Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (1): 42-53.doi: 10.19799/j.cnki.2095-4239.2024.0621
• Energy Storage Materials and Devices • Previous Articles Next Articles
Jie LU1( ), Xian DU2, Yupu SHI2, Zhuo LI3, Na CAO3, Xuntao DU4, Huiling DU2(
), Xian DU2, Yupu SHI2, Zhuo LI3, Na CAO3, Xuntao DU4, Huiling DU2( )
)
Received:2024-06-21
Revised:2024-07-24
Online:2025-01-28
Published:2025-02-25
Contact:
Huiling DU
E-mail:18105016001@stu.xust.edu.cn;hldu@xust.edu.cn
CLC Number:
Jie LU, Xian DU, Yupu SHI, Zhuo LI, Na CAO, Xuntao DU, Huiling DU. PANI-coated vanadium compound as high-stable aqueous zinc-ion batteries cathode material[J]. Energy Storage Science and Technology, 2025, 14(1): 42-53.
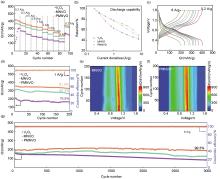
Fig.4
(a) Rate capacities at current densities range from 0.2 to 8 A/g for three samples, (b) capacity retention of zin-ion storage capability for three samples, (c) GCD curves at different current densities of PMNVO,(d) cycling performance at 1 A/g for three samples; contour plots depicting the evolution of dQ/dV derived from dezincification during cycling for MNVO (e) and PMNVO (f), (g) long-term cycling performances at 8 A/g for three samples"
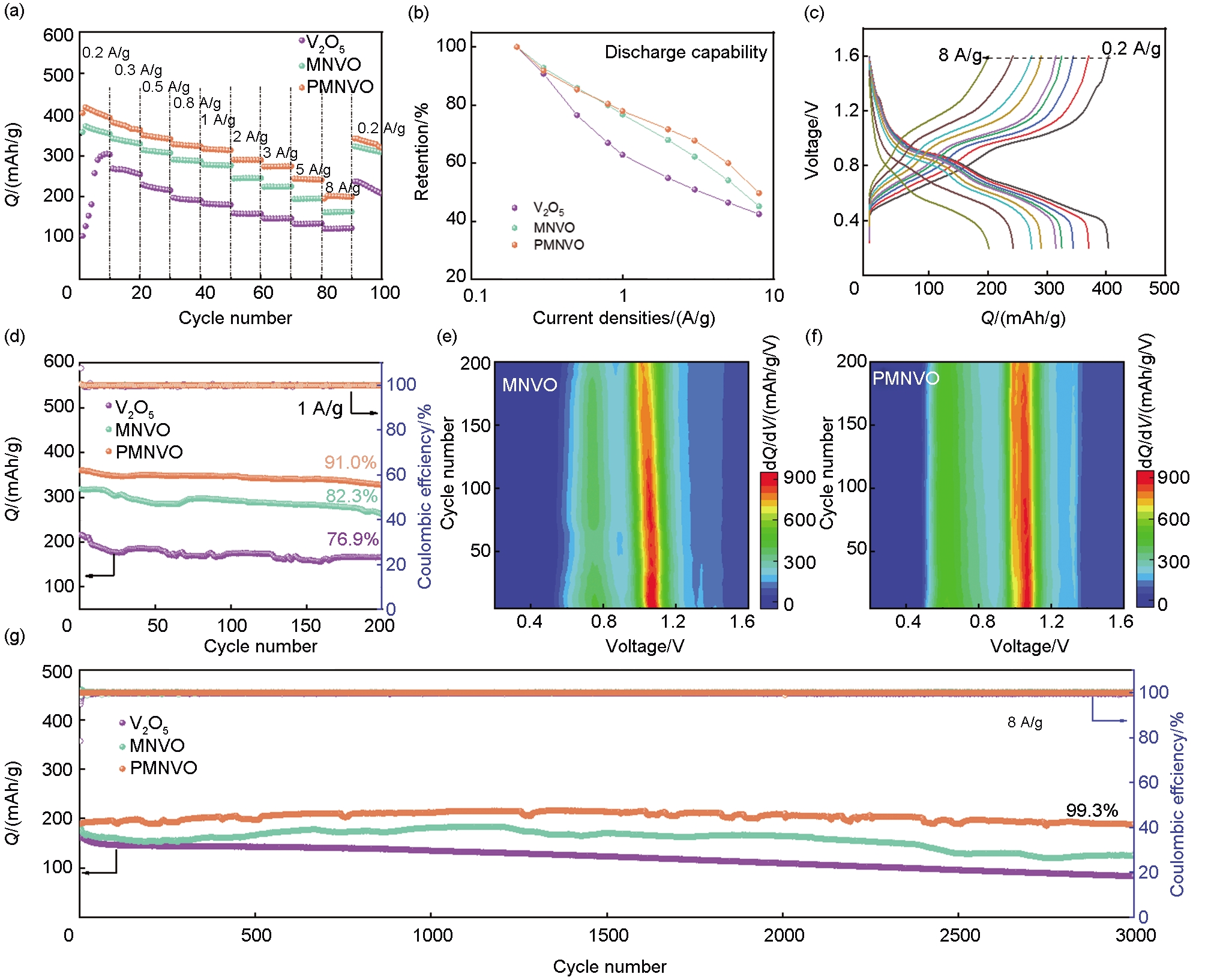
Table 2
Electrochemical properties of similar cathode materials in AZIBs"
| Materials | Current collector | Electrolyte | Current density/(A/g) /Specific capacity/(mAh/g) | Cycle numbers/ Capacity retention/% | References |
|---|---|---|---|---|---|
| Mn(VO3)2-NaV8O20@PANI | stainless steel mesh | 3 mol/L ZnSO4 | 0.2/417.6 1/360.3 | 1A/g 200/91.0 % 8A/g 3000/99.3 % | This work |
| VOH@PANI | Ti sheets | 3 mol/L Zn(CF3SO3)2 | 1/228 | 2A/g 2000/98 % | [ |
| PVM | Ti foil | 3 mol/L Zn(CF3SO3)2 | 0.2/265 | 10A/g 2000/68.3 % | [ |
| KPVO | Ti foil | 3 mol/L Zn(CF3SO3)2 | 0.2/400 | 5A/g 3000/89.5 % | [ |
| CoVO@PANI60 | Ti foil | 2 mol/L Zn(CF3SO3)2 | 0.2/407 | 10A/g 1500/90.4 % | [ |
| PANI-VOH | Ti sheets | 3 mol/L Zn(CF3SO3)2 | 0.1/363 | 5A/g 2000/52.4 % | [ |
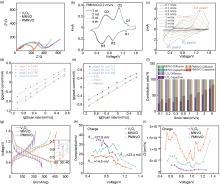
Fig.7
(a) The Nyquist plots for three samples, (b) CV curves at a scan rate of 0.2 mV/s for PMNVO, (c) CV curves at different scan rates for PMNVO, (d) lg (i) versus lg (v) plots of the PMNVO electrode at specific peak currents, (e) lg (i) versus lg (v) plots at reduction peak 3 currents of all three samples, (f) contribution ratio of capacitive capacities and diffusion-limited capacities at different scan rates for three samples, (g) voltage profiles for GITT measurements (inset showing the detailed overpotential in the magnified area) for three samples, (h) summary of overpotentials from the GITT results for three samples, (i) Zn2+ diffusion coefficients corresponding to different electrodes during charge"

