Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (4): 1424-1444.doi: 10.19799/j.cnki.2095-4239.2024.1078
• Energy Storage Materials and Devices • Previous Articles Next Articles
Bohua WEN1( ), Haijun MENG2, Yonglong CHEN1, Xiaohui LI3, Jiayan LUO3, Lin LIN4, Lan ZHANG5
), Haijun MENG2, Yonglong CHEN1, Xiaohui LI3, Jiayan LUO3, Lin LIN4, Lan ZHANG5
Received:2024-11-18
Revised:2024-12-10
Online:2025-04-28
Published:2025-05-20
Contact:
Bohua WEN
E-mail:bohuawen@sz.tsinghua.edu.cn
CLC Number:
Bohua WEN, Haijun MENG, Yonglong CHEN, Xiaohui LI, Jiayan LUO, Lin LIN, Lan ZHANG. Research progress on high specific-energy solid-state lithium-sulfur batteries[J]. Energy Storage Science and Technology, 2025, 14(4): 1424-1444.

Fig. 3
Charge transportation and interfacial stability in SLSB. (a) The influence of Mass transfer and reaction rate on the discharge product and the corresponding volume change; (b) Charge transportation under various ϕ(SE)/ϕ(C) and the corresponding discharge capacity in the 10th cycle; (c) The function of redox meditator (RM) on Li2S; (d) Low density SE with reasonable dispersion state could promote the kinetics performances of SLSB"
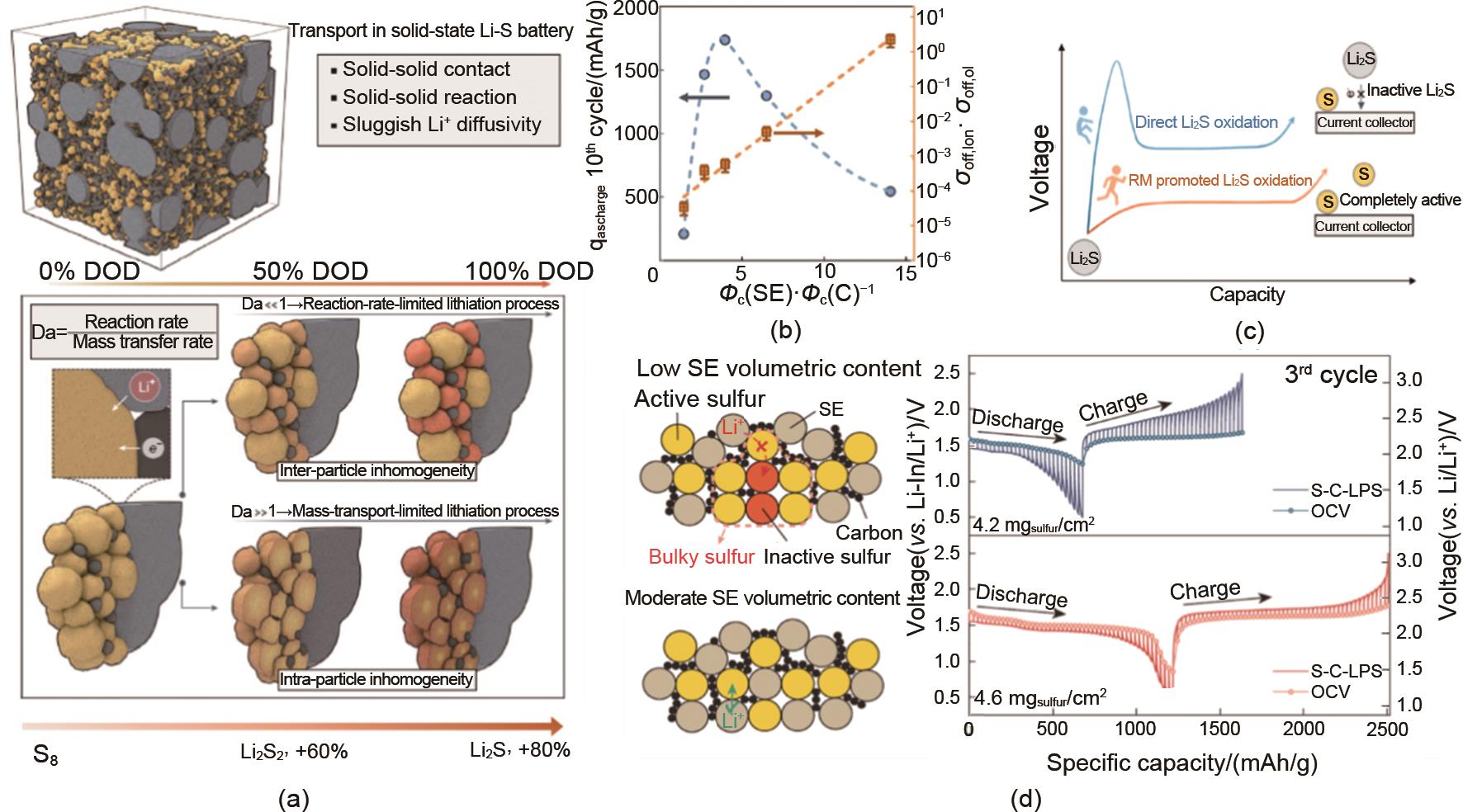

Fig. 4
(a) Average discharge potential, reversible capacity and specific energy density of typical metal sulfides; (b) Electronic conductivity of typical cathode material for Li-S battery and some typical electrode materials for lithium ion batteries; (c) Energy storage mechanism of S9.3I and the HAADF result of its lithiation product"
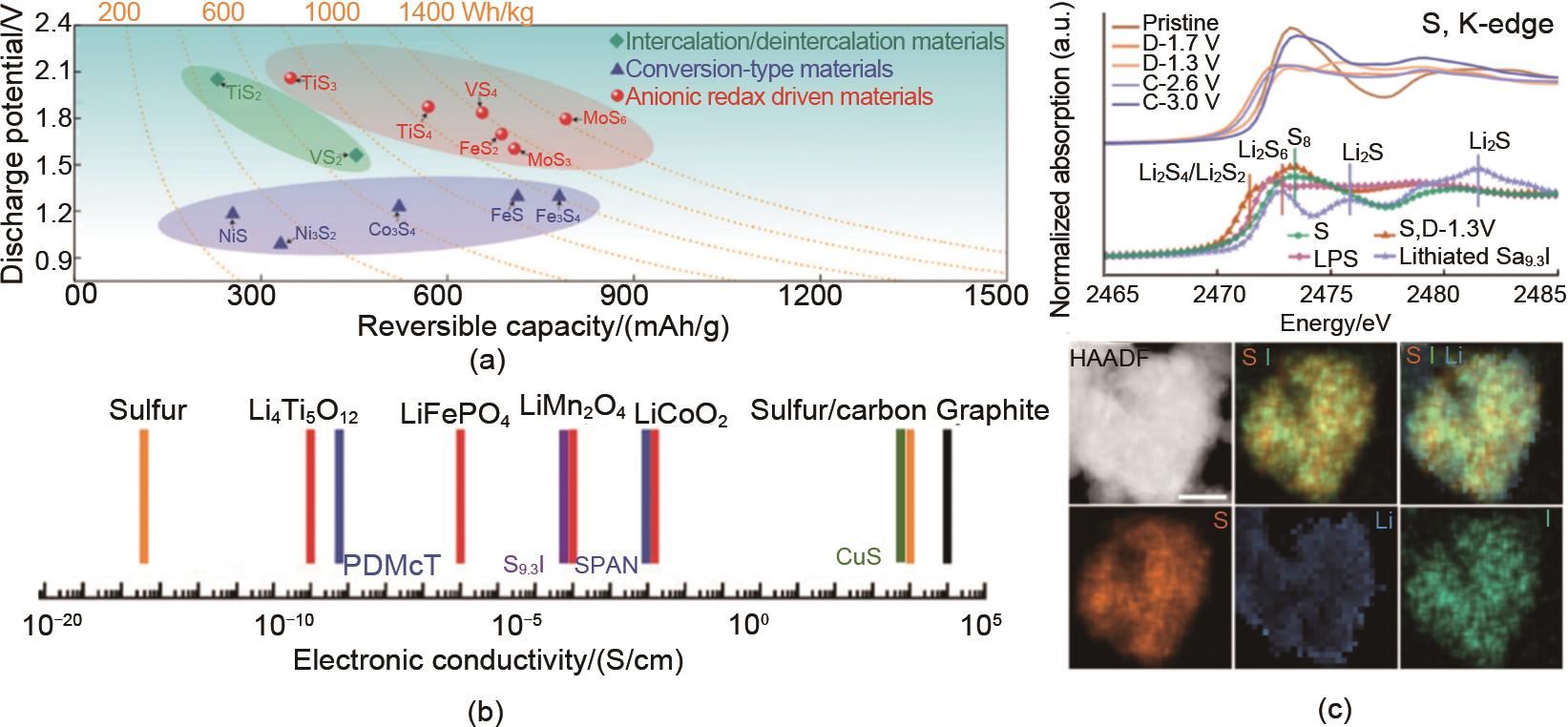
Fig. 5
Typical issues and stabilization strategies at the anode in solid-state lithium-sulfur batteries: (a) The generation of dendrite and dead lithium in the cycling process; (b) Stabilization of the anode using lithium alloying strategies; (c) Construction of an interlayer between the lithium anode and the solid-state electrolyte; (d) Fabrication of lithium/carbon composite anodes"
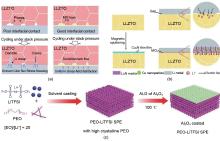
Fig. 7
Design of Interlayers on the surface of anodes in SLSB: (a) Construction of the MIS interlayer to stabilize the anode and enhance the limiting current density; (b) The MCL interlayer stabilizes the electric field distribution; (c) The Al2O3 interlayer improves the stability of polymer solid-state electrolytes in lithium-sulfur batteries"
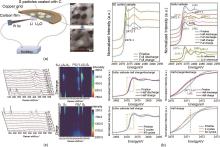
Fig. 8
Understanding the reaction mechanism of ASLSBs. (a) Schematic diagram of the electrochemical device set for in situ TEM observation of the solid-state Li-S nanobattery. Typical ADF-STEM images of S sample before and after lithiation; (b) Ex-situ sulfur K-edge XANES profiles of SE control and cathode in different charge/discharge states and references. The zoom-in spectra show the feature of Li2S2 in XANES profiles comparing the cathode in a pristine state, and half charge states after 2 and 40 cycles; (c) Waterfall plots of Raman spectra and the corresponding intensity mapping of the sulfur cathode at various voltages during the discharge and charge process operating at 60 ℃"
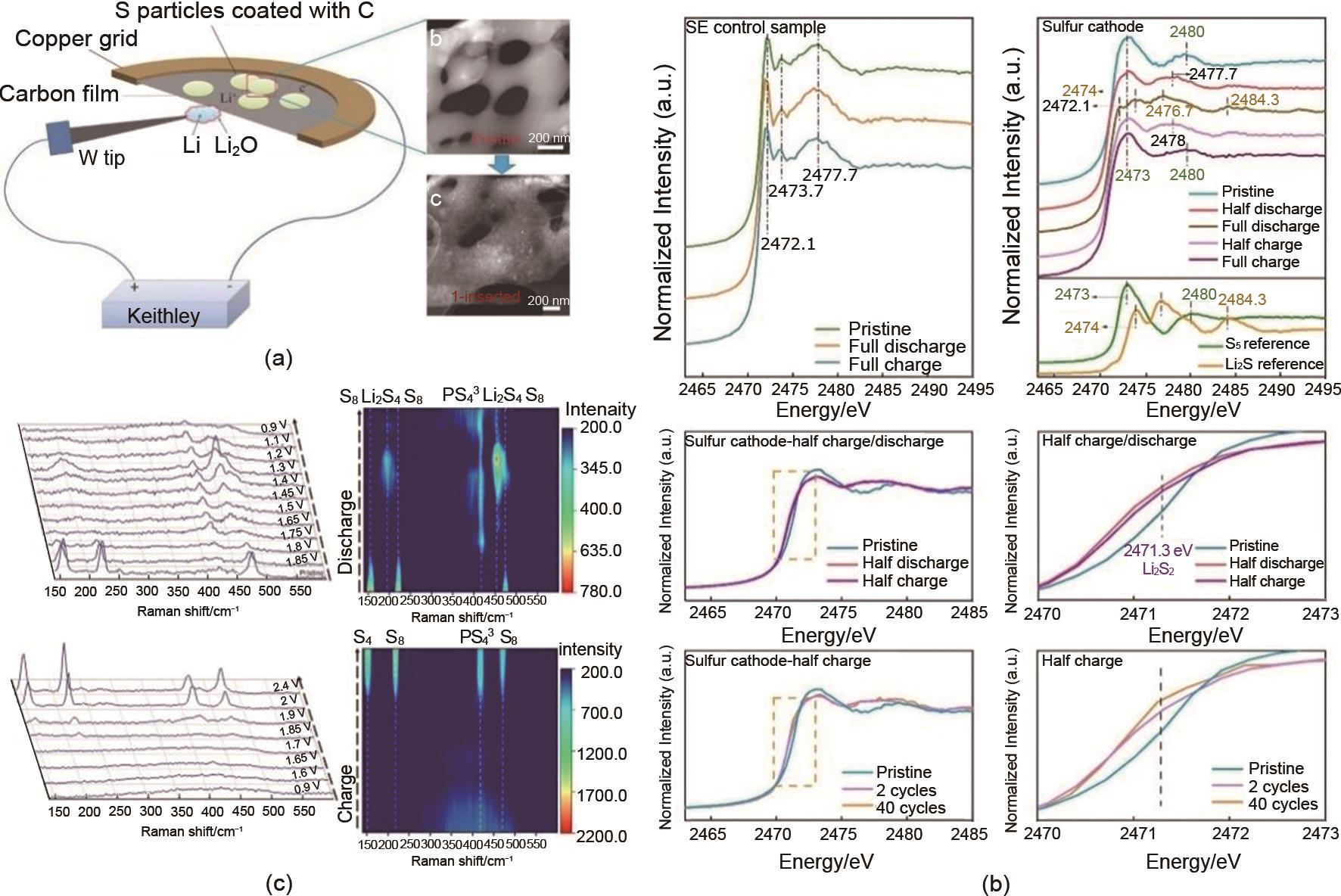
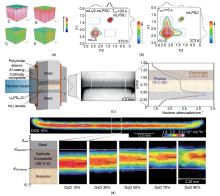
Fig. 9
(a) ToF-SIMS depth profiles and chemical element distribution of C, S, Ti, and P elements of H-C/LATP-20@S cathode after 50 cycles; (b) Two-dimensional 6Li-6Li exchange (2D-EXSY) NMR spectra of the Li2S/LPSCI and Li2S/LiI/LPSCI composite cathode at a mixing time of 10 s and the temperature of 293 K; (c) Cell design, representative neutron radiography, and the position-dependent neutron attenuation quantified within the white box in before and after the initial discharge visualizes the variation in the distribution of the Li concentration; (d) Neutron radiography image at 10% DoD, and progression of the point of maximum rate of attenuation change (reaction front) toward higher d as DoD increases"

