Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (9): 2971-2984.doi: 10.19799/j.cnki.2095-4239.2023.0305
• Energy Storage Test: Methods and Evaluation • Previous Articles Next Articles
Yonghui ZHANG1,2( ), Jie FU1, Xianfeng LI2(
), Jie FU1, Xianfeng LI2( ), Changkun ZHANG2(
), Changkun ZHANG2( )
)
Received:2023-05-04
Revised:2023-06-02
Online:2023-09-05
Published:2023-09-16
Contact:
Xianfeng LI, Changkun ZHANG
E-mail:zhangyonghui@dicp.ac.cn;lixianfeng@dicp.ac.cn;zhangchk17@dicp.ac.cn
CLC Number:
Yonghui ZHANG, Jie FU, Xianfeng LI, Changkun ZHANG. Research progress on in-situ characterization techniques for aqueous organic flow batteries[J]. Energy Storage Science and Technology, 2023, 12(9): 2971-2984.

Fig. 3
(a) Experimentally determined fraction of DHAQ?3-radicals; (b) Insitupseudo-2D 1H NMR spectra of anolyte; (c) In situ pseudo-2D 1H NMR spectra of catholyte[44]; (d) and (e) In situ NMR spectra; (f) Proton labels of the several anthraquinone anions[46]; (g) Insitupseudo-2D 1H NMR spectra of MB; (h) Current and potential profiles; (i) Molecular structures of oxidation (red), radical (yellow) and reduction (blue)[26]"


Fig. 5
(a) Operando FTIR spectra of 2, 6-DHAQ during reduction and oxidation processes in the first cycle of CV scan; (b) Operando FTIR spectra of 1, 5-DHAQ during reduction and oxidation processes in the first cycle of CV scan; (c) The hydrogen bond-mediated degradation process of 2, 6-DHAQ and 1, 5-DHAQ [15]; (d) On-line ATR-FTIR spectra of DHPS electrolyte recorded duringcycling[51]"
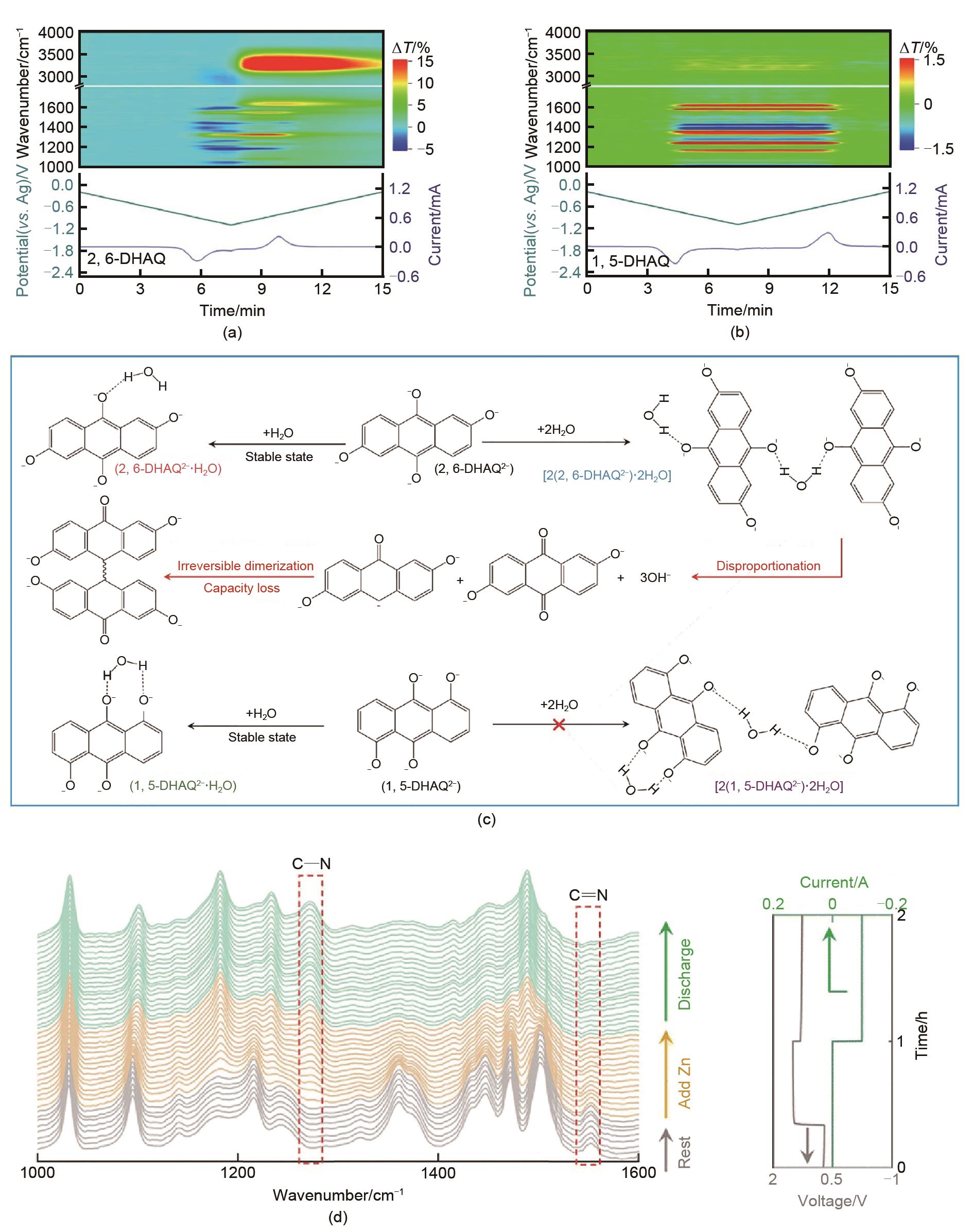
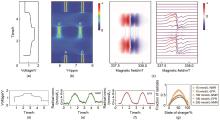
Fig. 9
(a) and (d) Voltage of a 10 mmol/L DHAQ versus 15 mmol/L K4[Fe(CN)6] and 3.75 mmol/L K3[Fe(CN)6] full cell as a function of time[61]; (b) NMR spectra of the anolyte in the aromatic region; (c) EPR spectra of the anolyte; (e), (f) Concentrations of DHAQ3–? radical anions as a function of time, estimated from the NMR spectra and EPR experiments, respectively; (g) Fractions of radicals as a function of state of charge for total concentrations of 10 mmol/L, 100 mmol/L, and 200 mmol/L DHAQ"

| 1 | Intergovernmental Panel on Climate Change. Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group Ⅲ to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change[C]. Cambridge University Press, doi: 10.1017/9781009157926. |
| 2 | Achieving net zero emissions with machine learning: The challenge ahead[J]. Nature Machine Intelligence, 2022, 4(8): 661-662. |
| 3 | LENNON A, LUNARDI M, HALLAM B, et al. The aluminium demand risk of terawatt photovoltaics for net zero emissions by 2050[J]. Nature Sustainability, 2022, 5(4): 357-363. |
| 4 | THOMPSON H. The geopolitics of fossil fuels and renewables reshape the world[J]. Nature, 2022, 603(7901): 364. |
| 5 | 陈海生, 李泓, 马文涛, 等. 2021年中国储能技术研究进展[J]. 储能科学与技术, 2022, 11(3): 1052-1076. |
| CHEN H S, LI H, MA W T, et al. Research progress of energy storage technology in China in 2021[J]. Energy Storage Science and Technology, 2022, 11(3): 1052-1076. | |
| 6 | 唐奡, 严川伟. 液流电池模拟仿真研究现状与展望[J]. 储能科学与技术, 2022, 11(9): 2866-2878. |