| 1 | DING Y, ZHANG L L, WANG X, et al. Vanadium-based cathodes for aqueous zinc ion batteries: Structure, mechanism and prospects[J]. Chinese Chemical Letters, 2023, 34(2): 107399. DOI: 10.1016/j.cclet.2022.03.122. |
| 2 | LIU M Q, WANG P, ZHANG W, et al. Strategies for pH regulation in aqueous zinc ion batteries[J]. Energy Storage Materials, 2024, 67: 103248. DOI: 10.1016/j.ensm.2024.103248. |
| 3 | LI X Y, WANG L, FU Y H, et al. Optimization strategies toward advanced aqueous zinc-ion batteries: From facing key issues to viable solutions[J]. Nano Energy, 2023, 116: 108858. DOI: 10.1016/j.nanoen.2023.108858. |
| 4 | YAN H B, LI S M, ZHONG J Y, et al. An electrochemical perspective of aqueous zinc metal anode[J]. Nano-Micro Letters, 2023, 16(1): 15. DOI: 10.1007/s40820-023-01227-x. |
| 5 | LI B, ZENG Y, ZHANG W S, et al. Separator designs for aqueous zinc-ion batteries[J]. Science Bulletin, 2024, 69(5): 688-703. DOI: 10.1016/j.scib.2024.01.011. |
| 6 | 李万瑞, 李文俊, 王小青, 等. 锌离子电池用锰/钒基氧化物异质结构正极的研究进展[J]. 储能科学与技术, 2024, 13(5): 1496-1515. DOI: 10.19799/j.cnki.2095-4239.2023.0854. |
| LI W R, LI W J, WANG X Q, et al. Research progress of manganese/vanadium-based oxide heterostructure cathodes for zinc-ion batteries[J]. Energy Storage Science and Technology, 2024, 13(5): 1496-1515. DOI: 10.19799/j.cnki.2095-4239. 2023.0854. | |
| 7 | 周江, 单路通, 唐博雅, 等. 水系可充锌电池的发展及挑战[J]. 科学通报, 2020, 65(32): 3562-3596. DOI: 10.1360/TB-2020-0352. |
| ZHOU J, SHAN L T, TANG B Y, et al. Development and challenges of aqueous rechargeable zinc batteries[J]. Chinese Science Bulletin, 2020, 65(32): 3562-3596. DOI: 10.1360/TB-2020-0352. | |
| 8 | JAVED M S, MATEEN A, ALI S, et al. The emergence of 2D MXenes based Zn-ion batteries: Recent development and prospects[J]. Small, 2022, 18(26): e2201989. DOI: 10.1002/smll.202201989. |
| 9 | HAO J N, ZHANG S J, WU H, et al. Advanced cathodes for aqueous Zn batteries beyond Zn2+ intercalation[J]. Chemical Society Reviews, 2024, 53(9): 4312-4332. DOI: 10.1039/d3cs00771e. |
| 10 | LI Z, DU H L, LU J, et al. Self-assembly of antimony sulfide nanowires on three-dimensional reduced GO with superior electrochemical lithium storage performances[J]. Chemical Physics Letters, 2021, 771: 138529. DOI: 10.1016/j.cplett. 2021. 138529. |