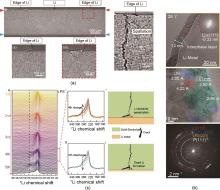
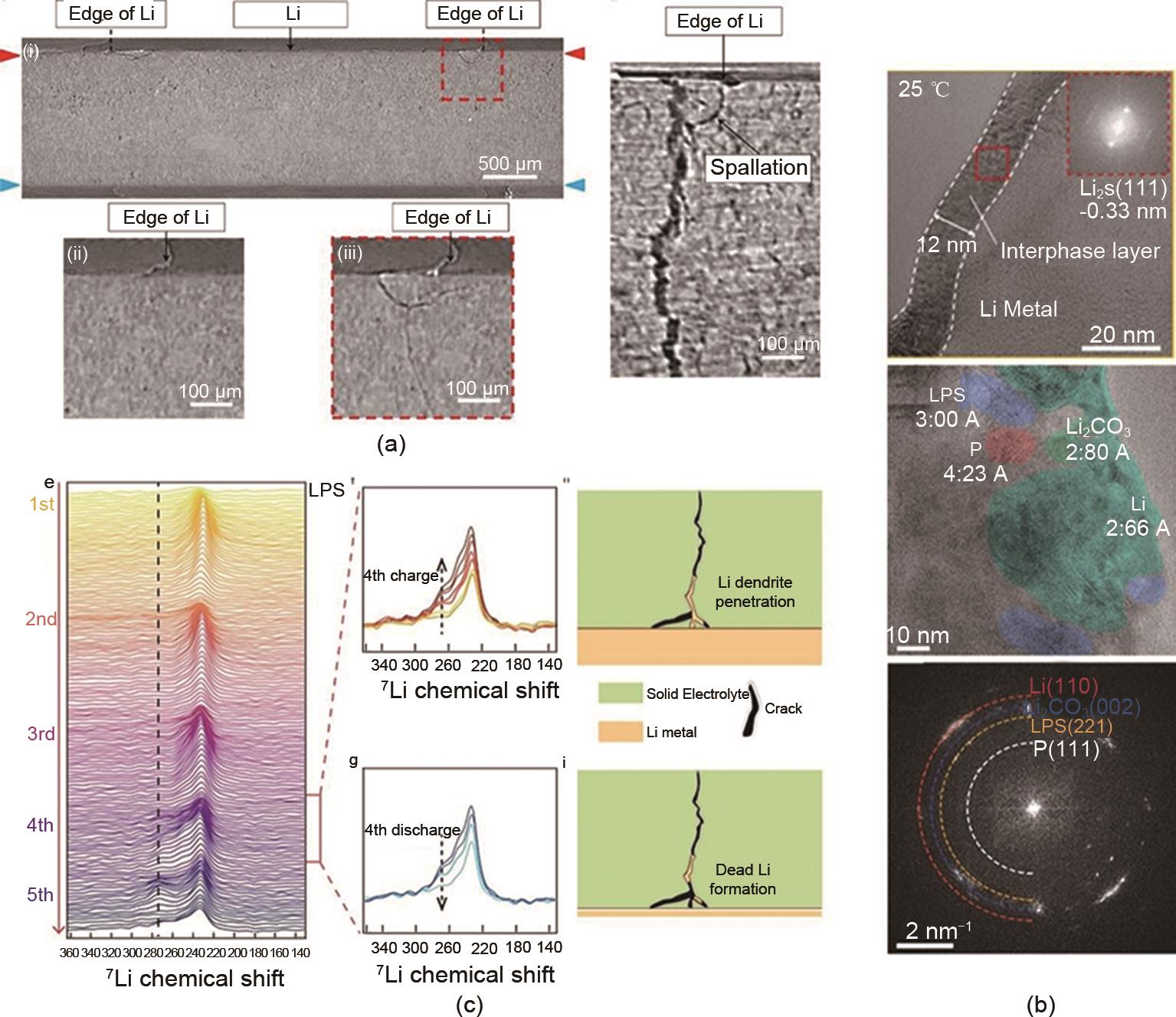
Fig. 10
Investigation of interactions between lithium metal anode and the SE: (a) XCT cross-sectional images illustrating the spallations at the edge of the lithium electrode, along with magnified views of vertical cracks formed at these regions; (b) Cryo-TEM observations revealing the evolution of the interfacial layer, providing insights into interfacial transformations; (c) Operando 7Li NMR stack spectra of AFB (with LPS) during the first five cycles, depicting the evolution of lithium signals and indicating the formation of “dead” lithium"
| 1 | BRUCE P G, FREUNBERGER S A, HARDWICK L J, et al. Li-O2 and Li-S batteries with high energy storage[J]. Nature Materials, 2011, 11(1): 19-29. DOI: 10.1038/nmat3191. |
| 2 | LEE J, ZHAO C, WANG C H, et al. Bridging the gap between academic research and industrial development in advanced all-solid-state lithium-sulfur batteries[J]. Chemical Society Reviews, 2024, 53(10): 5264-5290. DOI: 10.1039/d3cs00439b. |
| 3 | LIANG F, WANG S Z, LIANG Q, et al. Insight into all-solid-state Li-S batteries: Challenges, advances, and engineering design[J]. Advanced Energy Materials, 2024, 14(38): 2401959. DOI: 10.1002/aenm.202401959. |
| 4 | OHNO S, ZEIER W G. Toward practical solid-state lithium-sulfur batteries: Challenges and perspectives[J]. Accounts of Materials Research, 2021, 2(10): 869-880. DOI: 10.1021/accountsmr.1c00116. |
| 5 | CHEN Z R, LIANG Z T, ZHONG H Y, et al. Bulk/interfacial synergetic approaches enable the stable anode for high energy density all-solid-state lithium-sulfur batteries[J]. ACS Energy Letters, 2022, 7(8): 2761-2770. DOI: 10.1021/acsenergylett.2c01334. |
| 6 | HASSOUN J, SCROSATI B. Moving to a solid-state configuration: A valid approach to making lithium-sulfur batteries viable for practical applications[J]. Advanced Materials, 2010, 22(45): 5198-5201. DOI: 10.1002/adma.201002584. |
| 7 | YU X G, XIE J Y, YANG J, et al. All solid-state rechargeable lithium cells based on nano-sulfur composite cathodes[J]. Journal of Power Sources, 2004, 132(1/2): 181-186. DOI: 10.1016/j.jpowsour.2004.01.034. |
| 8 | HOSSEINI S M, VARZI A, ITO S, et al. High loading CuS-based cathodes for all-solid-state lithium sulfur batteries with enhanced volumetric capacity[J]. Energy Storage Materials, 2020, 27: 61-68. DOI: 10.1016/j.ensm.2020.01.022. |
| 9 | ZHOU J B, CHANDRAPPA M L H, TAN S, et al. Healable and conductive sulfur iodide for solid-state Li-S batteries[J]. Nature, 2024, 627(8003): 301-305. DOI: 10.1038/s41586-024-07101-z. |
| 10 | ESHETU G G, JUDEZ X, LI C M, et al. Lithium azide as an electrolyte additive for all-solid-state lithium-sulfur batteries[J]. Angewandte Chemie International Edition, 2017, 56(48): 15368-15372. DOI: 10.1002/anie.201709305. |
| 11 | SHENG O W, JIN C B, LUO J M, et al. Ionic conductivity promotion of polymer electrolyte with ionic liquid grafted oxides for all-solid-state lithium-sulfur batteries[J]. Journal of Materials Chemistry A, 2017, 5(25): 12934-12942. DOI: 10.1039/C7TA03699J. |
| 12 | TAO X Y, LIU Y Y, LIU W, et al. Solid-state lithium-sulfur batteries operated at 37 ℃ with composites of nanostructured Li7La3Zr2O12/carbon foam and polymer[J]. Nano Letters, 2017, 17(5): 2967-2972. DOI: 10.1021/acs.nanolett.7b00221. |
| 13 | CHEN L, FAN L Z. Dendrite-free Li metal deposition in all-solid-state lithium sulfur batteries with polymer-in-salt polysiloxane electrolyte[J]. Energy Storage Materials, 2018, 15: 37-45. DOI: 10.1016/j.ensm.2018.03.015. |