| TANG A, YAN C W. Modelling and simulation of flow batteries: Recent progress and prospects[J]. Energy Storage Science and Technology, 2022, 11(9): 2866-2878. | |
| 7 | 王瑄, 叶强. 全钒液流电池电堆局部供液不足导致副反应加剧的现象[J]. 储能科学与技术, 2022, 11(5): 1455-1467. |
| WANG X, YE Q. The aggravation of side reactions caused by insufficient localized liquid supply in an all-vanadium redox flow battery stack[J]. Energy Storage Science and Technology, 2022, 11(5): 1455-1467. | |
| 8 | 张华民, 王晓丽. 全钒液流电池技术最新研究进展[J]. 储能科学与技术, 2013, 2(3): 281-288. |
| ZHANG H M, WANG X L. Recent progress on vanadium flow battery technologies[J]. Energy Storage Science and Technology, 2013, 2(3): 281-288. | |
| 9 | LOURENSSEN K, WILLIAMS J, AHMADPOUR F, et al. Vanadium redox flow batteries: A comprehensive review[J]. Journal of Energy Storage, 2019, 25: 100844. |
| 10 | 王晓丽, 张宇, 张华民. 全钒液流电池储能技术开发与应用进展[J]. 电化学, 2015, 21(5): 433-440. |
| WANG X L, ZHANG Y, ZHANG H M. Latest progresses in vanadium flow battery technologies and applications[J]. Journal of Electrochemistry, 2015, 21(5): 433-440. | |
| 11 | 杨霖霖, 廖文俊, 苏青, 等. 全钒液流电池技术发展现状[J]. 储能科学与技术, 2013, 2(2): 140-145. |
| YANG L L, LIAO W J, SU Q, et al. The research & development status of vanadium redox flow battery[J]. Energy Storage Science and Technology, 2013, 2(2): 140-145. | |
| 12 | DING Y, ZHANG C K, ZHANG L Y, et al. Molecular engineering of organic electroactive materials for redox flow batteries[J]. Chemical Society Reviews, 2018, 47(1): 69-103. |
| 13 | CAO J Y, TIAN J Y, XU J A, et al. Organic flow batteries: Recent progress and perspectives[J]. Energy & Fuels, 2020, 34(11): 13384-13411. |
| 14 | AMINI K, KERR E F, GEORGE T Y, et al. An extremely stable, highly soluble monosubstituted anthraquinone for aqueous redox flow batteries[J]. Advanced Functional Materials, 2023, 33(13): 2211338. |
| 15 | HUANG S Q, ZHANG H, SALLA M, et al. Molecular engineering of dihydroxyanthraquinone-based electrolytes for high-capacity aqueous organic redox flow batteries[J]. Nature Communications, 2022, 13: 4746. |
| 16 | WU M, BAHARI M, FELL E M, et al. High-performance anthraquinone with potentially low cost for aqueous redox flow batteries[J]. Journal of Materials Chemistry A, 2021, 9(47): 26709-26716. |
| 17 | WU M, JING Y, WONG A A, et al. Extremely stable anthraquinone negolytes synthesized from common precursors[J]. Chem, 2020, 6(6): 1432-1442. |
| 18 | HU B, HU M W, LUO J A, et al. A stable, low permeable TEMPO catholyte for aqueous total organic redox flow batteries[J]. Advanced Energy Materials, 2022, 12(8): 2102577. |
| 19 | LIU Y H, GOULET M A, TONG L C, et al. A long-lifetime all-organic aqueous flow battery utilizing TMAP-TEMPO radical[J]. Chem, 2019, 5(7): 1861-1870. |
| 20 | ZHOU W B, LIU W J, QIN M, et al. Fundamental properties of TEMPO-based catholytes for aqueous redox flow batteries: Effects of substituent groups and electrolytes on electrochemical properties, solubilities and battery performance[J]. RSC Advances, 2020, 10(37): 21839-21844. |