| 11 | ZHANG Y W, DU H L, LU J, et al. Enhanced cyclic stability of Ga2O3@PDA-C nanospheres as pseudocapacitive anode materials for lithium-ion batteries[J]. Fuel, 2023, 334: 126683. DOI: 10.1016/j.fuel.2022.126683. |
| 12 | WANG D K, ZHOU C L, CAO B, et al. One-step synthesis of spherical Si/C composites with onion-like buffer structure as high-performance anodes for lithium-ion batteries[J]. Energy Storage Materials, 2020, 24: 312-318. DOI: 10.1016/j.ensm.2019.07.045. |
| 13 | PARKER J F, KO J S, ROLISON D R, et al. Translating materials-level performance into device-relevant metrics for zinc-based batteries[J]. Joule, 2018, 2(12): 2519-2527. DOI: 10.1016/j.joule.2018.11.007. |
| 14 | 唐梓巍, 师玉璞, 张雨禅, 等. 基于Informer神经网络的锂离子电池容量退化轨迹预测[J]. 储能科学与技术, 2024, 13(5): 1658-1666. DOI: 10.19799/j.cnki.2095-4239.2023.0812. |
| TANG Z W, SHI Y P, ZHANG Y S, et al. Prediction of lithium-ion battery capacity degradation trajectory based on Informer[J]. Energy Storage Science and Technology, 2024, 13(5): 1658-1666. DOI: 10.19799/j.cnki.2095-4239.2023.0812. | |
| 15 | YANG L, ZHU Y J, ZENG F L, et al. Synchronously promoting the electron and ion transport in high-loading Mn2.5V10O24∙5.9H2O cathodes for practical aqueous zinc-ion batteries[J]. Energy Storage Materials, 2024, 65: 103162. DOI: 10.1016/j.ensm. 2023. 103162. |
| 16 | ZHANG Y B, LI Z H, ZHAO B, et al. Ce ions and polyaniline co-intercalation into MOF-derived porous V2O5 nanosheets with a synergistic energy storage mechanism for high-capacity and super-stable aqueous zinc-ion batteries[J]. Journal of Materials Chemistry A, 2024, 12(3): 1725-1735. DOI: 10.1039/d3ta06554e. |
| 17 | HAN Z Y, GAO R H, WANG T S, et al. Machine-learning-assisted design of a binary descriptor to decipher electronic and structural effects on sulfur reduction kinetics[J]. Nature Catalysis, 2023, 6: 1073-1086. DOI: 10.1038/s41929-023-01041-z. |
| 18 | 刘起辉, 傅焰鹏, 罗京, 等. 低温水浴法制备钾离子预嵌层状MnO2及其储锌性能[J]. 储能科学与技术, 2023, 12(3): 768-776. DOI: 10.19799/j.cnki.2095-4239.2022.0612. |
| LIU Q H, FU Y P, LUO J, et al. Low-temperature solution synthesis of K+ preintercalated delta-MnO2 for high performance Zn-ion battery[J]. Energy Storage Science and Technology, 2023, 12(3): 768-776. DOI: 10.19799/j.cnki.2095-4239.2022.0612. | |
| 19 | HU Y X, DU H L, LU J, et al. Interface synergistic stabilization of zinc anodes via polyacrylic acid doped polyvinyl alcohol ultra-thin coating[J]. Journal of Energy Storage, 2024, 87: 111444. DOI: 10.1016/j.est.2024.111444. |
