Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (3): 781-794.doi: 10.19799/j.cnki.2095-4239.2021.0672
Previous Articles Next Articles
Zhiwei ZHAO( ), Zhi YANG, Zhangquan PENG(
), Zhi YANG, Zhangquan PENG( )
)
Received:2021-12-14
Revised:2022-01-06
Online:2022-03-05
Published:2022-03-11
Contact:
Zhangquan PENG
E-mail:zwzhao@dicp.ac.cn;zqpeng@dicp.ac.cn
CLC Number:
Zhiwei ZHAO, Zhi YANG, Zhangquan PENG. Application of time-of-flight secondary ion mass spectrometry in lithium-based rechargeable batteries[J]. Energy Storage Science and Technology, 2022, 11(3): 781-794.
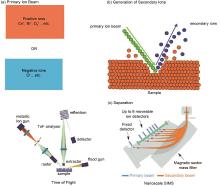
Fig. 1
Principles of secondary ion mass spectrometry (SIMS) analysis: (a) SIMS primary ion beam: positive ions or negative ions; (b) the primary ion beam strikes the sample surface during sputtering and then produces secondary ions; (c) secondary ions are accelerated towards the detector, either through a flight tube (ToF-SIMS) or through magnetic separation using a quadrupole (NanoSIMS)[23]"
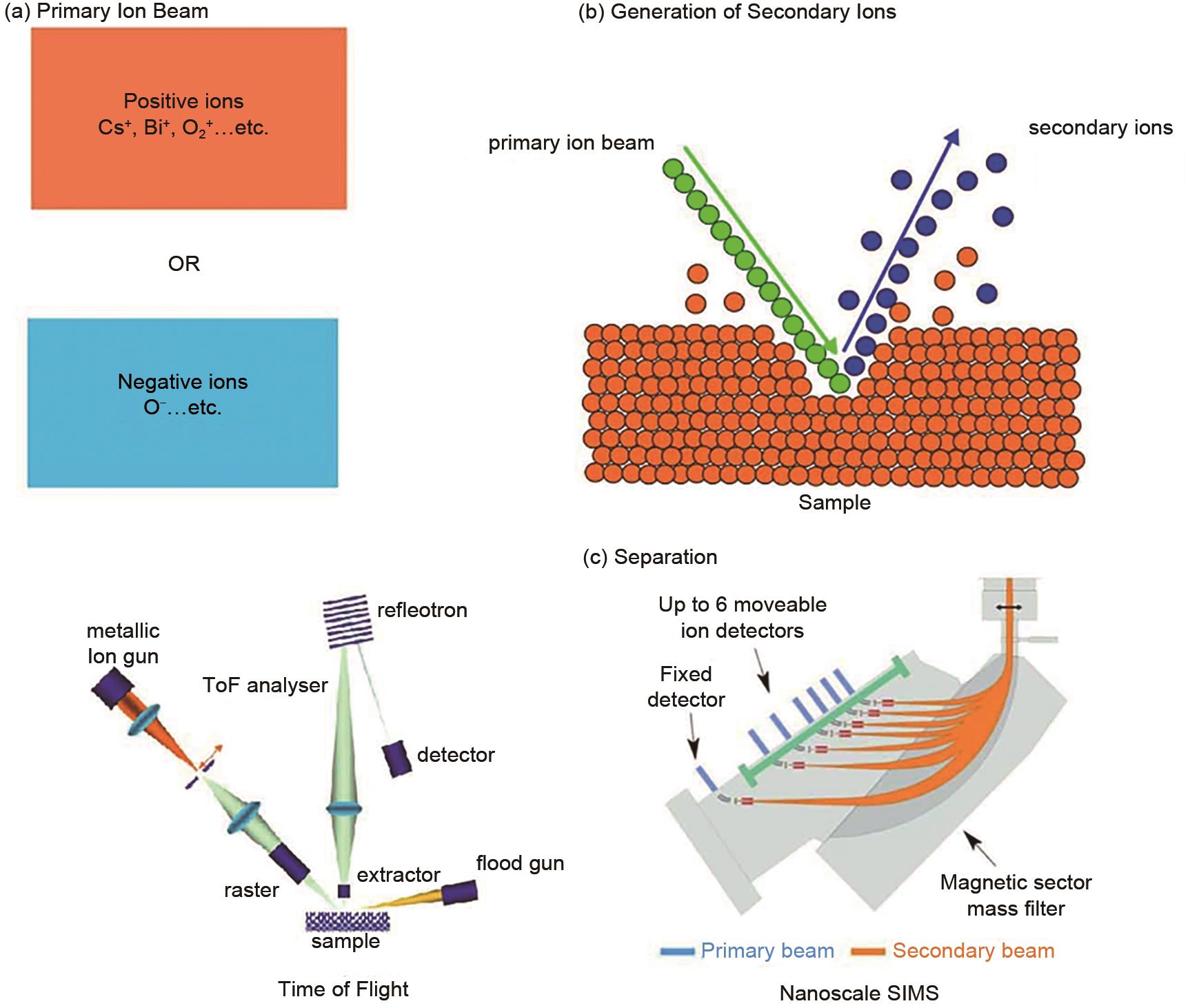
Fig. 2
The first in situ electrochemical ToF-SIMS analysis device, including (a) side view, (b) top view of the Pt counter electrode and reference electrode, (c) the top view of the Au working electrode, (d) the photograph of the device and (e) EC-cell assembly on the ToF-SIMS stage; (f) a schematic illustration of the aperture evolution during in situ measurements[26]"
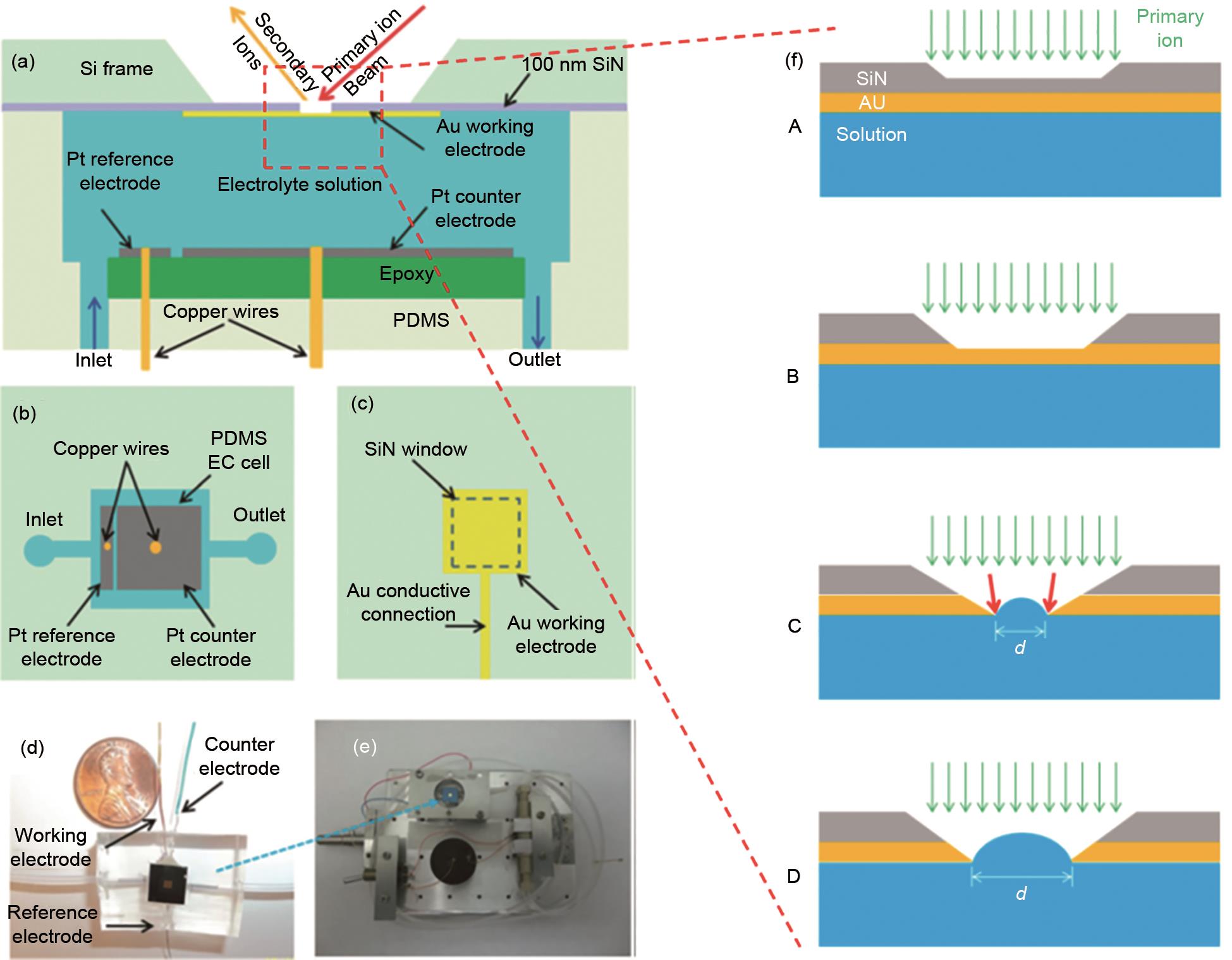
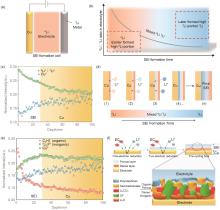
Fig. 5