| 14 | ESHETU G G, JUDEZ X, LI C M, et al. Ultrahigh performance all solid-state lithium sulfur batteries: Salt anion's chemistry-induced anomalous synergistic effect[J]. Journal of the American Chemical Society, 2018, 140(31): 9921-9933. DOI: 10.1021/jacs. 8b04612. |
| 15 | SONG Y X, SHI Y, WAN J, et al. Direct tracking of the polysulfide shuttling and interfacial evolution in all-solid-state lithium-sulfur batteries: A degradation mechanism study[J]. Energy & Environmental Science, 2019, 12(8): 2496-2506. DOI: 10.1039/C9EE00578A. |
| 16 | LIU Y, LIU H W, LIN Y T, et al. Mechanistic investigation of polymer-based all-solid-state lithium/sulfur battery[J]. Advanced Functional Materials, 2021, 31(41): 2104863. DOI: 10.1002/adfm. 202104863. |
| 17 | HAKARI T, FUJITA Y, DEGUCHI M, et al. Solid electrolyte with oxidation tolerance provides a high-capacity Li2S-based positive electrode for all-solid-state Li/S batteries[J]. Advanced Functional Materials, 2022, 32(5): 2106174. DOI: 10.1002/adfm.202106174. |
| 18 | KIM J T, SU H, ZHONG Y, et al. All-solid-state lithium-sulfur batteries through a reaction engineering lens[J]. Nature Chemical Engineering, 2024, 1(6): 400-410. DOI: 10.1038/s44286-024-00079-5. |
| 19 | DEWALD G F, OHNO S, HERING J G C, et al. Analysis of charge carrier transport toward optimized cathode composites for all-solid-state Li-S batteries[J]. Batteries & Supercaps, 2021, 4(1): 183-194. DOI: 10.1002/batt.202000194. |
| 20 | GAO X, ZHENG X L, TSAO Y, et al. All-solid-state lithium-sulfur batteries enhanced by redox mediators[J]. Journal of the American Chemical Society, 2021, 143(43): 18188-18195. DOI: 10.1021/jacs.1c07754. |
| 21 | WANG D W, JHANG L J, KOU R, et al. Realizing high-capacity all-solid-state lithium-sulfur batteries using a low-density inorganic solid-state electrolyte[J]. Nature Communications, 2023, 14(1): 1895. DOI: 10.1038/s41467-023-37564-z. |
| 22 | HOLEKEVI CHANDRAPPA M L, QI J, CHEN C, et al. Thermodynamics and kinetics of the cathode-electrolyte interface in all-solid-state Li-S batteries[J]. Journal of the American Chemical Society, 2022, 144(39): 18009-18022. DOI: 10.1021/jacs.2c07482. |
| 23 | HOU L P, YUAN H, ZHAO C Z, et al. Improved interfacial electronic contacts powering high sulfur utilization in all-solid-state lithium-sulfur batteries[J]. Energy Storage Materials, 2020, 25: 436-442. DOI: 10.1016/j.ensm.2019.09.037. |
| 24 | 叶戈, 袁洪, 赵辰孜, 等. 全固态锂硫电池正极中离子输运与电子传递的平衡[J]. 储能科学与技术, 2020, 9(2): 339-345. DOI: 10.19799/j.cnki.2095-4239.2020.0002. |
| YE G, YUAN H, ZHAO C Z, et al. Balance between ion migration and electron transport in composite cathodes for all-solid-state lithium-sulfur batteries[J]. Energy Storage Science and Technology, 2020, 9(2): 339-345. DOI: 10.19799/j.cnki.2095-4239.2020.0002. | |
| 25 | LIU Y Z, MENG X Y, WANG Z Y, et al. A Li2S-based all-solid-state battery with high energy and superior safety[J]. Science Advances, 2022, 8(1): eabl8390. DOI: 10.1126/sciadv.abl8390. |
| 26 | FUJITA Y, MÜNCH K, ASAKURA T, et al. Dynamic volume change of Li2S-based active material and the influence of stacking pressure on capacity in all-solid-state batteries[J]. Chemistry of Materials, 2024, 36(15): 7533-7540. DOI: 10.1021/acs.chemmater.4c01514. |
| 27 | LIN Z, LIU Z C, DUDNEY N J, et al. Lithium superionic sulfide cathode for all-solid lithium-sulfur batteries[J]. ACS Nano, 2013, 7(3): 2829-2833. DOI: 10.1021/nn400391h. |
| 28 | XU R C, YUE J, LIU S F, et al. Cathode-supported all-solid-state lithium-sulfur batteries with high cell-level energy density[J]. ACS Energy Letters, 2019, 4(5): 1073-1079. DOI: 10.1021/acsenergylett.9b00430. |