| 21 | HUANG M B, HU S Z, YUAN X Z, et al. Five-membered-heterocycle bridged viologen with high voltage and superior stability for flow battery[J]. Advanced Functional Materials, 2022, 32(16): 2111744. |
| 22 | LI H B, FAN H, HU B, et al. Spatial structure regulation: A rod-shaped viologen enables long lifetime in aqueous redox flow batteries[J]. Angewandte Chemie International Edition, 2021, 60(52): 26971-26977. |
| 23 | XU J C, PANG S, WANG X Y, et al. Ultrastable aqueous phenazine flow batteries with high capacity operated at elevated temperatures[J]. Joule, 2021, 5(9): 2437-2449. |
| 24 | ZHANG L Y, QIAN Y M, FENG R Z, et al. Reversible redox chemistry in azobenzene-based organic molecules for high-capacity and long-life nonaqueous redox flow batteries[J]. Nature Communications, 2020, 11: 3843. |
| 25 | KWON G, LEE S C, HWANG J, et al. Multi-redox molecule for high-energy redox flow batteries[J]. Joule, 2018, 2(9): 1771-1782. |
| 26 | ZHANG Y H, LI F, LI T Y, et al. Insights into an air-stable methylene blue catholyte towards kW-scale practical aqueous organic flow batteries[J]. Energy & Environmental Science, 2023, 16(1): 231-240. |
| 27 | WANG W. Reversible ketone hydrogenation and dehydrogenation for aqueous organic redox flow batteries[J]. ECS Meeting Abstracts, 2021, (5): 1821. |
| 28 | XU D H, ZHANG C J, LI Y D. Molecular engineering the naphthalimide compounds as high-capacity anolyte for nonaqueous redox flow batteries[J]. Chemical Engineering Journal, 2022, 439: 135766. |
| 29 | DING Y, YU G H. Molecular engineering enables better organic flow batteries[J]. Chem, 2017, 3(6): 917-919. |
| 30 | PARK M, RYU J, WANG W, et al. Material design and engineering of next-generation flow-battery technologies[J]. Nature Reviews Materials, 2017, 2: 16080. |
| 31 | WANG H, SAYED S Y, LUBER E J, et al. Redox flow batteries: How to determine electrochemical kinetic parameters[J]. ACS Nano, 2020, 14(3): 2575-2584. |
| 32 | LI Z Y, XU Y, MA K J, et al. In situ detection of electrochemical reaction by weak measurement[J]. Optics Express, 2021, 29(13): 19292. |
| 33 | 梁大宇, 包婷婷, 高田慧, 等. 锂离子电池固态电解质界面膜(SEI)的研究进展[J]. 储能科学与技术, 2018, 7(3): 418-423. |
| LIANG D Y, BAO T T, GAO T H, et al. Research progress of lithium ion battery solid-electrolyte interface (SEI)[J]. Energy Storage Science and Technology, 2018, 7(3): 418-423. | |
| 34 | 聂凯会, 耿振, 王其钰, 等. 锂电池研究中的循环伏安实验测量和分析方法[J]. 储能科学与技术, 2018, 7(3): 539-553. |
| NIE K H, GENG Z, WANG Q Y, et al. Experimental measurement and analysis methods of cyclic voltammetry for lithium batteries[J]. Energy Storage Science and Technology, 2018, 7(3): 539-553. | |
| 35 | 凌仕刚, 许洁茹, 李泓. 锂电池研究中的EIS实验测量和分析方法[J]. 储能科学与技术, 2018, 7(4): 732-749. |
| LING S G, XU J R, LI H. Experimental measurement and analysis methods of electrochemical impedance spectroscopy for lithium batteries[J]. Energy Storage Science and Technology, 2018, 7(4): 732-749. | |
| 36 | LI M, ODOM S A, PANCOAST A R, et al. Experimental protocols for studying organic non-aqueous redox flow batteries[J]. ACS Energy Letters, 2021, 6(11): 3932-3943. |
