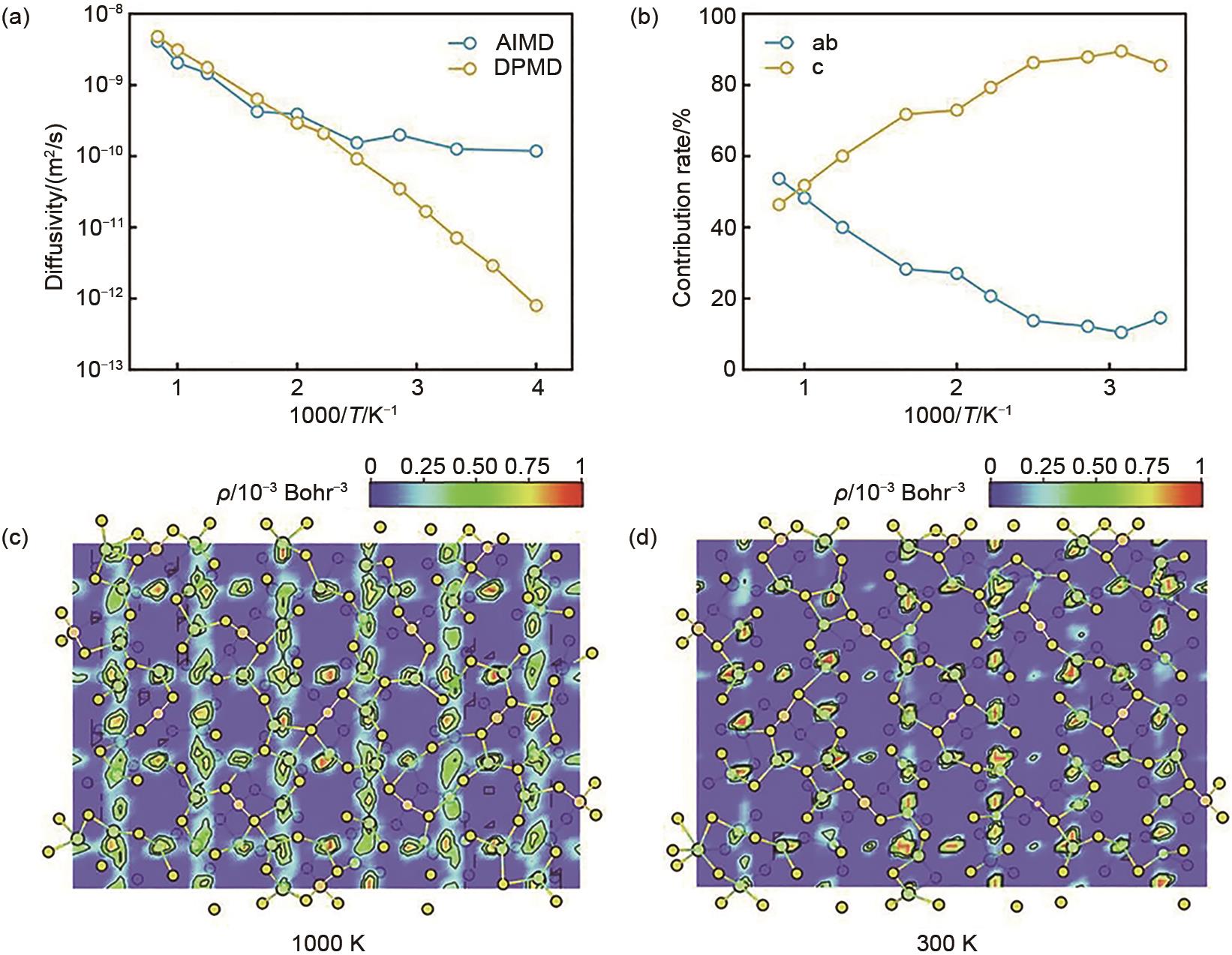

Fig. 11
Intra-layer lithium ion motion trajectories along the Li3TiCl6 (a) [010] and (b) [110] view directions; (c) Li atom displacement evolution with time corresponding to (a) and (b); Schematic diagram of the inter-layer lithium ion motion trajectories along the Li3TiCl6 (d) [010] and (e) [110] view directions; (f) Corresponding to (d) and (e) Li atom displacement evolution with time[44]"
| 1 | ZHANG L F, HAN J Q, WANG H, et al. Deep potential molecular dynamics: A scalable model with the accuracy of quantum mechanics[J]. Physical Review Letters, 2018, 120(14): 143001. DOI: 10.1103/PhysRevLett.120.143001. |
| 2 | BEHLER J, PARRINELLO M. Generalized neural-network representation of high-dimensional potential-energy surfaces[J]. Physical Review Letters, 2007, 98(14): 146401. DOI: 10.1103/PhysRevLett.98.146401. |
| 3 | SCHütt K, KINDERMANS P J, SAUCEDA FELIX H E, et al. Schnet: A continuous-filter convolutional neural network for modeling quantum interactions[J]. Advances in neural information processing systems, 2017, 30. |
| 4 | ZHANG D, BI H R, DAI F Z, et al. Pretraining of attention-based deep learning potential model for molecular simulation[J]. NPJ Computational Materials, 2024, 10: 94. DOI: 10.1038/s41524-024-01278-7. |
| 5 | ZHANG D, LIU X Z J, ZHANG X Y, et al. DPA-2: Towards a universal large atomic model for molecular and materials simulation[J]. arXiv, 2024. DOI: arxiv-2312.15492. |
| 6 | JIA W L, WANG H, CHEN M H, et al. Pushing the limit of molecular dynamics with ab initio accuracy to 100 million atoms with machine learning[C]//SC20: International Conference for High Performance Computing, Networking, Storage and Analysis. IEEE, 2020: 1-14. DOI: 10.1109/SC41405.2020.00009. |
| 7 | LI J H, WEI N, LI J L, et al. Physicochemical properties of cathode materials for failed lithium iron phosphate batteries[J]. China Powder Science and Technology, 2022, 28(6). |
| 8 | CHEN K, LIAO Q, LIU K, et al. Capacity degradation prediction of lithium-ion battery based on artificial bee colony and multi-kernel support vector regression[J]. Journal of Energy Storage, 2023, 72: 108160. DOI: 10.1016/j.est.2023.108160. |
| 9 | CHEN K, ZHOU S Y, LIU K, et al. State of charge estimation for lithium-ion battery based on whale optimization algorithm and multi-kernel relevance vector machine[J]. 2023, 158(10): 104110. DOI: 10.1063/5.0139376. |
| 10 | HUANG J X, ZHANG L F, WANG H, et al. Deep potential generation scheme and simulation protocol for the Li10GeP2S12-type superionic conductors[J]. 2021, 154(9): 094703. DOI: 10. 1063/5.0041849. |
| 11 | WANG F, MA Z B, CHENG J. Accelerating computation of acidity constants and redox potentials for aqueous organic redox flow batteries by machine learning potential-based molecular dynamics[J]. Journal of the American Chemical Society, 2024, 146(21): 14566-14575. DOI: 10.1021/jacs.4c01221. |