| 29 | FUJITA Y, SAKUDA A, HASEGAWA Y, et al. High capacity Li2S-Li2O-LiI positive electrodes with nanoscale ion-conduction pathways for all-solid-state Li/S batteries[J]. Small, 2023, 19(36): 2302179. DOI: 10.1002/smll.202302179. |
| 30 | YUAN H, NAN H X, ZHAO C Z, et al. Slurry-coated sulfur/sulfide cathode with Li metal anode for all-solid-state lithium-sulfur pouch cells[J]. Batteries & Supercaps, 2020, 3(7): 596-603. DOI: 10.1002/batt.202000051. |
| 31 | KIM H, CHOI H N, HWANG J Y, et al. Tailoring the interface between sulfur and sulfide solid electrolyte for high-areal-capacity all-solid-state lithium-sulfur batteries[J]. ACS Energy Letters, 2023, 8(10): 3971-3979. DOI: 10.1021/acsenergylett.3c01473. |
| 32 | MWIZERWA J P, ZHANG Q, HAN F D, et al. Sulfur-embedded FeS2 as a high-performance cathode for room temperature all-solid-state lithium-sulfur batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(16): 18519-18525. DOI: 10.1021/acsami. 0c01607. |
| 33 | SHI J M, LIU G Z, WENG W, et al. Co3S4@Li7P3S11 hexagonal platelets as cathodes with superior interfacial contact for all-solid-state lithium batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(12): 14079-14086. DOI: 10.1021/acsami.0c02085. |
| 34 | YANG M L, YAO Y, CHANG M Y, et al. High energy density sulfur-rich MoS6-based nanocomposite for room temperature all-solid-state lithium metal batteries[J]. Advanced Energy Materials, 2023, 13(28): 2300962. DOI: 10.1002/aenm.202300962. |
| 35 | ZHANG X Y, YU T, YANG S Y, et al. Organosulfur cathodes in lithium metal batteries: Bridging the gap between fundamentals and practical applications[J]. Advanced Functional Materials, 2024, 34(42): 2405122. DOI: 10.1002/adfm.202405122. |
| 36 | GAO J, GAO Y, HAO J H, et al. Activating redox kinetics of Li2S via Cu+, I- co-doping toward high-performance all-solid-state lithium sulfide-based batteries[J]. Small, 2024, 20(47): 2404171. DOI: 10.1002/smll.202404171. |
| 37 | XU G L, SUN H, LUO C, et al. Solid-state lithium/selenium-sulfur chemistry enabled via a robust solid-electrolyte interphase[J]. Advanced Energy Materials, 2019, 9(2): 1802235. DOI: 10.1002/aenm.201802235. |
| 38 | XU K L, LIU X J, LIANG J W, et al. Manipulating the redox kinetics of Li-S chemistry by tellurium doping for improved Li-S batteries[J]. ACS Energy Letters, 2018, 3(2): 420-427. DOI: 10.1021/acsenergylett.7b01249. |
| 39 | ZHANG Y, ORHAN O K, TAO L, et al. A high-performance tellurium-sulfur cathode in carbonate-based electrolytes[J]. Nano Energy, 2023, 107: 108141. DOI: 10.1016/j.nanoen.2022.108141. |
| 40 | ZHAO W, ZHANG Y, LIU Q S, et al. Entropy-modulated short-chain cathode for low-temperature all-solid-state Li-S batteries[J]. Angewandte Chemie International Edition, 2025, 64(1): e202413670. DOI: 10.1002/anie.202413670. |
| 41 | OYAMA N, TATSUMA T, SOTOMURA T. Organosulfur polymer batteries with high energy density[J]. Journal of Power Sources, 1997, 68(1): 135-138. DOI: 10.1016/S0378-7753(96)02586-4. |
| 42 | MOON H S, PARK J K. Electrochemical characteristics of the dimercaptan-poly(ethylene oxide) grafted polyaniline electrodes[J]. Solid State Ionics, 1999, 120(1/2/3/4): 1-12. DOI: 10.1016/S0167-2738(99)00003-X. |
| 43 | POPE J M, SATO T, SHOJI E, et al. Organosulfur/conducting polymer composite cathodes[J]. Journal of the Electrochemical Society, 2002, 149(7): A939. DOI: 10.1149/1.1482768. |
| 44 | WANG J L, YANG J, XIE J Y, et al. A novel conductive polymer-sulfur composite cathode material for rechargeable lithium batteries[J]. Advanced Materials, 2002, 14(13/14): 963-965. DOI: 10.1016/s0140-6701(03)91849-2. |
| 45 | WEI S Y, MA L, HENDRICKSON K E, et al. Metal-sulfur battery cathodes based on PAN-sulfur composites[J]. Journal of the American Chemical Society, 2015, 137(37): 12143-12152. DOI: 10.1021/jacs.5b08113. |