| 20 | LIU Y X, WANG T D, SUN Y N, et al. Fast and efficient in situ construction of low crystalline PEDOT-intercalated V2O5 nanosheets for high-performance zinc-ion battery[J]. Chemical Engineering Journal, 2024, 484: 149501. DOI: 10.1016/j.cej. 2024.149501. |
| 21 | ZHANG X F, ZHANG L, JIA X Y, et al. Design strategies for aqueous zinc metal batteries with high zinc utilization: From metal anodes to anode-free structures[J]. Nano-Micro Letters, 2024, 16(1): 75. DOI: 10.1007/s40820-023-01304-1. |
| 22 | LUO D, ZHU H, XIA Y, et al. A Li-rich layered oxide cathode with negligible voltage decay[J]. Nature Energy, 2023, 8: 1078-1087. DOI: 10.1038/s41560-023-01289-6. |
| 23 | LU J, DU H L, LIU H, et al. In situ constructing heterogeneous Mn(VO3)2/NaVO3 nanoribbons as high-performance cathodes for aqueous zinc-ion batteries[J]. Journal of Alloys and Compounds, 2024, 975: 172934. DOI: 10.1016/j.jallcom.2023.172934. |
| 24 | FAN L L, LI Z H, KANG W M, et al. Highly stable aqueous rechargeable Zn-ion battery: The synergistic effect between NaV6O15 and V2O5 in skin-core heterostructured nanowires cathode[J]. Journal of Energy Chemistry, 2021, 55: 25-33. DOI: 10.1016/j.jechem.2020.06.075. |
| 25 | RUAN P C, LIANG S Q, LU B G, et al. Design strategies for high-energy-density aqueous zinc batteries[J]. Angewandte Chemie (International Ed), 2022, 61(17): e202200598. DOI: 10.1002/anie. 202200598. |
| 26 | GUO Z H, WANG J X, YU P, et al. Toward full utilization and stable cycling of polyaniline cathode for nonaqueous rechargeable batteries[J]. Advanced Energy Materials, 2023, 13(38): 2301520. DOI: 10.1002/aenm.202301520. |
| 27 | LIU H, JIANG L, CAO B, et al. Van der waals interaction-driven self-assembly of V2O5 nanoplates and MXene for high-performing zinc-ion batteries by suppressing vanadium dissolution[J]. ACS Nano, 2022, 16(9): 14539-14548. DOI: 10.1021/acsnano.2c04968. |
| 28 | HU Y X, DU H L, LU J, et al. Achieving dendrite-free and long cyclic stability of zinc anode via CeO2 doped PEO interface modification[J]. Fuel, 2023, 354: 129417. DOI: 10.1016/j.fuel. 2023.129417. |
| 29 | ZHONG X Q, KONG Z Z, LIU Q F, et al. Design strategy of high stability vertically aligned RGO@V2O5 heterostructure cathodes for flexible quasi-solid-state aqueous zinc-ion batteries[J]. ACS Applied Materials & Interfaces, 2023, 15(50): 58333-58344. DOI: 10.1021/acsami.3c12161. |
| 30 | CUI S S, ZHANG D, GAN Y. Traditional electrochemical Zn2+ intercalation/extraction mechanism revisited: Unveiling ion-exchange mediated irreversible Zn2+ intercalation for the δ-MnO2 cathode in aqueous Zn ion batteries[J]. Advanced Energy Materials, 2024, 14(7): 2302655. DOI: 10.1002/aenm.202302655. |