| 12 | KRESSE G, FURTHMÜLLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Computational Materials Science, 1996, 6(1): 15-50. DOI: 10.1016/0927-0256(96)00008-0. |
| 13 | VANDEVONDELE J, KRACK M, MOHAMED F, et al. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach[J]. Computer Physics Communications, 2005, 167(2): 103-128. DOI: 10.1016/j.cpc. 2004.12.014. |
| 14 | FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 09, Revision E.01[M]. Gaussian, Inc., Wallingford CT, 2013. |
| 15 | GIANNOZZI P, BARONI S, BONINI N, et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials[J]. Journal of Physics Condensed Matter, 2009, 21(39): 395502. DOI: 10.1088/0953-8984/21/39/395502. |
| 16 | WANG H, ZHANG L F, HAN J Q, et al. DeePMD-kit: A deep learning package for many-body potential energy representation and molecular dynamics[J]. Computer Physics Communications, 2018, 228: 178-184. DOI: 10.1016/j.cpc.2018.03.016. |
| 17 | THOMPSON A P, AKTULGA H M, BERGER R, et al. LAMMPS-A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales[J]. Computer Physics Communications, 2022, 271: 108171. DOI: 10.1016/j.cpc. 2021.108171. |
| 18 | LARSEN A H, MORTENSEN J J, BLOMQVIST J, et al. The atomic simulation environment-A Python library for working with atoms[J]. Journal of Physics Condensed Matter, 2017, 29(27): 273002. DOI: 10.1088/1361-648X/aa680e. |
| 19 | CERIOTTI M, MORE J, MANOLOPOULOS D E. I-PI: A Python interface for ab initio path integral molecular dynamics simulations[J]. Computer Physics Communications, 2014, 185(3): 1019-1026. DOI: 10.1016/j.cpc.2013.10.027. |
| 20 | ABRAHAM M J, MURTOLA T, SCHULZ R, et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers[J]. SoftwareX, 2015, 1: 19-25. DOI: 10.1016/j.softx.2015.06.001. |
| 21 | HUA H M, WANG F, WANG F, et al. Machine learning molecular dynamics insight into high interface stability and fast kinetics of low-cost magnesium chloride amine electrolyte for rechargeable magnesium batteries[J]. Energy Storage Materials, 2024, 70: 103470. DOI: 10.1016/j.ensm.2024.103470. |
| 22 | WANG J J, SHEN H, YANG R Y, et al. A deep learning interatomic potential developed for atomistic simulation of carbon materials[J]. Carbon, 2022, 186: 1-8. DOI: 10.1016/j.carbon. 2021.09.062. |
| 23 | OBEID M M, LIU J H, DU P H, et al. A 3D metallic porous sulfurized carbon anode identified by global structure search for Na-ion batteries with fast diffusion kinetics[J]. Journal of Energy Storage, 2024, 82: 110587. DOI: 10.1016/j.est.2024.110587. |
| 24 | XU N, SHI Y, HE Y, et al. A deep-learning potential for crystalline and amorphous Li-Si alloys[J]. The Journal of Physical Chemistry C, 2020, 124(30): 16278-16288. DOI: 10.1021/acs.jpcc.0c03333. |
