Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (1): 92-112.doi: 10.19799/j.cnki.2095-4239.2023.0740
Previous Articles Next Articles
Panqing WANG1( ), Yanjie HUANG1, Yipeng HE1, Qiheng CHEN1, Ti YIN1, Weihao CHEN1, Lei TAN2, Tianxiang NING1, Kangyu ZOU1(
), Yanjie HUANG1, Yipeng HE1, Qiheng CHEN1, Ti YIN1, Weihao CHEN1, Lei TAN2, Tianxiang NING1, Kangyu ZOU1( ), Lingjun LI1(
), Lingjun LI1( )
)
Received:2023-10-24
Revised:2023-10-31
Online:2024-01-05
Published:2024-01-22
Contact:
Kangyu ZOU, Lingjun LI
E-mail:wangpanqing@stu.csust.edu.cn;ky-zou@csust.edu.cn;lingjun.li@csust.edu.cn
CLC Number:
Panqing WANG, Yanjie HUANG, Yipeng HE, Qiheng CHEN, Ti YIN, Weihao CHEN, Lei TAN, Tianxiang NING, Kangyu ZOU, Lingjun LI. Research progress on the surface lithium residue of high-nickel cathode materials[J]. Energy Storage Science and Technology, 2024, 13(1): 92-112.
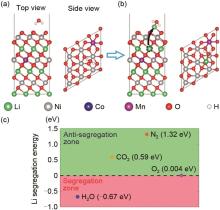
Fig. 3
(a), (b) Schematic diagram of the atomic structure and delithiated NCM of high-nickel ternary cathode materials (NCM) moving from the interior to the surface 3b site under the adsorption of gas molecules. The black dotted line indicates the migrating lithium ions, and the black arrow points to the surface site to which the subsurface lithium migrates. (c) Li migration energy diagram under H2O, O2, CO2 and N2 adsorption [15]"
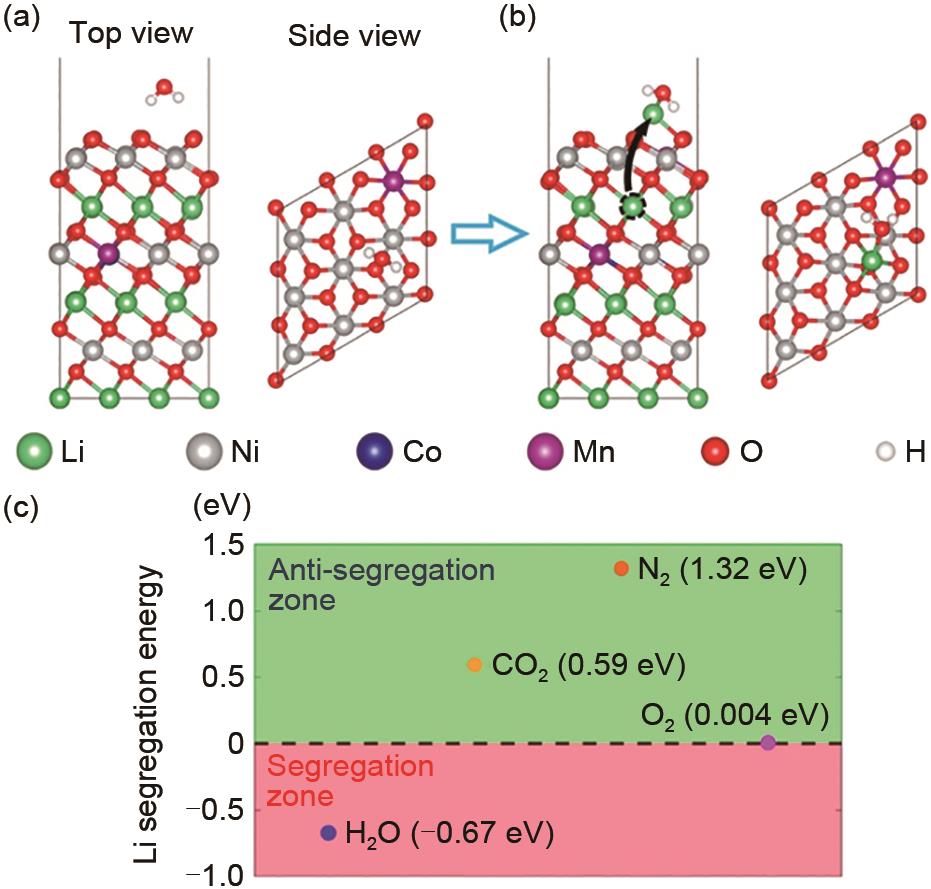

Fig. 7
(a) normalized depth profile of representative species in F-Sb-NCMA93[47]; (b) 11B NMR analysis of the supernatant after the completion of the chemical reaction between TPB, LiOH and Li2CO3[48]; TG-DSC curve of lithium tetraborate formed by the reaction of (c), (d) Li2CO3 with boric acid and LiOH with boric acid[49]"
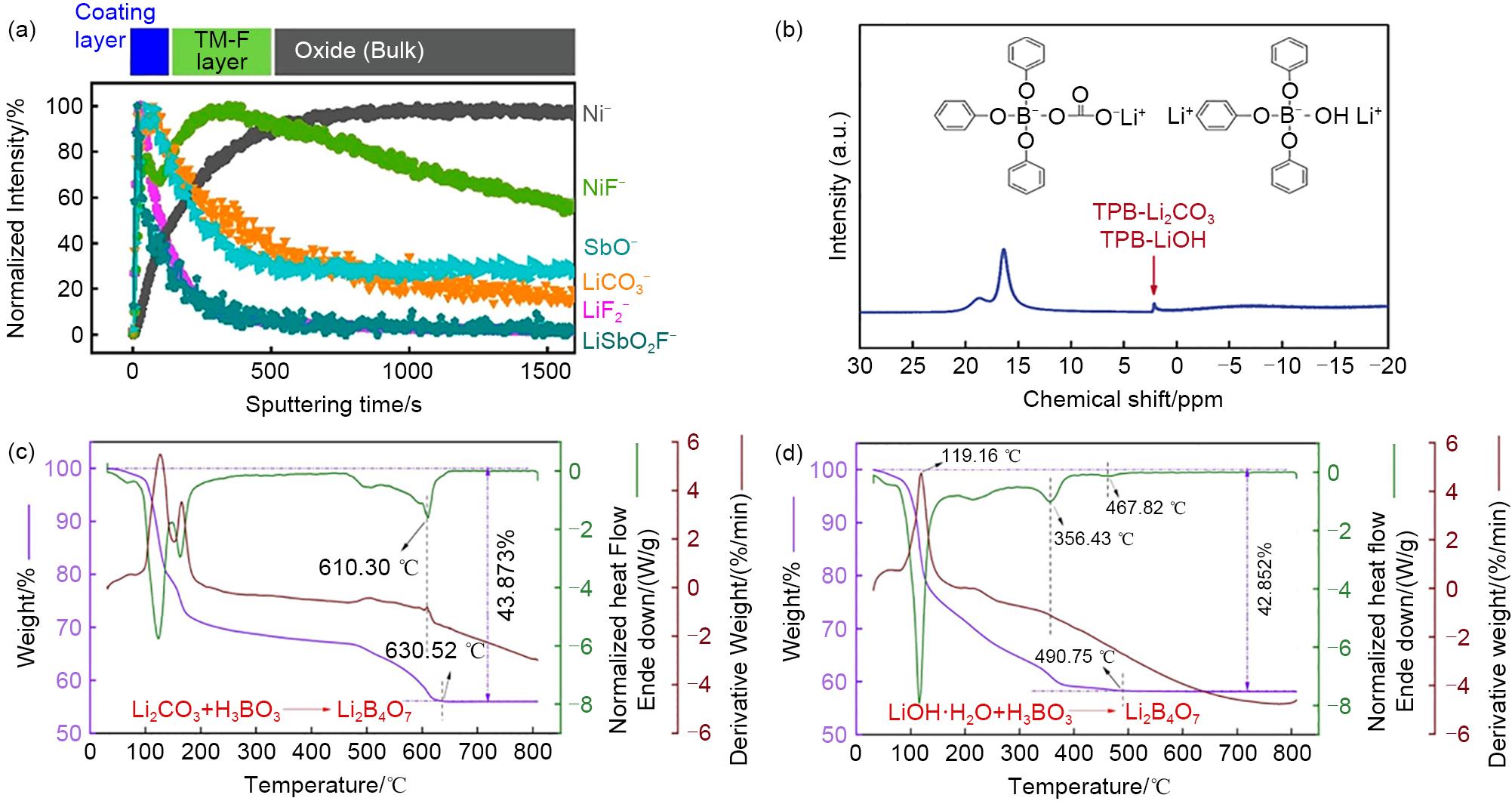
Fig. 9
(a)—(c) Comparison of drying time, particle size and electrochemical performance of cathode materials after drying by the two methods[52]; (d) Newly prepared sample F, (e) sample EF2 washed twice with ethanol, (f) sample ES2 first placed in air for 1 d and then washed twice with ethanol stored in air for 3 months before and after the first and thirtieth discharge curves[55]"

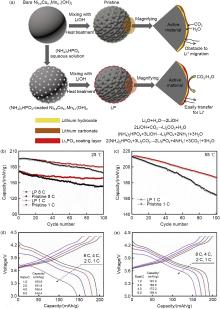
Fig. 12
Schematic diagram of the surface chemical reaction between the original sample and the Li3PO4-modified NCM811 cathode material (LP) (a); Cycling performance in the (b) 1 C and 8 C and (c) 1 C, 55 ℃ and 3.0—4.4 V voltage ranges; (d) Rate performance of the original sample and (e) the modified sample[81]"
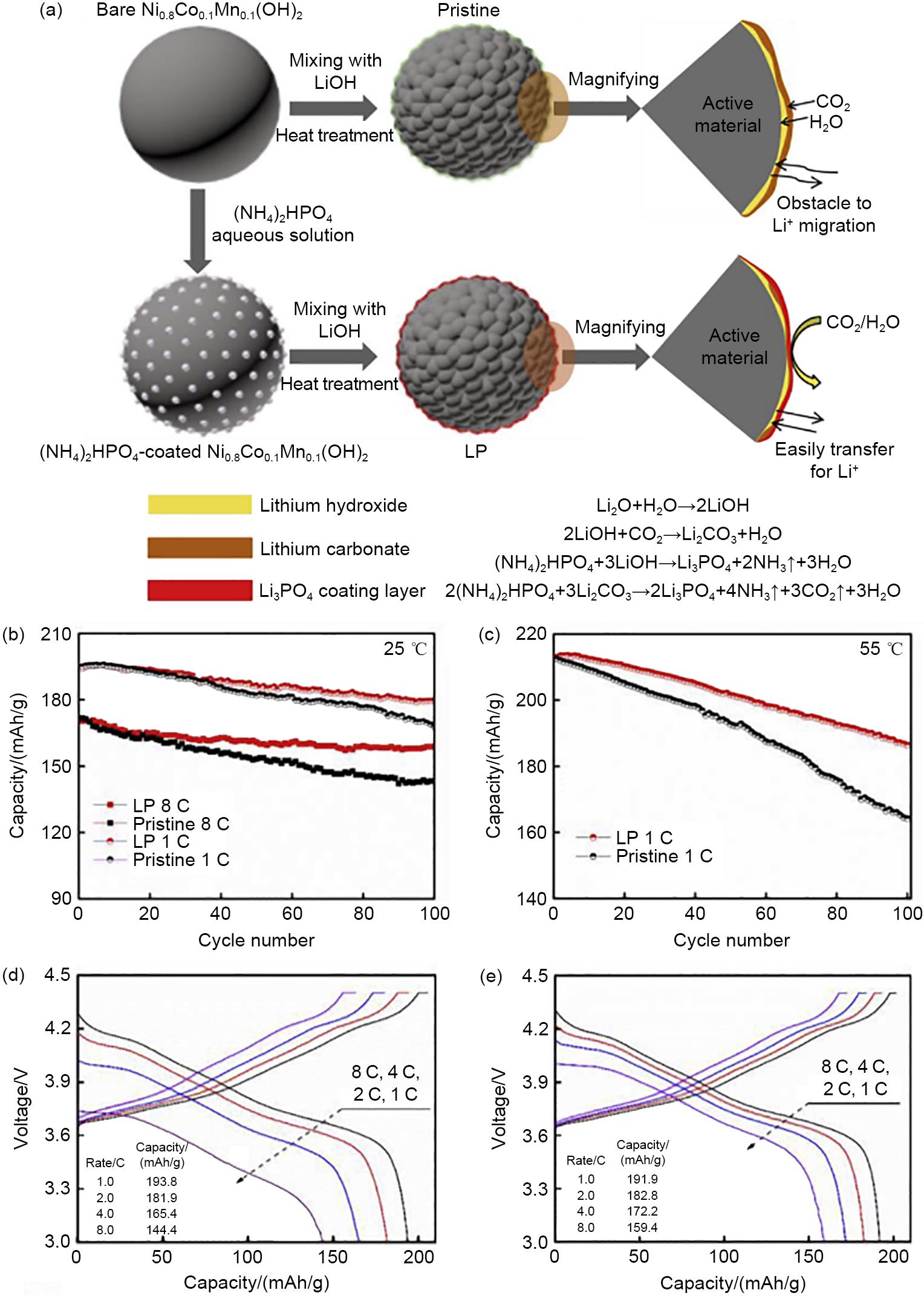
Fig. 13
(a) Schematic diagram of the synthesis process of SiO2-Li2SiO3 coated NCM811; Cycling performance of S0—S4 samples at (b), (c) 1 C and 25 ℃; Rate performance at (d), (e) different current densities, S0—S4 indicates unmodified, different LiOH content (1.07%, 1.09%, 1.11% and 1.13%) and EPS-modified NCM811 cathode material[2]"

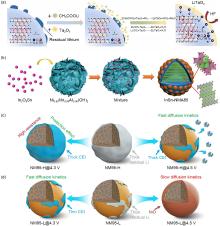
Fig. 15