| 31 | LI M, LIU M Z, LU Y Y, et al. A dual active site organic–inorganic poly(O-phenylenediamine)/NH4V3O8 composite cathode material for aqueous zinc-ion batteries[J]. Advanced Functional Materials, 2024, 34(19): 2312789. DOI: 10.1002/adfm.202312789. |
| 32 | DAI Y H, ZHANG C Y, LI J W, et al. Inhibition of vanadium cathodes dissolution in aqueous Zn-ion batteries[J]. Advanced Materials, 2024, 36(14): 2310645. DOI: 10.1002/adma. 202310645. |
| 33 | ZHANG T, REN M, HUANG Y H, et al. Negative lattice expansion in an O3-type transition-metal oxide cathode for highly stable sodium-ion batteries[J]. Angewandte Chemie (International Ed), 2024, 63(8): e202316949. DOI: 10.1002/anie.202316949. |
| 34 | LIANG J X, HOU Y P, SUN J, et al. Overpotential regulation of vanadium-doped chitosan carbon aerogel cathode promotes heterogeneous electro-Fenton degradation efficiency[J]. Applied Catalysis B: Environmental, 2022, 317: 121794. DOI: 10.1016/j.apcatb.2022.121794. |
| 35 | TIAN G F, LING D D, ZHANG D H, et al. Reversible NH4+ intercalation/de-intercalation with phosphate promotion effect in tunnel (NH4)0.25WO3[J]. Chemical Engineering Journal, 2024, 482: 149160. DOI: 10.1016/j.cej.2024.149160. |
| 36 | ZHAO F J, LI J W, CHUTIA A, et al. Highly stable manganese oxide cathode material enabled by Grotthuss topochemistry for aqueous zinc ion batteries[J]. Energy & Environmental Science, 2024, 17(4): 1497-1508. DOI: 10.1039/D3EE04161A. |
| 37 | PENG Z, FENG Z M, ZHOU X L, et al. Polymer engineering for electrodes of aqueous zinc ion batteries[J]. Journal of Energy Chemistry, 2024, 91: 345-369. DOI: 10.1016/j.jechem. 2023. 12.012. |
| 38 | HUANG L L, LI J H, GAO X Y, et al. Realizing high-performance of quinone-based cathode via multiple active centers for aqueous zinc ion batteries[J]. Journal of Power Sources, 2024, 591: 233896. DOI: 10.1016/j.jpowsour.2023.233896. |
| 39 | QIU Y, SUN Z H, GUO Z H, et al. Ion-molecule co-confining ammonium vanadate cathode for high-performance aqueous zinc-ion batteries[J]. Small, 2024, 20(22): e2311029. DOI: 10.1002/smll.202311029. |
| 40 | ZHENG Y, ZHOU T F, ZHANG C F, et al. Boosted charge transfer in SnS/SnO2 heterostructures: Toward high rate capability for sodium-ion batteries[J]. Angewandte Chemie (International Ed), 2016, 55(10): 3408-3413. DOI: 10.1002/anie.201510978. |
| 41 | ZHANG B, XU B H, QIN H Z, et al. Highly active and stable Cu9S5-MoS2 heterostructures nanocages enabled by dual-functional Cu electrocatalyst with enhanced potassium storage[J]. Journal of Materials Science & Technology, 2023, 143: 107-116. DOI: 10.1016/j.jmst.2022.10.011. |