| 37 | ROZNYATOVSKAYA N V, ROZNYATOVSKY V A, HÖHNE C C, et al. The role of phosphate additive in stabilization of sulphuric-acid-based vanadium(V) electrolyte for all-vanadium redox-flow batteries[J]. Journal of Power Sources, 2017, 363: 234-243. |
| 38 | ABBAS S, HWANG J, KIM H, et al. Enzyme-inspired formulation of the electrolyte for stable and efficient vanadium redox flow batteries at high temperatures[J]. ACS Applied Materials & Interfaces, 2019, 11(30): 26842-26853. |
| 39 | LIU W Q, ZHAO Z M, LI T Y, et al. A high potential biphenol derivative cathode: Toward a highly stable air-insensitive aqueous organic flow battery[J]. Science Bulletin, 2021, 66(5): 457-463. |
| 40 | PAN M G, LU Y, LU S Y, et al. The dual role of bridging phenylene in an extended bipyridine system for high-voltage and stable two-electron storage in redox flow batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(37): 44174-44183. |
| 41 | ZHANG C K, ZHANG L Y, DING Y, et al. Eutectic electrolytes for high-energy-density redox flow batteries[J]. ACS Energy Letters, 2018, 3(12): 2875-2883. |
| 42 | DING Y, ZHANG C K, ZHANG L Y, et al. Insights into hydrotropic solubilization for hybrid ion redox flow batteries[J]. ACS Energy Letters, 2018, 3(11): 2641-2648. |
| 43 | LV Y Q, ZHAO M, DU Y D, et al. Engineering a self-adaptive electric double layer on both electrodes for high-performance zinc metal batteries[J]. Energy & Environmental Science, 2022, 15(11): 4748-4760. |
| 44 | ZHAO E W, LIU T, JÓNSSON E, et al. In situ NMR metrology reveals reaction mechanisms in redox flow batteries[J]. Nature, 2020, 579(7798): 224-228. |
| 45 | ZHAO E W, SHELLARD E J K, KLUSENER P A A, et al. In situ bulk magnetization measurements reveal the state of charge of redox flow batteries[J]. Chemical Communications, 2022, 58(9): 1342-1345. |
| 46 | JING Y, ZHAO E W, GOULET M A, et al. In situ electrochemical recomposition of decomposed redox-active species in aqueous organic flow batteries[J]. Nature Chemistry, 2022, 14(10): 1103-1109. |
| 47 | FULFER K D, KURODA D G. Solvation structure and dynamics of the lithium ion in organic carbonate-based electrolytes: A time-dependent infrared spectroscopy study[J]. The Journal of Physical Chemistry C, 2016, 120(42): 24011-24022. |
| 48 | CHANG N N, LI T Y, LI R, et al. An aqueous hybrid electrolyte for low-temperature zinc-based energy storage devices[J]. Energy & Environmental Science, 2020, 13(10): 3527-3535. |
| 49 | DUAN W T, VEMURI R S, MILSHTEIN J D, et al. A symmetric organic-based nonaqueous redox flow battery and its state of charge diagnostics by FTIR[J]. Journal of Materials Chemistry A, 2016, 4(15): 5448-5456. |
| 50 | LI J T, ZHOU Z Y, BROADWELL I, et al. In-situ infrared spectroscopic studies of electrochemical energy conversion and storage[J]. Accounts of Chemical Research, 2012, 45(4): 485-494. |
| 51 | HUANG S Q, YUAN Z Z, SALLA M, et al. A redox-mediated zinc electrode for ultra-robust deep-cycle redox flow batteries[J]. Energy & Environmental Science, 2023, 16(2): 438-445. |
| 52 | NOLTE O, GEITNER R, HAGER M D, et al. IR spectroscopy as a method for online electrolyte state assessment in RFBs[J]. Advanced Energy Materials, 2021, 11(28): 2100931. |