(a) Schematic diagram of the NCA-LTO3 reaction process[42]; (b) schematic diagram of the synthetic route of InSn-NMA85[82]; (c) Schematic diagram of the change of RLCs after activation of LiNi0.95Mn0.05O2 (NM95-H) and (d) LiNi0.95Mn0.05O2 (NM95-L) cathode with low RLCs content at 2.7—4.3 V and 2.7—4.5 V with high surface RLCs content[31]"

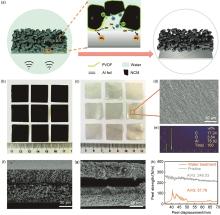
Fig. 16
Description of the separation process and results (a) Schematic diagram of the separation process and possible separation mechanisms, optical photograph of (b) degraded electrode sheet and (c) aluminum foil with a size of 2 cm×2 cm after separation; (d) Mapping results of SEM images and (e) separated aluminum foil; Scanning electron microscopy images of electrodes before (f) and (g) before water treatment; (h) Flowering strength of each electrode before and after water treatment[84]"


Fig. 17
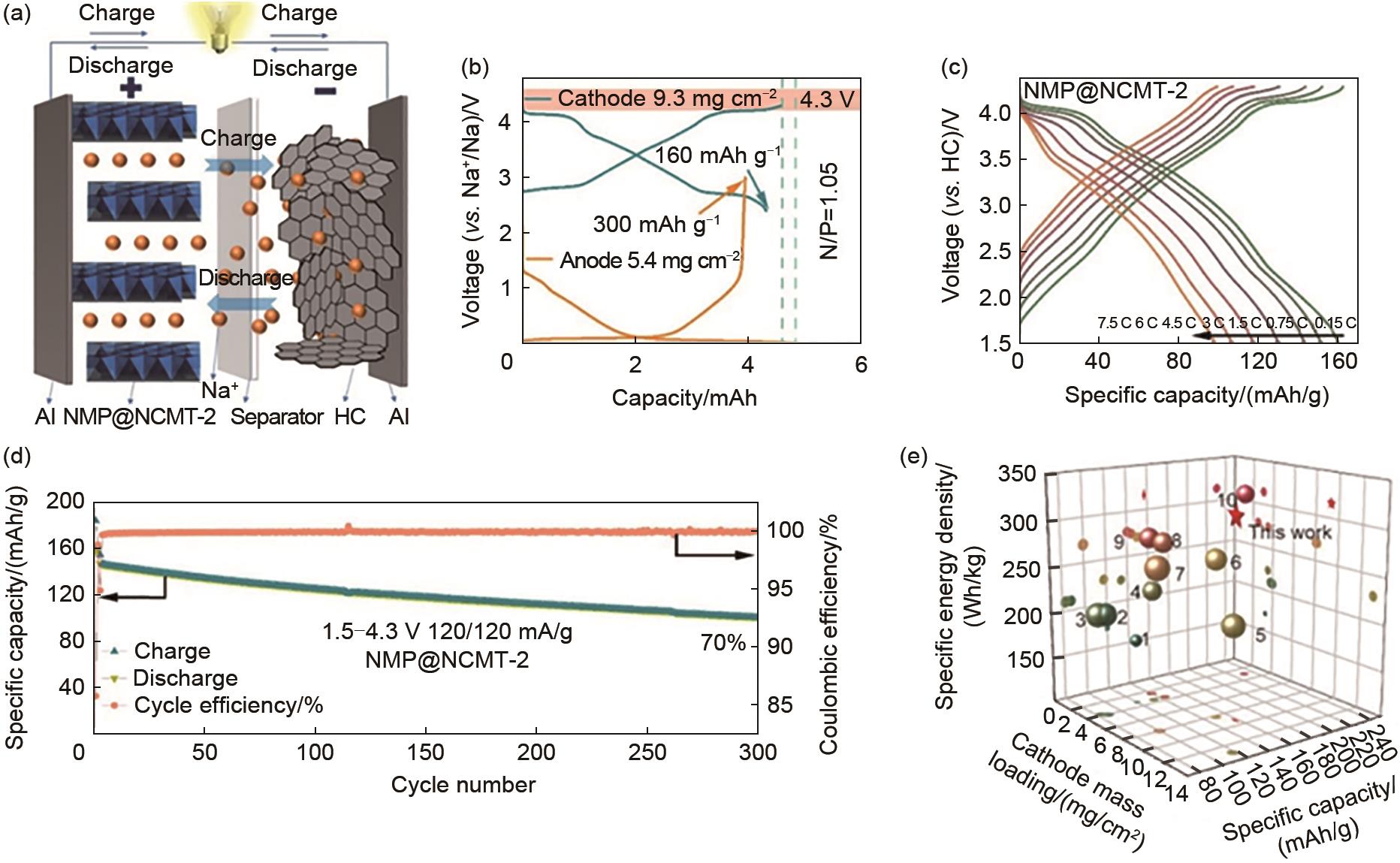
(a) Schematic diagram of the NMP@NCMT-2||HC full battery; (b) GCD patterns of NMP@NCMT-2 and hard carbon (HC) with a capacity ratio of 1.05∶1 for the electrodes; (c) GCD plot of a whole cell at different current densities; (d) Cycling performance of the full battery at 1.2 C; (e) Comparison of electrochemical performance of NMP@NCMT-2//HC with other sodium-ion battery cathode materials for whole batteries; Specific energy density is based on the total mass load (the size of the shape is proportional to the cyclic stability)[43]"
| 1 | OH S W, MYUNG S T, OH S M, et al. Double carbon coating of LiFePO4 as high rate electrode for rechargeable lithium batteries[J]. Advanced Materials, 2010, 22(43): 4842-4845. |
| 2 | LI Y J, ZHANG D Y, YAN Y X, et al. Enhanced electrochemical properties of SiO2-Li2SiO3-coated NCM811 cathodes by reducing surface residual lithium[J]. Journal of Alloys and Compounds, 2022, 923: 166317. |
| 3 | 栗志展, 秦金磊, 梁嘉宁, 等. 高镍三元层状锂离子电池正极材料: 研究进展、挑战及改善策略[J]. 储能科学与技术, 2022, 11(9): 2900-2920. |
| LI Z Z, QIN J L, LIANG J N, et al. High-nickel ternary layered cathode materials for lithium-ion batteries: Research progress, challenges and improvement strategies[J]. Energy Storage Science and Technology, 2022, 11(9): 2900-2920. | |
| 4 | 李金涛, 牟粤, 王静, 等. 高镍正极材料的稳定改性方法研究综述[J]. 储能科学与技术, 2023, 12(5): 1636-1654. |
| LI J T, MU Y, WANG J, et al. Investigation of the structural evolution and interface behavior in cathode materials for Li-ion batteries[J]. Energy Storage Science and Technology, 2023, 12(5): 1636-1654. | |
| 5 | CHO D H, JO C H, CHO W, et al. Effect of residual lithium compounds on layer Ni-rich Li[Ni0.7Mn0.3]O2[J]. Journal of the Electrochemical Society, 2014, 161(6): A920-A926. |
| 6 | LIU H S, ZHANG Z R, GONG Z L, et al. Origin of deterioration for LiNiO2 cathode material during storage in air[J]. Electrochemical and Solid-State Letters, 2004, 7(7): A190. |
| 7 | KIM Y, KIM D, KANG S. Experimental and first-principles thermodynamic study of the formation and effects of vacancies in layered lithium nickel cobalt oxides[J]. Chemistry of Materials, 2011, 23(24): 5388-5397. |