| 46 | WANG S, ZHOU J B, FENG S J, et al. Polythiocyanogen as cathode materials for high temperature all-solid-state lithium-sulfur batteries[J]. ACS Energy Letters, 2023, 8(6): 2699-2706. DOI: 10.1021/acsenergylett.3c00659. |
| 47 | TAN S, RAHMAN M M, WU Z H, et al. Structural and interphasial stabilities of sulfurized polyacrylonitrile (SPAN) cathode[J]. ACS Energy Letters, 2023, 8(6): 2496-2504. DOI: 10.1021/acsenergylett.3c00281. |
| 48 | WANG S, LU B Y, CHENG D Y, et al. Structural transformation in a sulfurized polymer cathode to enable long-life rechargeable lithium-sulfur batteries[J]. Journal of the American Chemical Society, 2023, 145(17): 9624-9633. DOI: 10.1021/jacs.3c00628. |
| 49 | KIM H, SADAN M K, KIM C, et al. Enhanced reversible capacity of sulfurized polyacrylonitrile cathode for room-temperature Na/S batteries by electrochemical activation[J]. Chemical Engineering Journal, 2021, 426: 130787. DOI: 10.1016/j.cej.2021.130787. |
| 50 | PEREZ BELTRAN S, BALBUENA P B. Sulfurized polyacrylonitrile (SPAN): Changes in mechanical properties during electrochemical lithiation[J]. The Journal of Physical Chemistry C, 2021, 125(24): 13185-13194. DOI: 10.1021/acs.jpcc.1c02966. |
| 51 | LI M R, FRERICHS J E, KOLEK M, et al. Solid-state lithium-sulfur battery enabled by thio-LiSICON/polymer composite electrolyte and sulfurized polyacrylonitrile cathode[J]. Advanced Functional Materials, 2020, 30(14): 1910123. DOI: 10.1002/adfm.201910123. |
| 52 | LI C, ZHANG Q, SHENG J Z, et al. A quasi-intercalation reaction for fast sulfur redox kinetics in solid-state lithium-sulfur batteries[J]. Energy & Environmental Science, 2022, 15(10): 4289-4300. DOI: 10.1039/D2EE01820A. |
| 53 | GUO Y W, ZUO W J, WU X K, et al. Practical SPAN||Li cells enabled by in situ polymerized electrolyte[J]. Materials Today Energy, 2024, 45: 101662. DOI: 10.1016/j.mtener.2024.101662. |
| 54 | ZHANG Y Y, SUN Y L, PENG L F, et al. Se as eutectic accelerator in sulfurized polyacrylonitrile for high performance all-solid-state lithium-sulfur battery[J]. Energy Storage Materials, 2019, 21: 287-296. DOI: 10.1016/j.ensm.2018.12.010. |
| 55 | ZHANG W, ZHANG Y Y, PENG L F, et al. Elevating reactivity and cyclability of all-solid-state lithium-sulfur batteries by the combination of tellurium-doping and surface coating[J]. Nano Energy, 2020, 76: 105083. DOI: 10.1016/j.nanoen.2020.105083. |
| 56 | BHARGAV A, MANTHIRAM A. 2,5-Dimercapto-1,3,4-thiadiazole (DMCT)-based polymers for rechargeable metal-sulfur batteries[J]. Energy & Environmental Materials, 2023, 6(6): e12446. DOI: 10.1002/eem2.12446. |
| 57 | PAN H, ZHANG M H, CHENG Z, et al. Carbon-free and binder-free Li-Al alloy anode enabling an all-solid-state Li-S battery with high energy and stability[J]. Science Advances, 2022, 8(15): eabn4372. DOI: 10.1126/sciadv.abn4372. |
| 58 | DUAN C, CHENG Z, LI W, et al. Realizing the compatibility of a Li metal anode in an all-solid-state Li-S battery by chemical iodine-vapor deposition[J]. Energy & Environmental Science, 2022, 15(8): 3236-3245. DOI: 10.1039/D2EE01358D. |
| 59 | LEE Y G, FUJIKI S, JUNG C, et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes[J]. Nature Energy, 2020, 5(4): 299-308. DOI: 10.1038/s41560-020-0575-z. |
| 60 | BI C X, ZHAO M, HOU L P, et al. Anode material options toward 500 Wh·kg-1 lithium-sulfur batteries[J]. Advanced Science, 2022, 9(2): 2103910. DOI: 10.1002/advs.202103910. |
| 61 | TU Z Y, CHOUDHURY S, ZACHMAN M J, et al. Fast ion transport at solid-solid interfaces in hybrid battery anodes[J]. Nature Energy, 2018, 3(4): 310-316. DOI: 10.1038/s41560-018-0096-1. |