(a) schematic of a model cell; (b) Li isotope ratio variation trend over time in electrolyte during SEI formation process; (c) SIMS depth profiles of the 6Li∶ 7Li ratio and Cu+ of SEI on Cu electrode surface; (d) schematic of 6Li∶ 7Li isotope ratio variation over time on SEI of Cu electrode surface; (e) the depth profile of secondary ion of C2H2-(organic), Li2F-(inorganic) and Cu+ on Cu electrode; (f) schematic of SEI formation mechanism[37]"
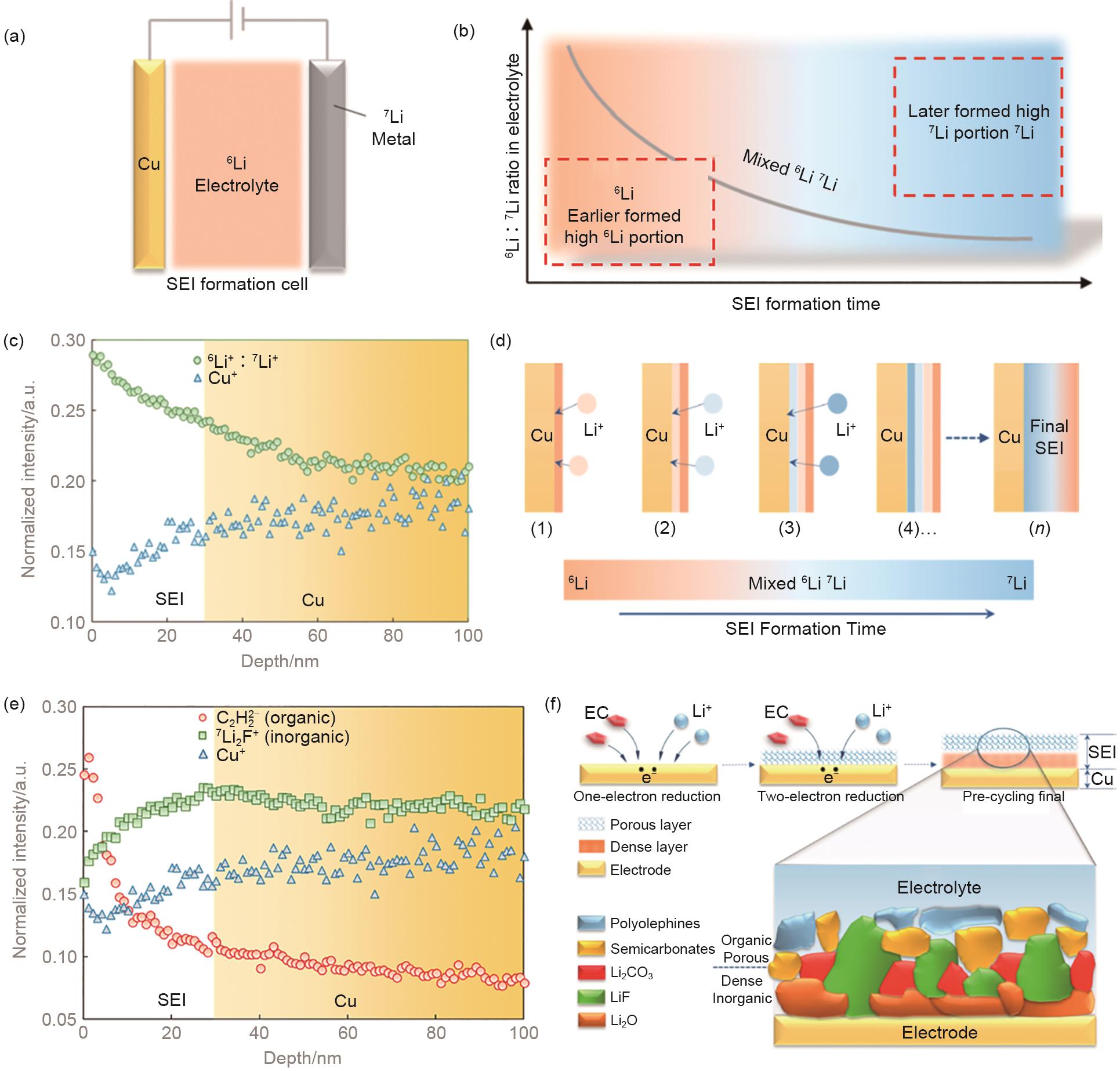
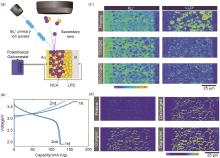
Fig. 7
(a) schematic illustration of in situ ToF-SIMS measurement performed on an all-solid-state lithium-ion battery; (b) Charge and discharge profiles for the first and second cycle; (c) evolution of the distribution of 6Li+ and Li2O+ fragments during cycling; (d) evolution of the intensity of PO2- and PO3- secondary ion fragments during the cycling of the all-solid-state lithium-ion battery [42]"

Fig. 8
(a) depth profiles of LiO-, LiS- and H- secondary ions that represents respectively Li2O, Li2S and various hydrogen-containing interphasial species, at 5, 40 and 300 cycles on lithium anode; (b) depth profiles of LiH-, LiOH- and C2H3- secondary ions that represents respectively LiH, LiOH and various organic interphasial species, at 5, 40, and 300 cycles on lithium anode; (c), (d) 3D reconstructions of the SIMS signal for LiH- and LiS- at 5, 40, and 300 cycles[45]"
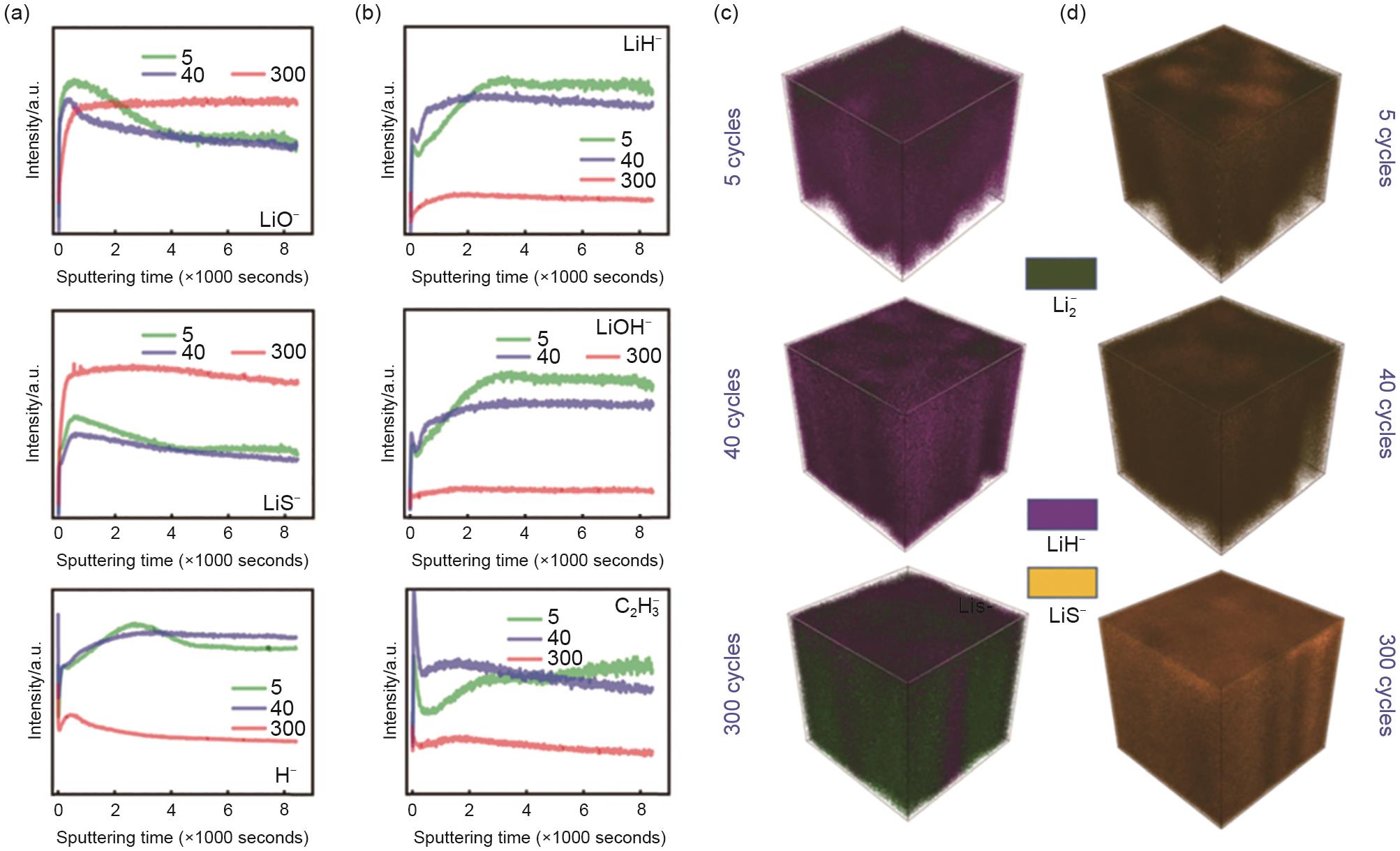

Fig. 9
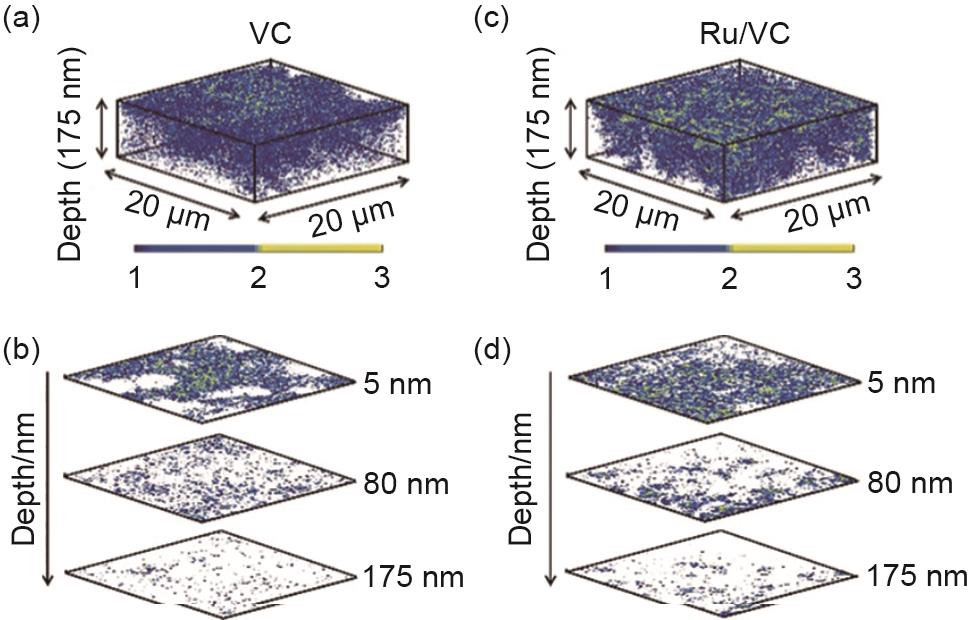