| 42 | WANG Y, NIU S Y, GONG S S, et al. Fused functional organic material with the alternating conjugation of quinone-pyrazine as cathode for aqueous zinc ion batteries[J]. Small Methods, 2024, 8(7): e2301301. DOI: 10.1002/smtd.202301301. |
| 43 | LIU S C, ZHU H, ZHANG B H, et al. Tuning the kinetics of zinc-ion insertion/extraction in V2O5 by in situ polyaniline intercalation enables improved aqueous zinc-ion storage performance[J]. Advanced Materials, 2020, 32(26): 2001113. DOI: 10.1002/adma.202001113. |
| 44 | LIANG X W, YANG Y Y, DI W F, et al. Three-dimensional mixed-valence polyoxovanadates@polyaniline architecture towards high-performance rechargeable aqueous zinc-ion batteries cathode[J]. Chemical Engineering Journal, 2024, 495: 153255. DOI: 10.1016/j.cej.2024.153255. |
| 45 | SUN J J, ZHAO Y F, LIU Y Y, et al. Synthesis of V2O5·nH2O nanobelts@polyaniline core-shell structures with highly efficient Zn2+ storage[J]. Journal of Colloid and Interface Science, 2023, 633: 923-931. DOI: 10.1016/j.jcis.2022.11.153. |
| 46 | LI X, LI Y, XIE S Y, et al. Zinc-based energy storage with functionalized carbon nanotube/polyaniline nanocomposite cathodes[J]. Chemical Engineering Journal, 2022, 427: 131799. DOI: 10.1016/j.cej.2021.131799. |
| 47 | DU M, LIU C F, ZHANG F, et al. Tunable layered (Na, Mn)V8O20· nH2O cathode material for high-performance aqueous zinc ion batteries[J]. Advanced Science, 2020, 7(13): 2000083. DOI: 10.1002/advs.202000083. |
| 48 | ZHU H, MA L, JIANG J, et al. Green synthesis of polypyrrole coated manganesee(II) vanadate nanoflower composite as cathode materials[J]. International Journal of Electrochemical Science, 2020, 15(1): 371-381. DOI: 10.20964/2020.01.17. |
| 49 | LI Y, LI X, DUAN H, et al. Aerogel-structured MnO2 cathode assembled by defect-rich ultrathin nanosheets for zinc-ion batteries[J]. Chemical Engineering Journal, 2022, 441: 136008. DOI: 10.1016/j.cej.2022.136008. |
| 50 | ZHANG Y F, XU L, JIANG H M, et al. Polyaniline-expanded the interlayer spacing of hydrated vanadium pentoxide by the interface-intercalation for aqueous rechargeable Zn-ion batteries[J]. Journal of Colloid and Interface Science, 2021, 603: 641-650. DOI: 10.1016/j.jcis.2021.06.141. |
| 51 | ZENG J, ZHANG Z H, GUO X S, et al. A conjugated polyaniline and water co-intercalation strategy boosting zinc-ion storage performances for rose-like vanadium oxide architectures[J]. Journal of Materials Chemistry A, 2019, 7(37): 21079-21084. DOI: 10.1039/C9TA08086D. |