| 62 | WAN J, SONG Y X, CHEN W P, et al. Micromechanism in all-solid-state alloy-metal batteries: Regulating homogeneous lithium precipitation and flexible solid electrolyte interphase evolution[J]. Journal of the American Chemical Society, 2021, 143(2): 839-848. DOI: 10.1021/jacs.0c10121. |
| 63 | JI W X, ZHANG X X, LIU M, et al. High-performance all-solid-state Li-S batteries enabled by an all-electrochem-active prelithiated Si anode[J]. Energy Storage Materials, 2022, 53: 613-620. DOI: 10.1016/j.ensm.2022.10.003. |
| 64 | WANG T R, DUAN J, ZHANG B, et al. A self-regulated gradient interphase for dendrite-free solid-state Li batteries[J]. Energy & Environmental Science, 2022, 15(3): 1325-1333. DOI: 10.1039/D1EE03604A. |
| 65 | SANTHOSHA A L, MEDENBACH L, BUCHHEIM J R, et al. The indium-lithium electrode in solid-state lithium-ion batteries: Phase formation, redox potentials, and interface stability[J]. Batteries & Supercaps, 2019, 2(6): 497. DOI: 10.1002/batt.201900073. |
| 66 | YE Y D, XIE H Y, YANG Y H, et al. Solid-solution or intermetallic compounds: Phase dependence of the Li-alloying reactions for Li-metal batteries[J]. Journal of the American Chemical Society, 2023. DOI: 10.1021/jacs.3c08711. |
| 67 | KRAUSKOPF T, MOGWITZ B, ROSENBACH C, et al. Diffusion limitation of lithium metal and Li-Mg alloy anodes on LLZO type solid electrolytes as a function of temperature and pressure[J]. Advanced Energy Materials, 2019, 9(44): 1902568. DOI: 10.1002/aenm.201902568. |
| 68 | QU J L, XIAO J W, WANG T S, et al. High rate transfer mechanism of lithium ions in lithium-tin and lithium-indium alloys for lithium batteries[J]. The Journal of Physical Chemistry C, 2020, 124(45): 24644-24652. DOI: 10.1021/acs.jpcc.0c07880. |
| 69 | LU P S, XIA Y, SUN G C, et al. Realizing long-cycling all-solid-state L i - I n TiS2 batteries using Li6+ xMxAs1- xS5I (M=Si, Sn) sulfide solid electrolytes[J]. Nature Communications, 2023, 14: 4077. DOI: 10.1038/s41467-023-39686-w. |
| 70 | ZHANG X L, ZHANG H Y, GENG Y, et al. A multifunctional nano filler for solid polymer electrolyte toward stable cycling for lithium-metal anodes in lithium-sulfur batteries[J]. Chemical Engineering Journal, 2022, 444: 136328. DOI: 10.1016/j.cej.2022.136328. |
| 71 | GE M Z, CAO C Y, BIESOLD G M, et al. Silicon anodes: Recent advances in silicon-based electrodes: From fundamental research toward practical applications[J]. Advanced Materials, 2021, 33(16): 2004577. DOI: 10.1002/adma.2004577. |
| 72 | TAN D H S, CHEN Y T, YANG H D, et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes[J]. Science, 2021, 373(6562): 1494-1499. DOI: 10.1126/science.abg7217. |
| 73 | LYU Z W, LIU J, LI C, et al. High-areal-capacity all-solid-state Li-S battery enabled by dry process technology[J]. eTransportation, 2024, 19: 100298. DOI: 10.1016/j.etran.2023.100298. |
| 74 | KIM M, KIM M J, OH Y S, et al. Design strategies of Li-Si alloy anode for mitigating chemo-mechanical degradation in sulfide-based all-solid-state batteries[J]. Advanced Science, 2023, 10(24): 2301381. DOI: 10.1002/advs.202301381. |
| 75 | ZHU J H, LUO J Y, LI J Y, et al. A porous Li-Al alloy anode toward high-performance sulfide-based all-solid-state lithium batteries[J]. Advanced Materials, 2024, 36(39): 2407128. DOI: 10.1002/adma.202407128. |
| 76 | SUZUKI N, YASHIRO N, FUJIKI S, et al. Highly cyclable all-solid-state battery with deposition-type lithium metal anode based on thin carbon black layer[J]. Advanced Energy and Sustainability Research, 2021, 2(11): 2100066. DOI: 10.1002/aesr.202100066. |
| 77 | WANG Z X, LU Y, ZHAO C Z, et al. Suppressing Li voids in all-solid-state lithium metal batteries through Li diffusion regulation[J]. Joule, 2024, 8(10): 2794-2810. DOI: 10.1016/j.joule. 2024.07.007. |