| 8 | SEONG W M, KIM Y, MANTHIRAM A. Impact of residual lithium on the adoption of high-nickel layered oxide cathodes for lithium-ion batteries[J]. Chemistry of Materials, 2020, 32(22): 9479-9489. |
| 9 | HEISKANEN S K, KIM J, LUCHT B L. Generation and evolution of the solid electrolyte interphase of lithium-ion batteries[J]. Joule, 2019, 3(10): 2322-2333. |
| 10 | SUSAI F A, SCLAR H, MAITI S, et al. Stabilized behavior of LiNi0.85Co0.10Mn0.05O2 cathode materials induced by their treatment with SO2[J]. ACS Applied Energy Materials, 2020, 3(4): 3609-3618. |
| 11 | LEE K T, JEONG S, CHO J. Roles of surface chemistry on safety and electrochemistry in lithium ion batteries[J]. Accounts of Chemical Research, 2013, 46(5): 1161-1170. |
| 12 | ZHANG M J, HU X B, LI M F, et al. Lithium-ion batteries: Cooling induced surface reconstruction during synthesis of high-Ni layered oxides[J]. Advanced Energy Materials, 2019, 9(43): 1901915. |
| 13 | ZOU K Y, JIANG M Z, ZHAO Z X, et al. Mechanistic insights into suppressing microcracks by regulating grain size of precursor for high-performance Ni-rich cathodes[J]. Chemical Engineering Journal, 2023, 476: 146793. |
| 14 | FAENZA N V, BRUCE L, LEBENS-HIGGINS Z W, et al. Editors' choice—Growth of ambient induced surface impurity species on layered positive electrode materials and impact on electrochemical performance[J]. Journal of the Electrochemical Society, 2017, 164(14): A3727-A3741. |
| 15 | ZOU L F, HE Y, LIU Z Y, et al. Unlocking the passivation nature of the cathode-air interfacial reactions in lithium ion batteries[J]. Nature Communications, 2020, 11: 3204. |
| 16 | SHENG H, MENG X H, XIAO D D, et al. An air-stable high-nickel cathode with reinforced electrochemical performance enabled by convertible amorphous Li2CO3 modification[J]. Advanced Materials, 2022, 34(12): e2108947. |
| 17 | 杨慧平. 掺杂与包覆双重修饰锂离子电池高镍正极材料的研究[D]. 长沙: 长沙理工大学, 2019. |
| YANG H P. Study on dual modification (doped and coated) of nickel-rich cathode materials for lithium ion batteries[D]. Changsha: Changsha University of Science & Technology, 2019. | |
| 18 | HAWLEY W B, LI J L. Beneficial rheological properties of lithium-ion battery cathode slurries from elevated mixing and coating temperatures[J]. Journal of Energy Storage, 2019, 26: 100994. |
| 19 | KEPPELER M, ROESSLER S, BRAUNWARTH W. Production research as key factor for successful establishment of battery production on the example of large-scale automotive cells containing nickel-rich LiNi0.8Mn0.1Co0.1O2 electrodes[J]. Energy Technology, 2020, 8(6): 202000183. |
| 20 | LIU F, HASHIM N A, LIU Y T, et al. Progress in the production and modification of PVDF membranes[J]. Journal of Membrane Science, 2011, 375(1/2): 1-27. |
| 21 | XIAO L, DAVENPORT D M, ORMSBEE L, et al. Polymerization and functionalization of membrane pores for water related applications[J]. Industrial & Engineering Chemistry Research, 2015, 54(16): 4174-4182. |