3D distribution of secondary ion 18O- on the discharge electrode with ToF-SIMS depth scan: (a) 3D distribution of 18O- on the carbon (VC) electrode; (b) selected layers from the reconstructed 3D image of 18O- on the VC electrode at three depths; (c) 3D distribution of 18O- on the carbon load with Ru (Ru/VC) electrode; (d) selected layers from the reconstructed 3D image of 18O- on the Ru/VC electrode at three depths[48]"
| 1 | ARMAND M, TARASCON J M. Building better batteries[J]. Nature, 2008, 451(7179): 652-657. |
| 2 | LI M, LU J, CHEN Z, et al. 30 years of lithium-ion batteries[J]. Advanced Materials, 2018, doi: 10.1002/adma.201800561. |
| 3 | LU J, WU T, AMINE K. State-of-the-art characterization techniques for advanced lithium-ion batteries[J]. Nature Energy, 2017, 2: doi: 10.1038/nenergy.2017.11. |
| 4 | BRUCE P G, FREUNBERGER S A, HARDWICK L J, et al. Li-O2 and Li-S batteries with high energy storage[J]. Nature Materials, 2012, 11(1): 19-29. |
| 5 | PENG Z Q, FREUNBERGER S A, CHEN Y H, et al. A reversible and higher-rate Li-O2 battery[J]. Science, 2012, 337(6094): 563-566. |
| 6 | ASADI M, SAYAHPOUR B, ABBASI P, et al. A lithium-oxygen battery with a long cycle life in an air-like atmosphere[J]. Nature, 2018, 555(7697): 502-506. |
| 7 | LUNTZ A C, MCCLOSKEY B D. Nonaqueous Li-air batteries: A status report[J]. Chemical Reviews, 2014, 114(23): 11721-11750. |
| 8 | KWAK W J, ROSY, SHARON D, et al. Lithium-oxygen batteries and related systems: Potential, status, and future[J]. Chemical Reviews, 2020, 120(14): 6626-6683. |