| 25 | FU F J, WANG X X, ZHANG L F, et al. Unraveling the atomic-scale mechanism of phase transformations and structural evolutions during (de)lithiation in Si anodes[J]. Advanced Functional Materials, 2023, 33(37): 2303936. DOI: 10.1002/adfm.202303936. |
| 26 | JIAO J Y, LAI G M, ZHAO L, et al. Self-healing mechanism of lithium in lithium metal[J]. Advanced Science, 2022, 9(12): e2105574. DOI: 10.1002/advs.202105574. |
| 27 | PHUTHI M K, YAO A M, BATZNER S, et al. Accurate surface and finite-temperature bulk properties of lithium metal at large scales using machine learning interaction potentials[J]. ACS Omega, 2024, 9(9): 10904-10912. DOI: 10.1021/acsomega.3c10014. |
| 28 | LAI G M, JIAO J Y, FANG C, et al. A deep neural network interface potential for Li-Cu systems[J]. Advanced Materials Interfaces, 2022, 9(27): 2201346. DOI: 10.1002/admi.202201346. |
| 29 | LAI G M, JIAO J Y, FANG C, et al. The mechanism of Li deposition on the Cu substrates in the anode-free Li metal batteries[J]. Small, 2023, 19(3): 2205416. DOI: 10.1002/smll. 202205416. |
| 30 | GARCIA J C, GABRIEL J, PAULSON N H, et al. Insights from computational studies on the anisotropic volume change of LixNiO2 at high states of charge (x<0.25)[J]. The Journal of Physical Chemistry C, 2021, 125(49): 27130-27139. DOI: 10. 1021/acs.jpcc.1c08022. |
| 31 | HU T P, DAI F Z, ZHOU G B, et al. Unraveling the dynamic correlations between transition metal migration and the oxygen dimer formation in the highly delithiated LixCoO2 cathode[J]. The Journal of Physical Chemistry Letters, 2023, 14(15): 3677-3684. DOI: 10.1021/acs.jpclett.3c00506. |
| 32 | HU Y C, WANG X X, LI P, et al. Understanding the sluggish and highly variable transport kinetics of lithium ions in LiFePO4[J]. Science China Chemistry, 2023, 66(11): 3297-3306. DOI: 10. 1007/s11426-023-1662-9. |
| 33 | LIN M, LIU X S, XIANG Y X, et al. Unravelling the fast alkali-ion dynamics in paramagnetic battery materials combined with NMR and deep-potential molecular dynamics simulation[J]. Angewandte Chemie International Edtion, 2021, 60(22): 12547-12553. DOI: 10.1002/anie.202102740. |
| 34 | LIN M, XIONG J F, SU M T, et al. A machine learning protocol for revealing ion transport mechanisms from dynamic NMR shifts in paramagnetic battery materials[J]. Chemical Science, 2022, 13(26): 7863-7872. DOI: 10.1039/d2sc01306a. |
| 35 | LIN M, FU R Q, XIANG Y X, et al. Combining NMR and molecular dynamics simulations for revealing the alkali-ion transport in solid-state battery materials[J]. Current Opinion in Electrochemistry, 2022, 35: 101048. DOI: 10.1016/j.coelec.2022.101048. |
| 36 | MARCOLONGO A, BINNINGER T, ZIPOLI F, et al. Simulating diffusion properties of solid-state electrolytes via a neural network potential: Performance and training scheme[J]. ChemSystemsChem, 2020, 2(3). DOI: 10.1002/syst.201900031. |