| 22 | BRESSER D, BUCHHOLZ D, MORETTI A, et al. Alternative binders for sustainable electrochemical energy storage-the transition to aqueous electrode processing and bio-derived polymers[J]. Energy & Environmental Science, 2018, 11(11): 3096-3127. |
| 23 | PARIMALAM B S, MACINTOSH A D, KADAM R, et al. Decomposition reactions of anode solid electrolyte interphase (SEI) components with LiPF6[J]. The Journal of Physical Chemistry C, 2017, 121(41): 22733-22738. |
| 24 | RENFREW S E, MCCLOSKEY B D. Residual lithium carbonate predominantly accounts for first cycle CO2 and CO outgassing of Li-stoichiometric and Li-rich layered transition-metal oxides[J]. Journal of the American Chemical Society, 2017, 139(49): 17853-17860. |
| 25 | SU Y F, LI L W, CHEN G, et al. Strategies of removing residual lithium compounds on the surface of Ni-rich cathode materials[J]. Chinese Journal of Chemistry, 2021, 39(1): 189-198. |
| 26 | BI Y J, WANG T, LIU M, et al. Stability of Li2CO3 in cathode of lithium ion battery and its influence on electrochemical performance[J]. RSC Advances, 2016, 6(23): 19233-19237. |
| 27 | TASAKI K, GOLDBERG A, LIAN J J, et al. Solubility of lithium salts formed on the lithium-ion battery negative electrode surface in organic solvents[J]. Journal of the Electrochemical Society, 2009, 156(12): A1019. |
| 28 | 袁绍辉, 曾子豪, 董煜, 等. 富镍三元层状正极材料表面残碱去除工艺: 研究进展、挑战及展望[J]. 中国有色金属学报, 2023, 33(5): 1554-1584. |
| YUAN S H, ZENG Z H, DONG Y, et al. Removal process of residual alkali on surface of nickel-rich ternary layered cathode materials: Research progress, challenges and prospects[J]. The Chinese Journal of Nonferrous Metals, 2023, 33(5): 1554-1584. | |
| 29 | LIU W, OH P, LIU X E, et al. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries[J]. Angewandte Chemie International Edition, 2015, 54(15): 4440-4457. |
| 30 | BENEDETTI-PICHLER A A, CEFOLA M. Warder's method for the titration of carbonates[J]. Industrial & Engineering Chemistry Analytical Edition, 1939, 11(6): 327-332. |
| 31 | WANG C, TAN L, YI H L, et al. Unveiling the impact of residual Li conversion and cation ordering on electrochemical performance of Co-free Ni-rich cathodes[J]. Nano Research, 2022, 15(10): 9038-9046. |
| 32 | ZHOU C X, WANG P B, ZHANG B, et al. Formation and effect of residual lithium compounds on Li-rich cathode material Li1.35[Ni0.35Mn0.65]O2[J]. ACS Applied Materials & Interfaces, 2019, 11(12): 11518-11526. |
| 33 | MOSHTEV R, ZLATILOVA P, VASILEV S, et al. Synthesis, XRD characterization and electrochemical performance of overlithiated LiNiO2[J]. Journal of Power Sources, 1999, 81/82: 434-441. |
| 34 | PRITZL D, TEUFL T, FREIBERG A T S, et al. Editors' choice—Washing of nickel-rich cathode materials for lithium-ion batteries: Towards a mechanistic understanding[J]. Journal of the Electrochemical Society, 2019, 166(16): A4056-A4066. |