| 9 | GAO H, GALLANT B M. Advances in the chemistry and applications of alkali-metal-gas batteries[J]. Nature Reviews Chemistry, 2020, 4(11): 566-583. |
| 10 | XU R, LU J, AMINE K. Progress in mechanistic understanding and characterization techniques of Li-S batteries[J]. Advanced Energy Materials, 2015, 5(16): doi: 10.1002/aenm.201500408. |
| 11 | ZAERA F. Probing liquid/solid interfaces at the molecular level[J]. Chemical Reviews, 2012, 112(5): 2920-2986. |
| 12 | LI X N, WANG H Y, YANG H B, et al. In situ/operando characterization techniques to probe the electrochemical reactions for energy conversion[J]. Small Methods, 2018, 2(6): doi: 10.1002/smtd.201700395. |
| 13 | LI H Y, GUO S H, ZHOU H S. In-situ/operando characterization techniques in lithium-ion batteries and beyond[J]. Journal of Energy Chemistry, 2021, 59: 191-211. |
| 14 | CHENG X B, ZHANG R, ZHAO C Z, et al. A review of solid electrolyte interphases on lithium metal anode[J]. Advanced Science, 2015, 3(3): doi: 10.1002/advs.201500213. |
| 15 | BHATTACHARYYA R, KEY B, CHEN H, et al. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries[J]. Nature Materials, 2010, 9(6): 504-510. |
| 16 | HUANG J Y, ZHONG L, WANG C M, et al. In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode[J]. Science, 2010, 330(6010): 1515-1520. |
| 17 | LIU X, WANG D, LIU G, et al. Distinct charge dynamics in battery electrodes revealed by in situ and operando soft X-ray spectroscopy[J]. Nature Communications, 2013, 4: doi: 10.1038/ncomms3568. |
| 18 | EBNER M, MARONE F, STAMPANONI M, et al. Visualization and quantification of electrochemical and mechanical degradation in Li ion batteries[J]. Science, 2013, 342(6159): 716-720. |
