Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (4): 1110-1120.doi: 10.19799/j.cnki.2095-4239.2021.0505
• Special issue of International Outstanding Young Scientists for Energy Storage • Previous Articles Next Articles
Ying SUN1( ), Qin ZHAO1, Bosi YIN1(
), Qin ZHAO1, Bosi YIN1( ), Tianyi MA2(
), Tianyi MA2( )
)
Received:2021-09-27
Revised:2021-11-01
Online:2022-04-05
Published:2022-04-11
Contact:
Bosi YIN,Tianyi MA
E-mail:853950893@qq.com;yinbosi@lnu.edu.cn;tianyima@swin.edu.au
CLC Number:
Ying SUN, Qin ZHAO, Bosi YIN, Tianyi MA. Performance of PTCDI//δ-MnO2 aqueous ammonium-ion battery[J]. Energy Storage Science and Technology, 2022, 11(4): 1110-1120.

Fig. 3
Kinetic analysis and electrochemical performance of δ-MnO2 electrode : (a) The CV curves at different scan rates; (b) The corresponding plots of log (peak current) vs. log (scan rate) at each peak; (c) The capacitive contribution at different scan rates; (d) The galvanostatic discharge curves at different current densities; (e) The rate capability; (f) The galvanostatic charge/discharge curves at 0.5 A/g of δ-MnO2 and commercial MnO2 electrode"
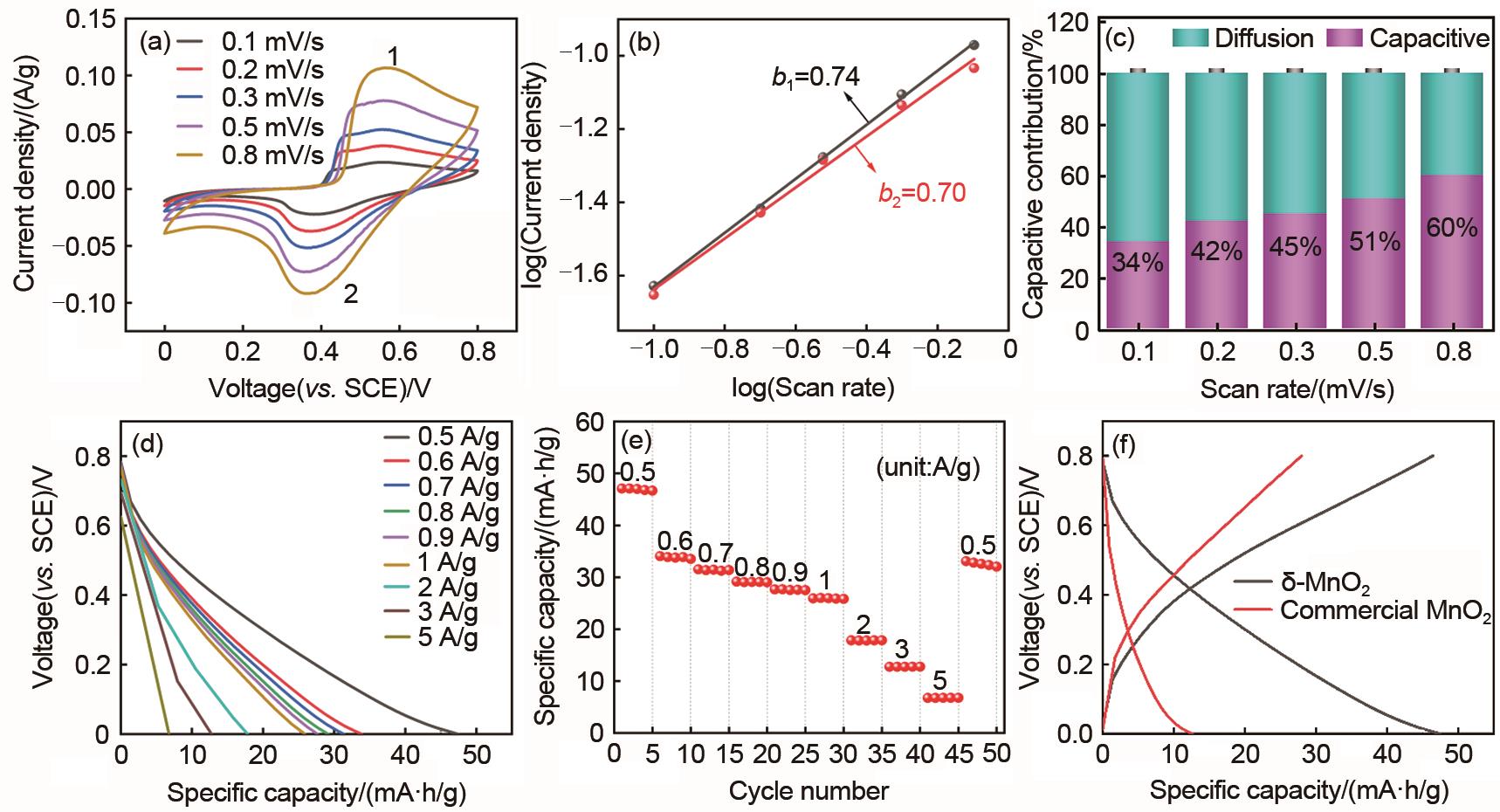

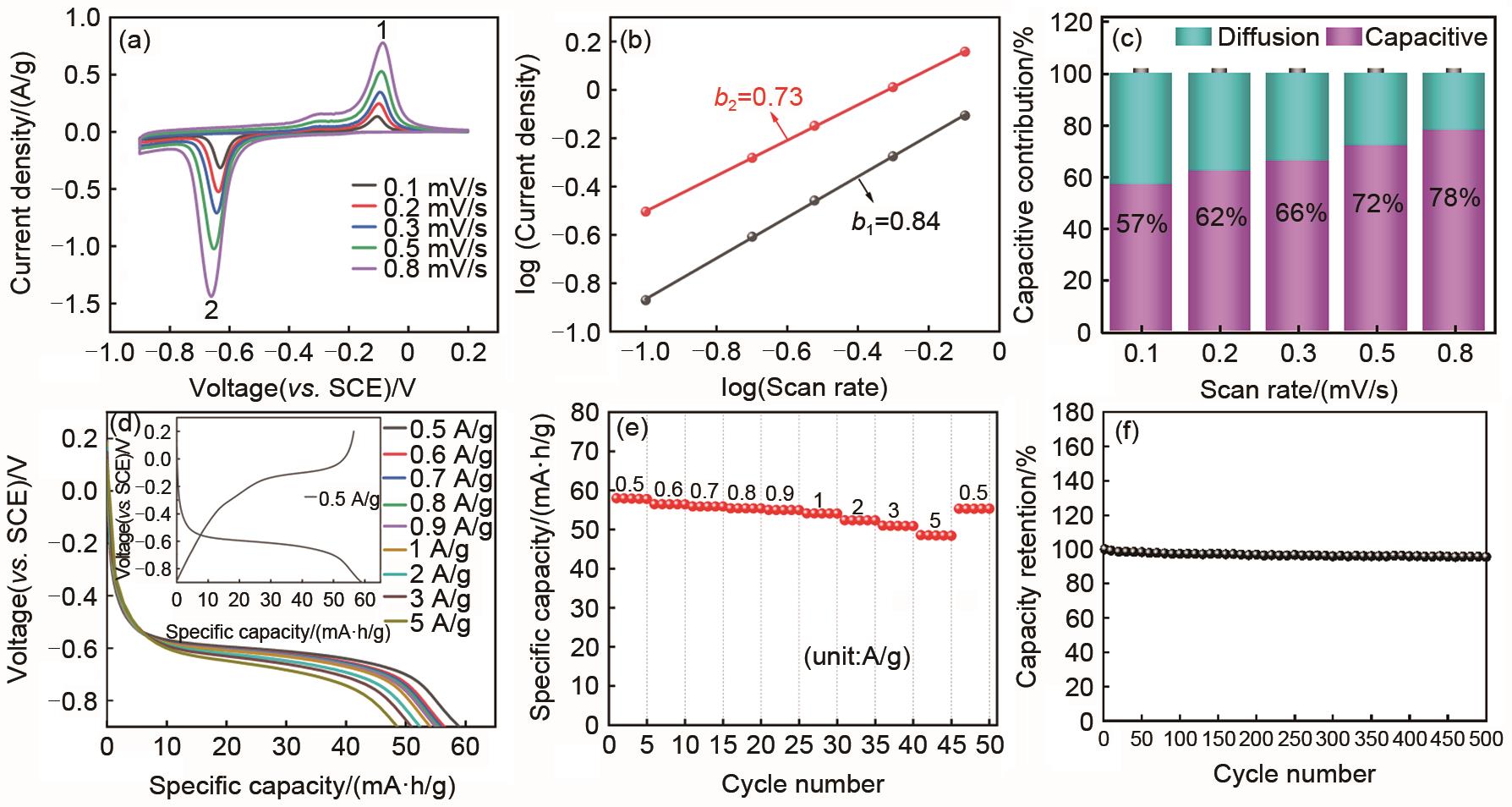