| 35 | SICKLINGER J, METZGER M, BEYER H, et al. Ambient storage derived surface contamination of NCM811 and NCM111: Performance implications and mitigation strategies[J]. Journal of the Electrochemical Society, 2019, 166(12): A2322-A2335. |
| 36 | KIM Y, PARK H, WARNER J H, et al. Unraveling the intricacies of residual lithium in high-Ni cathodes for lithium-ion batteries[J]. ACS Energy Letters, 2021, 6(3): 941-948. |
| 37 | XIA Y, ZHENG J M, WANG C M, et al. Designing principle for Ni-rich cathode materials with high energy density for practical applications[J]. Nano Energy, 2018, 49: 434-452. |
| 38 | KIM H, KIM M G, JEONG H Y, et al. A new coating method for alleviating surface degradation of LiNi0.6Co0.2Mn0.2O2 cathode material: Nanoscale surface treatment of primary particles[J]. Nano Letters, 2015, 15(3): 2111-2119. |
| 39 | LEE M J, NOH M, PARK M H, et al. The role of nanoscale-range vanadium treatment in LiNi0.8Co0.15Al0.05O2 cathode materials for Li-ion batteries at elevated temperatures[J]. Journal of Materials Chemistry A, 2015, 3(25): 13453-13460. |
| 40 | LIAO J Y, MANTHIRAM A. Surface-modified concentration-gradient Ni-rich layered oxide cathodes for high-energy lithium-ion batteries[J]. Journal of Power Sources, 2015, 282: 429-436. |
| 41 | MU L Q, YANG Z Z, TAO L, et al. The sensitive surface chemistry of Co-free, Ni-rich layered oxides: Identifying experimental conditions that influence characterization results[J]. Journal of Materials Chemistry A, 2020, 8(34): 17487-17497. |
| 42 | ZHANG H W, ZHANG X Y, ZENG T Y, et al. Conversion of residual lithium into fast ionic conductor coating to achieve one-step double modification strategy in LiNi0.8Co0.15Al0.05O2[J]. Journal of Alloys and Compounds, 2023, 931: 167638. |
| 43 | XU W L, DANG R B, ZHOU L, et al. Conversion of surface residual alkali to solid electrolyte to enable Na-ion full cells with robust interfaces[J]. Advanced Materials, 2023, 35(42): e2301314. |
| 44 | YANG X R, TANG S J, ZHENG C X, et al. From contaminated to highly lithiated interfaces: A versatile modification strategy for garnet solid electrolytes[J]. Advanced Functional Materials, 2023, 33(3): 2209120. |
| 45 | WANG W Z, WU L Y, LI Z W, et al. In situ tuning residual lithium compounds and constructing TiO2 coating for surface modification of a nickel-rich cathode toward high-energy lithium-ion batteries[J]. ACS Applied Energy Materials, 2020, 3(12): 12423-12432. |
| 46 | XIE Q A, LI W D, DOLOCAN A, et al. Insights into boron-based polyanion-tuned high-nickel cathodes for high-energy-density lithium-ion batteries[J]. Chemistry of Materials, 2019, 31(21): 8886-8897. |
| 47 | RYU H H, LIM H W, KANG G C, et al. Long-lasting Ni-rich NCMA cathodes via simultaneous microstructural refinement and surface modification[J]. ACS Energy Letters, 2023, 8(3): 1354-1361. |
| 48 | YIM T, JANG S H, HAN Y K. Triphenyl borate as a bi-functional additive to improve surface stability of Ni-rich cathode material[J]. Journal of Power Sources, 2017, 372: 24-30. |