| 19 | LIU H, STROBRIDGE F C, BORKIEWICZ O J, et al. Capturing metastable structures during high-rate cycling of LiFePO4 nanoparticle electrodes[J]. Science, 2014, 344(6191): doi: 10.1126/science.1252817. |
| 20 | ZHU Z H, ZHOU Y F, YAN P F, et al. In situ mass spectrometric determination of molecular structural evolution at the solid electrolyte interphase in lithium-ion batteries[J]. Nano Letters, 2015, 15(9): 6170-6176. |
| 21 | LIU P Y, LU M, ZHENG Q L, et al. Recent advances of electrochemical mass spectrometry[J]. The Analyst, 2013, 138(19): 5519-5539. |
| 22 | LU J S, HUA X, LONG Y T. Recent advances in real-time and in situ analysis of an electrode-electrolyte interface by mass spectrometry[J]. The Analyst, 2017, 142(5): 691-699. |
| 23 | BONNIN E A, RIZZOLI S O. Novel secondary ion mass spectrometry methods for the examination of metabolic effects at the cellular and subcellular levels[J]. Frontiers in Behavioral Neuroscience, 2020, 14: doi: 10.3389/fnbeh.2020.00124. |
| 24 | FLETCHER J S. Latest applications of 3D ToF-SIMS bio-imaging[J]. Biointerphases, 2015, 10(1): doi: 10.1116/1.4907727. |
| 25 | NUÑEZ J, RENSLOW R, CLIFF J B 3rd, et al. NanoSIMS for biological applications: Current practices and analyses[J]. Biointerphases, 2017, 13(3): doi: 10.1116/1.4993628. |
| 26 | LIU B W, YU X Y, ZHU Z H, et al. In situ chemical probing of the electrode-electrolyte interface by ToF-SIMS[J]. Lab on a Chip, 2014, 14(5): 855-859. |