| 37 | FU Z H, CHEN X, YAO N, et al. The chemical origin of temperature-dependent lithium-ion concerted diffusion in sulfide solid electrolyte Li10GeP2S12[J]. Journal of Energy Chemistry, 2022, 70: 59-66. DOI: 10.1016/j.jechem.2022.01.018. |
| 38 | MIYAGAWA T, KRISHNAN N, GRUMET M, et al. Accurate description of ion migration in solid-state ion conductors from machine-learning molecular dynamics[J]. Journal of Materials Chemistry A, 2024, 12(19): 11344-11361. DOI: 10.1039/D4TA00452C. |
| 39 | GIGLI L, TISI D, GRASSELLI F, et al. Mechanism of charge transport in lithium thiophosphate[J]. Chemistry of Materials, 2024, 36(3): 1482-1496. DOI: 10.1021/acs.chemmater.3c02726. |
| 40 | GUPTA M K, DING J X, OSTI N C, et al. Fast Na diffusion and anharmonic phonon dynamics in superionic Na3PS4[J]. Energy & Environmental Science, 2021, 14(12): 6554-6563. DOI: 10.1039/D1EE01509E. |
| 41 | ZHANG R Y, XU S F, WANG L Y, et al. Theoretical study on ion diffusion mechanism in W-doped K3SbS4 as solid-state electrolyte for K-ion batteries[J]. Inorganic Chemistry, 2024, 63(15): 6743-6751. DOI: 10.1021/acs.inorgchem.4c00074. |
| 42 | LEE J W, KIM J H, KIM J S, et al. Design of multicomponent argyrodite based on a mixed oxidation state as promising solid-state electrolyte using moment tensor potentials[J]. Journal of Materials Chemistry A, 2024, 12(12): 7272-7278. DOI: 10.1039/D4TA00361F. |
| 43 | GUPTA M K, KUMAR S, MITTAL R, et al. Soft-phonon anharmonicity, floppy modes, and Na diffusion in Na3FY(Y=S, Se, Te): Ab initio and machine-learned molecular dynamics simulations[J]. Physical Review B, 2022, 106: 014311. DOI: 10. 1103/physrevb.106.014311. |
| 44 | SELVARAJ S C, KOVERGA V, NGO A T. Exploring Li-ion transport properties of Li3TiCl6: A machine learning molecular dynamics study[J]. Journal of the Electrochemical Society, 2024, 171(5): 050544. DOI: 10.1149/1945-7111/ad4ac9. |
| 45 | ZHANG Z, MA Z Y, PEI Y. Li ion diffusion behavior of Li3OCl solid-state electrolytes with different defect structures: Insights from the deep potential model[J]. Physical Chemistry Chemical Physics, 2023, 25(19): 13297-13307. DOI: 10.1039/d2cp06073f. |
| 46 | LI H X, ZHOU X Y, WANG Y C, et al. Theoretical study of Na+ transport in the solid-state electrolyte Na3OBr based on deep potential molecular dynamics[J]. Inorganic Chemistry Frontiers, 2021, 8(2): 425-432. DOI: 10.1039/D0QI00921K. |
| 47 | LIU J H, WANG S, KAWAZOE Y, et al. A new spinel chloride solid electrolyte with high ionic conductivity and stability for Na-ion batteries[J]. ACS Materials Letters, 2023, 5(4): 1009-1017. DOI: 10.1021/acsmaterialslett.3c00119. |
| 48 | YOU Y W, ZHANG D X, WU F L, et al. Principal component analysis enables the design of deep learning potential precisely capturing LLZO phase transitions[J]. NPJ Computational Materials, 2024, 10: 57. DOI: 10.1038/s41524-024-01240-7. |