| 49 | LUO Z Y, HU G R, WANG W G, et al. Enhancing the electrochemical performance of Co-less Ni-rich LiNi0.925Co0.03Mn0.045O2 cathode material via Co-modification with Li2B4O7 coating and B3+ doping[J]. Journal of Power Sources, 2022, 548: 232092. |
| 50 | CHEN A Q, WANG K, LI J J, et al. The formation, detriment and solution of residual lithium compounds on Ni-rich layered oxides in lithium-ion batteries[J]. Frontiers in Energy Research, 2020, 8: 593009. |
| 51 | LI Y, SHI H C, HE J J, et al. Enhanced cyclability and reversibility of nickel-rich cathode for lithium-ion batteries via LiH2PO4 assisted saturated Li2CO3 washing[J]. Applied Surface Science, 2022, 593: 153409. |
| 52 | 邵佳伟. 高镍三元锂电池正极材料水洗后干燥过程及其装备研究[D]. 常州: 常州大学, 2022. |
| SHAO J W. Development of drying process equipment for high nickel ternary lithium ion battery cathode materials after washing[D]. Changzhou: Changzhou University, 2022. | |
| 53 | WONTAE L, SANGYOON L, EUNKANG L, et al. Destabilization of the surface structure of Ni-rich layered materials by water-washing process[J]. Energy Storage Materials, 2022, 44: 441-451. |
| 54 | ZHENG X B, LI X H, WANG Z X, et al. Investigation and improvement on the electrochemical performance and storage characteristics of LiNiO2-based materials for lithium ion battery[J]. Electrochimica Acta, 2016, 191: 832-840. |
| 55 | LIU W M, QIN M L, XU L, et al. Washing effect on properties of LiNi0.8Co0.15Al0.05O2 cathode material by ethanol solvent[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(8): 1626-1631. |
| 56 | CHEN J Y, SU B T, FAN J, et al. A low-temperature coating method with H3BO3 for enhanced electrochemical performance of Ni-rich LiNi0.82Co0.12Mn0.06O2 cathode[J]. Electrochimica Acta, 2022, 422: 140564. |
| 57 | WU F, DONG J Y, CHEN L, et al. Removing the intrinsic NiO phase and residual lithium for high-performance nickel-rich materials[J]. Energy Material Advances, 2023, 4: 0007. |
| 58 | NEUDECK S, STRAUSS F, GARCIA G, et al. Room temperature, liquid-phase Al2O3 surface coating approach for Ni-rich layered oxide cathode material[J]. Chemical Communications, 2019, 55(15): 2174-2177. |
| 59 | WATANABE T, YOKOKAWA T, YAMADA M, et al. Surface coating of a LiNixCoyAl1- x- yO2 (x>0.85) cathode with Li3PO4 for applying a water-based hybrid polymer binder during Li-ion battery preparation[J]. RSC Advances, 2021, 11(59): 37150-37161. |
| 60 | ZHOU P F, ZHANG Z, MENG H J, et al. SiO2-coated LiNi0.915Co0.075Al0.01O2 cathode material for rechargeable Li-ion batteries[J]. Nanoscale, 2016, 8(46): 19263-19269. |
| 61 | TAO T, CHEN C, YAO Y B, et al. Enhanced electrochemical performance of ZrO2 modified LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries[J]. Ceramics International, 2017, 43(17): 15173-15178. |
| 62 | CHEN Y P, ZHANG Y, CHEN B J, et al. An approach to application for LiNi0.6Co0.2Mn0.2O2 cathode material at high cutoff voltage by TiO2 coating[J]. Journal of Power Sources, 2014, 256: 20-27. |