| 27 | XU K. Electrolytes and interphases in Li-ion batteries and beyond[J]. Chemical Reviews, 2014, 114(23): 11503-11618. |
| 28 | NIE M Y, ABRAHAM D P, SEO D M, et al. Role of solution structure in solid electrolyte interphase formation on graphite with LiPF6 in propylene carbonate[J]. The Journal of Physical Chemistry C, 2013, 117(48): 25381-25389. |
| 29 | HE Y T, ZHANG Y H, YU P, et al. Ion association tailoring SEI composition for Li metal anode protection[J]. Journal of Energy Chemistry, 2020, 45: 1-6. |
| 30 | ZHOU L, CAO Z, WAHYUDI W, et al. Electrolyte engineering enables high stability and capacity alloying anodes for sodium and potassium ion batteries[J]. ACS Energy Letters, 2020, 5(3): 766-776. |
| 31 | BORODIN O, REN X M, VATAMANU J, et al. Modeling insight into battery electrolyte electrochemical stability and interfacial structure[J]. Accounts of Chemical Research, 2017, 50(12): 2886-2894. |
| 32 | CRESCE A, BORODIN O, XU K. Correlating Li+ solvation sheath structure with interphasial chemistry on graphite[J]. The Journal of Physical Chemistry C, 2012, 116(50): 26111-26117. |
| 33 | ZHANG Y Y, SU M, YU X F, et al. Investigation of ion-solvent interactions in nonaqueous electrolytes using in situ liquid SIMS[J]. Analytical Chemistry, 2018, 90(5): 3341-3348. |
| 34 | ZHOU Y, SU M, YU X, et al. Real-time mass spectrometric characterization of the solid-electrolyte interphase of a lithium-ion battery[J]. Nature Nanotechnology, 2020, 15(3): 224-230. |