| 49 | BALYAKIN I A, VLASOV M I, PERSHINA S V, et al. Neural network molecular dynamics study of LiGe2(PO4)3: Investigation of structure[J]. Computational Materials Science, 2024, 239: 112979. DOI: 10.1016/j.commatsci.2024.112979. |
| 50 | ZHOU R, LUO K, MARTIN S W, et al. Insights into lithium sulfide glass electrolyte structures and ionic conductivity via machine learning force field simulations[J]. ACS Applied Materials & Interfaces, 2024, 16(15): 18874-18887. DOI: 10.1021/acsami. 4c00618. |
| 51 | GUPTA S, YANG X C, CEDER G. What dictates soft clay-like lithium superionic conductor formation from rigid salts mixture[J]. Nature Communications, 2023, 14(1): 6884. DOI: 10.1038/s41467-023-42538-2. |
| 52 | YANG X C, GUPTA S, CHEN Y, et al. Fast room-temperature Mg-ion conduction in clay-like halide glassy electrolytes[J]. Advanced Energy Materials, 2024, 14(26): 2400163. DOI: 10.1002/aenm. 202400163. |
| 53 | ZHANG D X, YOU Y W, WU F L, et al. Exploring the relationship between composition and Li-ion conductivity in the amorphous Li-La-Zr-O system[J]. ACS Materials Letters, 2024, 6(5): 1849-1855. DOI: 10.1021/acsmaterialslett.3c01558. |
| 54 | DAI T, WU S Y, LU Y X, et al. Inorganic glass electrolytes with polymer-like viscoelasticity[J]. Nature Energy, 2023, 8: 1221-1228. DOI: 10.1038/s41560-023-01356-y. |
| 55 | WANG F, CHENG J. Automated workflow for computation of redox potentials, acidity constants, and solvation free energies accelerated by machine learning[J]. Journal of Chemical Physics, 2022, 157(2): 024103. DOI: 10.1063/5.0098330. |
| 56 | WANG F, SUN Y, CHENG J. Switching of redox levels leads to high reductive stability in water-in-salt electrolytes[J]. Journal of the American Chemical Society, 2023, 145(7): 4056-4064. DOI: 10.1021/jacs.2c11793. |
| 57 | ZHANG C Y, YUE S W, PANAGIOTOPOULOS A Z, et al. Why dissolving salt in water decreases its dielectric permittivity[J]. Physical Review Letters, 2023, 131(7): 076801. DOI: 10.1103/PhysRevLett.131.076801. |
| 58 | PANAGIOTOPOULOS A Z, YUE S W. Dynamics of aqueous electrolyte solutions: Challenges for simulations[J]. The Journal of Physical Chemistry B, 2023, 127(2): 430-437. DOI: 10.1021/acs.jpcb.2c07477. |
| 59 | ZHU D, SHENG L, HU T P, et al. Investigation of the degradation of LiPF6 - in polar solvents through deep potential molecular dynamics[J]. The Journal of Physical Chemistry Letters, 2024, 15(15): 4024-4030. DOI: 10.1021/acs.jpclett.4c00575. |
| 60 | LING Y L, LI K, WANG M, et al. Revisiting the structure, interaction, and dynamical property of ionic liquid from the deep learning force field[J]. Journal of Power Sources, 2023, 555: 232350. DOI: 10.1016/j.jpowsour.2022.232350. |
| 61 | XU M Y, ZHU T, ZHANG J Z H. Molecular dynamics simulation of zinc ion in water with an ab initio based neural network potential[J]. The Journal of Physical Chemistry A, 2019, 123(30): 6587-6595. DOI: 10.1021/acs.jpca.9b04087. |