| 63 | LI X X, SHI H B, WANG B, et al. Controllable TiO2 coating on the nickel-rich layered cathode through TiCl4 hydrolysis via fluidized bed chemical vapor deposition[J]. RSC Advances, 2019, 9(31): 17941-17949. |
| 64 | LIU X Z, LI H Q, YOO E, et al. Fabrication of FePO4 layer coated LiNi1/3Co1/3Mn1/3O2: Towards high-performance cathode materials for lithium ion batteries[J]. Electrochimica Acta, 2012, 83: 253-258. |
| 65 | XIONG X H, WANG Z X, YIN X, et al. A modified LiF coating process to enhance the electrochemical performance characteristics of LiNi0.8Co0.1Mn0.1O2 cathode materials[J]. Materials Letters, 2013, 110: 4-9. |
| 66 | KIM H B, PARK B C, MYUNG S T, et al. Electrochemical and thermal characterization of AlF3-coated Li[Ni0.8Co0.15Al0.05]O2 cathode in lithium-ion cells[J]. Journal of Power Sources, 2008, 179(1): 347-350. |
| 67 | LEE S H, YOON C S, AMINE K, et al. Improvement of long-term cycling performance of Li[Ni0.8Co0.15Al0.05]O2 by AlF3 coating[J]. Journal of Power Sources, 2013, 234: 201-207. |
| 68 | LI J W, WANG J J, LU X H, et al. Enhancing high-potential stability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode with PrF3 coating[J]. Ceramics International, 2021, 47(5): 6341-6351. |
| 69 | QIU H L, JIN D, WANG C Z, et al. Design of Li2FeSiO4 cathode material for enhanced lithium-ion storage performance[J]. Chemical Engineering Journal, 2020, 379: 122329. |
| 70 | MENG K, WANG Z X, GUO H J, et al. Improving the cycling performance of LiNi0.8Co0.1Mn0.1O2 by surface coating with Li2TiO3[J]. Electrochimica Acta, 2016, 211: 822-831. |
| 71 | KIM J M, XU Y B, ENGELHARD M H, et al. Facile dual-protection layer and advanced electrolyte enhancing performances of cobalt-free/nickel-rich cathodes in lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(15): 17405-17414. |
| 72 | WU F, DONG J Y, CHEN L, et al. High-voltage and high-safety nickel-rich layered cathode enabled by a self-reconstructive cathode/electrolyte interphase layer[J]. Energy Storage Materials, 2021, 41: 495-504. |
| 73 | YANG Z, LI Z M, HUANG Y X, et al. Artificial interface stabilized LiNi0.80Co0.15Al0.05O2@Polysiloxane cathode for stable cycling lithium-ion batteries[J]. Journal of Power Sources, 2020, 471: 228480. |
| 74 | YAO L, LIANG F Q, JIN J, et al. Improved electrochemical property of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode via in situ ZrO2 coating for high energy density lithium ion batteries[J]. Chemical Engineering Journal, 2020, 389: 124403. |
| 75 | QU X Y, YU Z L, RUAN D S, et al. Enhanced electrochemical performance of Ni-rich cathode materials with Li1.3Al0.3Ti1.7(PO4)3 coating[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(15): 5819-5830. |
| 76 | LIU X P, CHEN Q Q, LI Y W, et al. Synergistic modification of magnesium fluoride/sodium for improving the electrochemical performances of high-nickel ternary (NCM811) cathode materials[J]. Journal of the Electrochemical Society, 2019, 166(14): A3480-A3486. |