| 53 | LI L, SU Y H, JI Y L, et al. A long-lived water-soluble phenazine radical cation[J]. Journal of the American Chemical Society, 2023, 145(10): 5778-5785. |
| 54 | HU B, TANG Y J, LUO J, et al. Improved radical stability of viologen anolytes in aqueous organic redox flow batteries[J]. Chemical Communications, 2018, 54(50): 6871-6874. |
| 55 | YANG Z A, LUO Y W, GAO X A, et al. High-safety all-solid-state lithium-ion battery working at ambient temperature with in situ UV-curing polymer electrolyte on the electrode[J]. ChemElectroChem, 2020, 7(12): 2599-2607. |
| 56 | CHEN Z X, MEI S W, LI W J, et al. Study of multi-electron redox mechanism via electrochromic behavior in hexaazatrinaphthylene-based polymer as the cathode of lithium-organic batteries[J]. Journal of Materials Chemistry A, 2021, 9(47): 27010-27018. |
| 57 | WONG A A, AZIZ M J, RUBINSTEIN S. Direct visualization of electrochemical reactions and comparison of commercial carbon papers in operando by fluorescence microscopy using a quinone-based flow cell[J]. ECS Transactions, 2017, 77(11): 153-161. |
| 58 | WONG A A, RUBINSTEIN S M, AZIZ M J. Direct visualization of electrochemical reactions and heterogeneous transport within porous electrodes in operando by fluorescence microscopy[J]. Cell Reports Physical Science, 2021, 2(4): 100388. |
| 59 | XIN H J, WANG H, ZHANG W, et al. Frontispiece: In operando visualization and dynamic manipulation of electrochemical processes at the electrode-solution interface[J]. Angewandte Chemie International Edition, 2022, 61(36): doi: 10.1002/anie.202283661. |
| 60 | ZHAO E W, JÓNSSON E, JETHWA R B, et al. Coupled in situ NMR and EPR studies reveal the electron transfer rate and electrolyte decomposition in redox flow batteries[J]. Journal of the American Chemical Society, 2021, 143(4): 1885-1895. |
| 61 | KLOD S, DUNSCH L. A combination of in situ ESR and in situ NMR spectroelectrochemistry for mechanistic studies of electrode reactions: The case of p-benzoquinone[J]. Magnetic Resonance in Chemistry, 2011, 49(11): 725-729. |
| 62 | ZHI L P, LI T Y, LIU X Q, et al. Functional complexed zincate ions enable dendrite-free long cycle alkaline zinc-based flow batteries[J]. Nano Energy, 2022, 102: 107697. |
| 63 | MASUDA H, ISHIDA N, OGATA Y, et al. In situ visualization of Li concentration in all-solid-state lithium ion batteries using time-of-flight secondary ion mass spectrometry[J]. Journal of Power Sources, 2018, 400: 527-532. |
| 64 | YU Z Y, SHAO Y, MA L P, et al. Revealing the sulfur redox paths in a Li-S battery by an in situ hyphenated technique of electrochemistry and mass spectrometry[J]. Advanced Materials, 2022, 34(7): 2106618. |
| 65 | WANG J H, DING T, WU K F. Charge transfer from n-doped nanocrystals: Mimicking intermediate events in multielectron photocatalysis[J]. Journal of the American Chemical Society, 2018, 140(25): 7791-7794. |
| 66 | WANG J H, DING T, WU K F. Electron transfer into electron-accumulated nanocrystals: Mimicking intermediate events in multielectron photocatalysis Ⅱ[J]. Journal of the American Chemical Society, 2018, 140(32): 10117-10120. |