| 62 | LIU J C, LIU R X, CAO Y, et al. Solvation structures of calcium and magnesium ions in water with the presence of hydroxide: A study by deep potential molecular dynamics[J]. Physical Chemistry Chemical Physics, 2023, 25(2): 983-993. DOI: 10. 1039/d2cp04105g. |
| 63 | WANG F, CHENG J. Understanding the solvation structures of glyme-based electrolytes by machine learning molecular dynamics[J]. Chinese Journal of Structural Chemistry, 2023, 42(9): 100061. DOI: 10.1016/j.cjsc.2023.100061. |
| 64 | XU T R, LI X J, WANG Y, et al. Development of deep potentials of molten MgCl2-NaCl and MgCl2-KCl salts driven by machine learning[J]. ACS Applied Materials & Interfaces, 2023. DOI: 10.1021/acsami.2c19272. |
| 65 | QI S M, BO T, ZHANG L, et al. Machine-learning-driven simulations on microstructure, thermodynamic properties, and transport properties of LiCl-KCl-LiF molten salt[J]. Artificial Intelligence Chemistry, 2024, 2(1): 100027. DOI: 10.1016/j.aichem.2023.100027. |
| 66 | LE J B, CHEN A, LI L, et al. Modeling electrified Pt(111)-Had/water interfaces from ab initio molecular dynamics[J]. JACS Au, 2021, 1(5): 569-577. DOI: 10.1021/jacsau.1c00108. |
| 67 | LE J B, FAN Q Y, LI J Q, et al. Molecular origin of negative component of Helmholtz capacitance at electrified Pt(111)/water interface[J]. Science Advances, 2020, 6(41): eabb1219. DOI: 10.1126/sciadv.abb1219. |
| 68 | LI J Q, SUN Y, CHENG J. Theoretical investigation on water adsorption conformations at aqueous anatase TiO2/water interfaces[J]. Journal of Materials Chemistry A, 2023, 11(2): 943-952. DOI: 10.1039/D2TA07994A. |
| 69 | ZHUANG Y B, CHENG J. Deciphering the anomalous acidic tendency of terminal water at rutile(110)-water interfaces[J]. The Journal of Physical Chemistry C, 2023, 127(22): 10532-10540. DOI: 10.1021/acs.jpcc.3c01870. |
| 70 | BIN JASSAR M, MICHEL C, ABADA S, et al. A perspective on the molecular modeling of electrolyte decomposition reactions for solid electrolyte interphase growth in lithium-ion batteries[J]. Advanced Functional Materials, 2024, 34(30): 2313188. DOI: 10.1002/adfm.202313188. |
| 71 | REN F C, WU Y Q, ZUO W H, et al. Visualizing the SEI formation between lithium metal and solid-state electrolyte[J]. Energy & Environmental Science, 2024, 17(8): 2743-2752. DOI: 10.1039/D3EE03536K. |
| 72 | HU T P, XU L H, DAI F Z, et al. Impact of amorphous LiF coating layers on cathode-electrolyte interfaces in solid-state batteries[J]. Advanced Functional Materials, 2024: 2402993. DOI: 10.1002/adfm.202402993. |
| 73 | HU T P, TIAN J X, DAI F Z, et al. Impact of the local environment on Li ion transport in inorganic components of solid electrolyte interphases[J]. Journal of the American Chemical Society, 2023, 145(2): 1327-1333. DOI: 10.1021/jacs.2c11521. |
| 74 | CHEN M, GUO G C, HE L. Systematically improvable optimized atomic basis sets for ab initio calculations [J]. Journal of Physics: Condensed Matter, 2010, 22(44): 445501. |