| 35 | USHIROGATA K, SODEYAMA K, FUTERA Z, et al. Near-shore aggregation mechanism of electrolyte decomposition products to explain solid electrolyte interphase formation[J]. Journal of the Electrochemical Society, 2015, 162(14): A2670-A2678. |
| 36 | TAKENAKA N, SUZUKI Y, SAKAI H, et al. On electrolyte-dependent formation of solid electrolyte interphase film in lithium-ion batteries: Strong sensitivity to small structural difference of electrolyte molecules[J]. The Journal of Physical Chemistry C, 2014, 118(20): 10874-10882. |
| 37 | LIU Z, LU P, ZHANG Q L, et al. A bottom-up formation mechanism of solid electrolyte interphase revealed by isotope-assisted time-of-flight secondary ion mass spectrometry[J]. The Journal of Physical Chemistry Letters, 2018, 9(18): 5508-5514. |
| 38 | LU P, HARRIS S J. Lithium transport within the solid electrolyte interphase[J]. Electrochemistry Communications, 2011, 13(10): 1035-1037. |
| 39 | RANDAU S, WEBER D A, KÖTZ O, et al. Benchmarking the performance of all-solid-state lithium batteries[J]. Nature Energy, 2020, 5(3): 259-270. |
| 40 | BIELEFELD A, WEBER D A, JANEK J. Microstructural modeling of composite cathodes for all-solid-state batteries[J]. The Journal of Physical Chemistry C, 2019, 123(3): 1626-1634. |
| 41 | SAKUDA A, TAKEUCHI T, KOBAYASHI H. Electrode morphology in all-solid-state lithium secondary batteries consisting of LiNi1/3Co1/3Mn1/3O2 and Li2S-P2S5 solid electrolytes[J]. Solid State Ionics, 2016, 285: 112-117. |