| 52 | TAN S D, SANG Z Y, YI Z H, et al. Conductive coating, cation-intercalation, and oxygen vacancies co-modified vanadium oxides as high-rate and stable cathodes for aqueous zinc-ion batteries[J]. EcoMat, 2023, 5(4): e12326. DOI: 10.1002/eom2. 12326. |
| 53 | ZHENG C, JIAN B Q, ZHONG J R, et al. Single-crystalline Mn2V2O7 anodes with high rate and ultra-stable capability for sodium-ion batteries[J]. Journal of Alloys and Compounds, 2023, 934: 168018. DOI: 10.1016/j.jallcom.2022.168018. |
| 54 | WANG J J, ZHAO X Y, KANG J Z, et al. Li+, Na+ co-stabilized vanadium oxide nanobelts with a bilayer structure for boosted zinc-ion storage performance[J]. Journal of Materials Chemistry A, 2022, 10(40): 21531-21539. DOI: 10.1039/D2TA05803K. |
| 55 | LIU H, DU H L, ZHAO W, et al. Fast potassium migration in mesoporous carbon with ultrathin framework boosting superior rate performance for high-power potassium storage[J]. Energy Storage Materials, 2021, 40: 490-498. DOI: 10.1016/j.ensm. 2021.05.037. |
| 56 | LIU A N, WU F, ZHANG Y X, et al. Ultralarge layer spacing and superior structural stability of V2O5 as high-performance cathode for aqueous zinc-ion battery[J]. Nano Research, 2023, 16(7): 9461-9470. DOI: 10.1007/s12274-023-5676-0. |
| 57 | LUO P, YU G T, ZHANG W W, et al. "Triple-synergistic effect" of K+ and PANI co-intercalation enabling the high-rate capability and stability of V2O5 for aqueous zinc-ion batteries[J]. Journal of Colloid and Interface Science, 2024, 659: 267-275. DOI: 10.1016/j.jcis.2023.12.167. |
| 58 | WANG X D, LU Y, LI R, et al. High-performance aqueous zinc-ion batteries enabled by superlattice intercalation Zn3V2O7-C cathodes[J]. ACS Applied Energy Materials, 2023, 6(4): 2462-2470. DOI: 10.1021/acsaem.2c03787. |
| [1] | Wanrui LI, Wenjun LI, Xiaoqing WANG, Shengli LU, Xilian XU. Research progress of manganese/vanadium-based oxide heterostructure cathodes for zinc-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(5): 1496-1515. |
| [2] | Ting TING, Qihang LIN, Changyang LIU, Liuzhen BIAN, Chao SUN, QI Ji, Jihua PENG, Shengli AN. Research progress in modification of manganese dioxide as cathode materials for aqueous zinc-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(3): 754-767. |
| [3] | Miao WU, Guiqing ZHAO, Zhongzhu QIU, Baofeng WANG. Preparation and electrochemical properties of NiCo2O4 as a novel cathode material for aqueous zinc-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(3): 1019-1025. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