| [1] | Zenghui HAO, Xunliang LIU, Yuan MENG, Nan MENG, Zhi WEN. Effect of electrode interface microstructure on the performance of solid-state lithium-ion battery [J]. Energy Storage Science and Technology, 2023, 12(7): 2095-2104. |
| [2] | Maosong FAN, Mengmeng GENG, Guangjin ZHAO, Kai YANG, Fangfang WANG, Hao LIU. Research on battery sorting technology for echelon utilization based on multifrequency impedance [J]. Energy Storage Science and Technology, 2023, 12(7): 2202-2210. |
| [3] | Linze LI, Xiangwen ZHANG. SOH estimation for lithium-ion batteries based on combination of frequency impedance characteristics [J]. Energy Storage Science and Technology, 2023, 12(5): 1705-1712. |
| [4] | Fangfang WANG, Xiangming FENG, Guangjin ZHAO, Dawei XIA, Yuxia HU, Weihua CHEN. Identification of retired power lithium-ion batteries of chemical systems by electrochemical impedance spectroscopy [J]. Energy Storage Science and Technology, 2023, 12(2): 609-614. |
| [5] | Xiaoyu CHEN, Mengmeng GENG, Qiankun WANG, Jiani SHEN, Yijun HE, Zifeng MA. Electrochemical impedance feature selection and gaussian process regression based on the state-of-health estimation method for lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2995-3002. |
| [6] | Chenkun LI, Shuai WANG, Jun HUANG. Method for solving physical model of electrochemical impedance spectroscopy [J]. Energy Storage Science and Technology, 2022, 11(3): 912-920. |
| [7] | Mengmeng GENG, Maosong FAN, Kai YANG, Guangjin ZHAO, Zhen TAN, Fei GAO, Mingjie ZHANG. Fast estimation method for state-of-health of retired batteries based on electrochemical impedance spectroscopy and neural network [J]. Energy Storage Science and Technology, 2022, 11(2): 673-678. |
| [8] | Zhuo XU, Xichao LI, Longzhou JIA, Bing CHEN, Zuoqiang DAI, Lili ZHENG. Effect of overcharge cycle on capacity attenuation and safety of lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(12): 3978-3986. |
| [9] | SUN Shuwei, ZHAO Huiling, YU Caiyan, BAI Ying. Experimental measurement and analysis of Raman/infrared methods for lithium batteries [J]. Energy Storage Science and Technology, 2019, 8(5): 975-996. |
| [10] | LING Shigang, XU Jieru, LI Hong. Experimental measurement and analysis methods of electrochemical impedance spectroscopy for lithium batteries [J]. Energy Storage Science and Technology, 2018, 7(4): 732-749. |
| [11] | XU Gengzhao, LIU Zhenghui, ZHONG Haijian, FAN Yingmin, HUANG Zengli, XU Ke. Scanning near-field optics-electrical microscope [J]. Energy Storage Science and Technology, 2014, 3(6): 614-619. |
| [12] | MA Hongyun, FAN Yongsheng, HONG Weichen, WANG Baoguo. Principle and application of electrochemical impedance spectroscopy method [J]. Energy Storage Science and Technology, 2014, 3(5): 544-549. |
| [13] | WANG Yongchen, NI Jiangfeng, WANG Haibo, GAO Lijun, HU Daozhong, WANG Zidong. Sorting methods of lithium ion batteries consistency [J]. Energy Storage Science and Technology, 2013, 2(5): 522-527. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