| [1] | Jing XU, Yuqi WANG, Xiao FU, Qifan YANG, Jingchen LIAN, Liqi WANG, Ruijuan XIAO. Discovery of new battery materials based on a big data approach [J]. Energy Storage Science and Technology, 2024, 13(9): 2920-2932. |
| [2] | Lijie YANG. Research on the application of phase change energy storage materials in construction engineering [J]. Energy Storage Science and Technology, 2024, 13(5): 1471-1473. |
| [3] | Hongpei NIU. Research on the application of phase change energy storage materials in energy saving building design [J]. Energy Storage Science and Technology, 2024, 13(3): 847-849. |
| [4] | Wen PEI. Preparation and thermal properties analysis of phase change energy storage materials in marine logistics [J]. Energy Storage Science and Technology, 2024, 13(3): 844-846. |
| [5] | Jie JU, Ruifang CHEN, Gang WEI. Application of new phase change energy storage materials in building engineering [J]. Energy Storage Science and Technology, 2023, 12(12): 3883-3885. |
| [6] | Siqi SHI, Zhangwei TU, Xinxin ZOU, Shiyu SUN, Zhengwei YANG, Yue LIU. Applying data-driven machine learning to studying electrochemical energy storage materials [J]. Energy Storage Science and Technology, 2022, 11(3): 739-759. |
| [7] | Bowen YUE, Jiahuan TONG, Yuwen LIU, Feng HUO. Simulation calculation method and application of ionic liquid electrolyte [J]. Energy Storage Science and Technology, 2022, 11(3): 897-911. |
| [8] | Niangzhi LIN, Chuanchang LI. Phase change materials for energy storage in cold-chain transportation [J]. Energy Storage Science and Technology, 2021, 10(3): 1040-1050. |
| [9] | WAN Qian, XIAO Haonan, QIAN Jing, HE Zhengbin, YI Songlin. Influence of iron foam on paraffin phase change heat storage process [J]. Energy Storage Science and Technology, 2020, 9(1): 94-100. |
| [10] | LI Chuan1, LI Qi2, JIANG Zhu1, CAO Hui1, QIAO Geng3, LI Yongliang1, LEI Xianzhang3, DING Yulong1. Charging and discharging behavior of carbonate-based salt composite phase change material modules [J]. Energy Storage Science and Technology, 2017, 6(4): 655-661. |
| [11] | ZHAO Liang, WANG Haiyang, FANG Xiangchen, WANG Gang, XU Hong. Modification of fly ash as a carrier of paraffin wax based phase change energy storage material for waste heat recovery [J]. Energy Storage Science and Technology, 2013, 2(6): 598-602. |
| [12] | LENG Guanghui, WU Jianfeng, XU Xiaohong. Encapsulation of PCM in ceramic thermal energy storage materials [J]. Energy Storage Science and Technology, 2012, 1(2): 123-130. |
| [13] | GE Zhiwei, YE Feng, Mathieu Lasfargues, YANG Jun, DING Yulong. Recent progress and prospective of medium and high temperatures thermal energy storage materials [J]. Energy Storage Science and Technology, 2012, 1(2): 89-102. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