| 42 | YAMAGISHI Y, MORITA H, NOMURA Y, et al. Visualizing lithium distribution and degradation of composite electrodes in sulfide-based all-solid-state batteries using operando time-of-flight secondary ion mass spectrometry[J]. ACS Applied Materials & Interfaces, 2021, 13(1): 580-586. |
| 43 | LIU J, BAO Z, CUI Y, et al. Pathways for practical high-energy long-cycling lithium metal batteries[J]. Nature Energy, 2019, 4(3): 180-186. |
| 44 | CHEN S R, NIU C J, LEE H, et al. Critical parameters for evaluating coin cells and pouch cells of rechargeable Li-metal batteries[J]. Joule, 2019, 3(4): 1094-1105. |
| 45 | NANDA S, MANTHIRAM A. Lithium degradation in lithium-sulfur batteries: Insights into inventory depletion and interphasial evolution with cycling[J]. Energy & Environmental Science, 2020, 13(8): 2501-2514. |
| 46 | ZHAO Z W, HUANG J, PENG Z Q. Achilles' heel of lithium-air batteries: Lithium carbonate[J]. Angewandte Chemie, 2018, 57(15): 3874-3886. |
| 47 | WANG J W, ZHANG Y L, GUO L M, et al. Identifying reactive sites and transport limitations of oxygen reactions in aprotic lithium-O2 batteries at the stage of sudden death[J]. Angewandte Chemie, 2016, 55(17): 5201-5205. |
| 48 | WANG Y, LU Y C. Isotopic labeling reveals active reaction interfaces for electrochemical oxidation of lithium peroxide[J]. Angewandte Chemie, 2019, 131(21): 7036-7040. |
| [1] | Yun TANG, Fang YUE, Kaimo GUO, Lanchun LI, Wei CHEN. International development trend analysis of next-generation electrochemical energy storage technology [J]. Energy Storage Science and Technology, 2022, 11(1): 89-97. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