| 78 | SU Y B, YE L H, FITZHUGH W, et al. A more stable lithium anode by mechanical constriction for solid state batteries[J]. Energy & Environmental Science, 2020, 13(3): 908-916. DOI: 10.1039/C9EE04007B. |
| 79 | PAN L D, ZHAO W B, ZHAI L Q, et al. Hierarchical carbon interlayer design as interfacial stabilizer and in-situ solid-electrolyte infiltrate for high-performance solid-state Li-S batteries[J]. Chem & Bio Engineering, 2024, 1(4): 340-348. DOI: 10.1021/cbe.4c00040. |
| 80 | PANG Y P, PAN J Y, YANG J H, et al. Electrolyte/electrode interfaces in all-solid-state lithium batteries: A review[J]. Electrochemical Energy Reviews, 2021, 4(2): 169-193. DOI: 10.1007/s41918-020-00092-1. |
| 81 | TAN D H S, BANERJEE A, CHEN Z, et al. From nanoscale interface characterization to sustainable energy storage using all-solid-state batteries[J]. Nature Nanotechnology, 2020, 15(3): 170-180. DOI: 10.1038/s41565-020-0657-x. |
| 82 | HU X, YU J H, WANG Y, et al. A lithium intrusion-blocking interfacial shield for wide-pressure-range solid-state lithium metal batteries[J]. Advanced Materials, 2024, 36(7): 2308275. DOI: 10.1002/adma.202308275. |
| 83 | HUO H Y, CHEN Y, LI R Y, et al. Design of a mixed conductive garnet/Li interface for dendrite-free solid lithium metal batteries[J]. Energy & Environmental Science, 2020, 13(1): 127-134. DOI: 10.1039/C9EE01903K. |
| 84 | FAN Z J, DING B, ZHANG T F, et al. Solid/solid interfacial architecturing of solid polymer electrolyte-based all-solid-state lithium-sulfur batteries by atomic layer deposition[J]. Small, 2019, 15(46): 1903952. DOI: 10.1002/smll.201903952. |
| 85 | KIM K J, BALAISH M, WADAGUCHI M, et al. Solid-state Li-metal batteries: Challenges and horizons of oxide and sulfide solid electrolytes and their interfaces[J]. Advanced Energy Materials, 2021, 11(1): 2002689. DOI: 10.1002/aenm.202002689. |
| 86 | ABDELHAMID A A, CHEONG J L, YING J Y. Li7La3Zr2O12 sheet-based framework for high-performance lithium-sulfur hybrid quasi-solid battery[J]. Nano Energy, 2020, 71: 104633. DOI: 10.1016/j.nanoen.2020.104633. |
| 87 | WAN H L, WANG Z Y, ZHANG W R, et al. Interface design for all-solid-state lithium batteries[J]. Nature, 2023, 623(7988): 739-744. DOI: 10.1038/s41586-023-06653-w. |
| 88 | PEI F, WU L, ZHANG Y, et al. Interfacial self-healing polymer electrolytes for long-cycle solid-state lithium-sulfur batteries[J]. Nature Communications, 2024, 15(1): 351. DOI: 10.1038/s41467-023-43467-w. |
| 89 | YIN X S, WANG L, KIM Y, et al. Thermal conductive 2D boron nitride for high-performance all-solid-state lithium-sulfur batteries[J]. Advanced Science, 2020, 7(19): 2001303. DOI: 10.1002/advs.202001303. |
| 90 | JUDEZ X, ESHETU G G, GRACIA I, et al. Understanding the role of nano-aluminum oxide in all-solid-state lithium-sulfur batteries[J]. ChemElectroChem, 2019, 6(2): 326-330. DOI: 10.1002/celc.201801390. |
| 91 | NAGAO M, HAYASHI A, TATSUMISAGO M. High-capacity Li2S-nanocarbon composite electrode for all-solid-state rechargeable lithium batteries[J]. Journal of Materials Chemistry, 2012, 22(19): 10015-10020. DOI: 10.1039/C2JM16802B. |
| 92 | YANG Z Z, ZHU Z Y, MA J, et al. Phase separation of Li2S/S at nanoscale during electrochemical lithiation of the solid-state lithium-sulfur battery using in situ TEM[J]. Advanced Energy Materials, 2016, 6(20): 1600806. DOI: 10.1002/aenm.201600806. |
| 93 | CAO D X, SUN X, LI F, et al. Understanding electrochemical reaction mechanisms of sulfur in all-solid-state batteries through operando and theoretical studies[J]. Angewandte Chemie International Edition, 2023, 62(20): e202302363. DOI: 10.1002/anie.202302363. |
| 94 | GU J B, HU W X, WU Y Q, et al. Asymmetric sulfur redox paths in sulfide-based all-solid-state lithium-sulfur batteries[J]. Chemistry of Materials, 2024, 36(9): 4403-4416. DOI: 10.1021/acs.chemmater.3c03317. |