| 77 | ZHAO E Y, CHEN M M, HU Z B, et al. Improved cycle stability of high-capacity Ni-rich LiNi0.8Mn0.1Co0.1O2 at high cut-off voltage by Li2SiO3 coating[J]. Journal of Power Sources, 2017, 343: 345-353. |
| 78 | MA Y, LV F, WANG Z Q, et al. Open-channel[011]facet CeO2 with a shorter pathway of Li+ migration as a modification material for LiNi0.8Co0.1Mn0.1O2 toward high-rate lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(23): 8795-8802. |
| 79 | SHEN Y B, WU Y Q, ZHANG D Y, et al. Stabilization of high-voltage layered oxide cathode by utilizing residual lithium to form NASICON-type nanoscale functional coating[J]. Nano Research, 2023, 16(4): 5973-5982. |
| 80 | LI L J, FU L Z, LI M, et al. B-doped and La4NiLiO8-coated Ni-rich cathode with enhanced structural and interfacial stability for lithium-ion batteries[J]. Journal of Energy Chemistry, 2022, 71: 588-594. |
| 81 | ZHU J, LI Y J, XUE L L, et al. Enhanced electrochemical performance of Li3PO4 modified Li[Ni0.8Co0.1Mn0.1]O2 cathode material via lithium-reactive coating[J]. Journal of Alloys and Compounds, 2019, 773: 112-120. |
| 82 | LV Y, HUANG S F, LU S R, et al. Engineering of cobalt-free Ni-rich cathode material by dual-element modification to enable 4.5 V-class high-energy-density lithium-ion batteries[J]. Chemical Engineering Journal, 2023, 455: 140652. |
| 83 | ZHANG Y X, ZHAO Y, HU W, et al. Microstructure and surface engineering through indium modification on Ni-rich layered cathode materials for enhanced electrochemical performance of lithium-ion batteries[J]. Journal of Alloys and Compounds, 2023, 934: 167862. |
| 84 | FAN M, CHANG X, GUO Y J, et al. Increased residual lithium compounds guided design for green recycling of spent lithium-ion cathodes[J]. Energy & Environmental Science, 2021, 14(3): 1461-1468. |
| 85 | HE K, ZHANG Z Y, ALAI L G, et al. A green process for exfoliating electrode materials and simultaneously extracting electrolyte from spent lithium-ion batteries[J]. Journal of Hazardous Materials, 2019, 375: 43-51. |
| 86 | SHIM J H, JUNG M H, YANG M J, et al. Fine-tuned synthesis for reducing residual lithium in Ni-rich cathode materials for lithium-ion batteries[J]. ACS Applied Energy Materials, 2023, 6(11): 5952-5958. |
| [1] | Miao LI, Yongli YU, Jianyang WU, Min LEI, Henghui ZHOU. Design of high-energy-density LiFePO4 cathode materials [J]. Energy Storage Science and Technology, 2023, 12(7): 2045-2058. |
| [2] | Haiyan HU, Shulei CHOU, Yao XIAO. Layered oxide cathode materials based on molecular orbital hybridization for high voltage sodium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(4): 1093-1102. |
| [3] | LIANG Dayu, BAO Tingting, GAO Tianhui, ZHANG Jian. Research progress of lithium ion battery solid-electrolyte interface(SEI) [J]. Energy Storage Science and Technology, 2018, 7(3): 418-423. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