| 95 | YU P W, SUN S R, SUN C H, et al. Active regulation volume change of micrometer-size Li2S cathode with high materials utilization for all-solid-state Li/S batteries through an interfacial redox mediator[J]. Advanced Functional Materials, 2024, 34(8): 2306939. DOI: 10.1002/adfm.202306939. |
| 96 | KIM J T, RAO A, NIE H Y, et al. Manipulating Li2S2/Li2S mixed discharge products of all-solid-state lithium sulfur batteries for improved cycle life[J]. Nature Communications, 2023, 14: 6404. DOI: 10.1038/s41467-023-42109-5. |
| 97 | LI J, XIE F X, PANG W W, et al. Regulate transportation of ions and polysulfides in all-solid-state Li-S batteries using ordered-MOF composite solid electrolyte[J]. Science Advances, 2024, 10(11): eadl3925. DOI: 10.1126/sciadv.adl3925. |
| 98 | MENG X Y, LIU Y Z, MA Y F, et al. Diagnosing and correcting the failure of the solid-state polymer electrolyte for enhancing solid-state lithium-sulfur batteries[J]. Advanced Materials, 2023, 35(22): 2212039. DOI: 10.1002/adma.202212039. |
| 99 | WANG M K, SU H, ZHONG Y, et al. Localized S-Li2S conversion with accelerated kinetics mediated by mixed conductive shell for high-performance solid-state lithium-sulfur battery[J]. Advanced Energy Materials, 2024, 14(9): 2302255. DOI: 10.1002/aenm. 202302255. |
| 100 | LIU M, WANG C, ZHAO C L, et al. Quantification of the Li-ion diffusion over an interface coating in all-solid-state batteries via NMR measurements[J]. Nature Communications, 2021, 12(1): 5943. DOI: 10.1038/s41467-021-26190-2. |
| 101 | BRADBURY R, DEWALD G F, KRAFT M A, et al. Visualizing reaction fronts and transport limitations in solid-state Li-S batteries via operando neutron imaging[J]. Advanced Energy Materials, 2023, 13(17): 2203426. DOI: 10.1002/aenm. 202203426. |
| 102 | BRADBURY R, KARDJILOV N, DEWALD G F, et al. Visualizing lithium ion transport in solid-state Li-S batteries using 6Li contrast enhanced neutron imaging[J]. Advanced Functional Materials, 2023, 33(38): 2302619. DOI: 10.1002/adfm.202302619. |
| 103 | NING Z Y, JOLLY D S, LI G C, et al. Visualizing plating-induced cracking in lithium-anode solid-electrolyte cells[J]. Nature Materials, 2021, 20(8): 1121-1129. DOI: 10.1038/s41563-021-00967-8. |
| 104 | SUN M H, LIU T F, YUAN Y F, et al. Visualizing lithium dendrite formation within solid-state electrolytes[J]. ACS Energy Letters, 2021, 6(2): 451-458. DOI: 10.1021/acsenergylett.0c02314. |
| 105 | LUO S T, LIU X Y, ZHANG X, et al. Nanostructure of the interphase layer between a single Li dendrite and sulfide electrolyte in all-solid-state Li batteries[J]. ACS Energy Letters, 2022, 7(9): 3064-3071. DOI: 10.1021/acsenergylett.2c01543. |
| 106 | LIANG Z T, XIANG Y X, WANG K J, et al. Understanding the failure process of sulfide-based all-solid-state lithium batteries via operando nuclear magnetic resonance spectroscopy[J]. Nature Communications, 2023, 14(1): 259. DOI: 10.1038/s41467-023-35920-7. |
| 107 | HAN F D, WESTOVER A S, YUE J, et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes[J]. Nature Energy, 2019, 4(3): 187-196. DOI: 10.1038/s41560-018-0312-z. |
| [1] | Jinhui GAO, Yinglong CHEN, Fanhui MENG, Meichao DING, Li WANG, Gang XU, Xiangming HE. Research on in-situ optical microscopic observation in lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(1): 53-59. |
| [2] | YE Ge, YUAN Hong, ZHAO Chenzi, ZHU Gaolong, XU Lei, HOU Lipeng, CHENG Xinbing, HE Chuanxin, NAN Haoxiong, LIU Quanbin, HUANG Jiaqi, ZHANG Qiang. Balance between ion migration and electron transport in composite cathodes for all-solid-state lithium-sulfur batteries [J]. Energy Storage Science and Technology, 2020, 9(2): 339-345. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