Fig. 7
Kinetic analysis and electrochemical performance of PTCDI electrode: (a) The CV curves at different scan rates; (b) The corresponding plots of log (peak current) vs. log (scan rate) at each peak; (c) The capacitive contribution at different scan rates; (d) The galvanostatic discharge curves at different current densities [inset in (d): The galvanostatic charge/discharge curves at 0.5 A/g]; (e) The rate capability; (f) Cycling stability at the current density of 0.5 A/g"
| 1 | GÜR T M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage[J]. Energy & Environmental Science, 2018, 11(10): 2696-2767. |
| 2 | LIU Y, SHI Y H, XU X H. Evolution and application of all-in-one electrochemical energy storage system[J]. Energy Storage Materials, 2021, 41: 677-696. |
| 3 | ZHOU G, XU L, HU G, et al. Nanowires for electrochemical energy storage[J]. Chemical Reviews, 2019, 119(20): 11042-11109. |
| 4 | LIU Z X, LI H F, ZHU M S, et al. Towards wearable electronic devices: A quasi-solid-state aqueous lithium-ion battery with outstanding stability, flexibility, safety and breathability[J]. Nano Energy, 2018, 44: 164-173. |
| 5 | ZHOU X, ZHAO A, CHEN Z X, et al. Research progress of tunnel-structural Na0.44MnO2 cathode for sodium-ion batteries: A mini review[J]. Electrochemistry Communications, 2021, 122: doi:10. 1016/j.elecom.2020.106897. |
| 6 | SCHMIDT A, RAMOS M K, FERREIRA C M, et al. Molybdenum-based materials/carbon nanotubes nanocomposites prepared as thin and transparent films for aqueous K-ion batteries[J]. Electrochimica Acta, 2021, 387: doi: 10.1016/j.electacta.2021.138500. |
| 7 | SHI Z Y, WU J H, NI M Z, et al. Superior performance of calcium birnessite by electrochemical conversion as cathode for aqueous calcium ion battery[J]. Materials Research Bulletin, 2021, 144: doi: 10.1016/j.materresbull.2021.111475. |
| 8 | ZHANG D L, CHEN Q, ZHANG J H, et al. MgMn2O4/multiwalled carbon nanotubes composite fabricated by electrochemical conversion as a high-performance cathode material for aqueous rechargeable magnesium ion battery[J]. Journal of Alloys and Compounds, 2021, 873: doi: 10.1016/j.jallcom.2021.159872. |
| 9 | 衡永丽, 谷振一, 郭晋芝, 等. Na3V2(PO4)3@C用作水系锌离子电池正极材料的研究[J]. 储能科学与技术, 2021, 10(3): 938-944. |
| HENG Y L, GU Z Y, GUO J Z, et al. Na3V2(PO4)3@C cathode material for aqueous zinc-ion batteries[J]. Energy Storage Science and Technology, 2021, 10(3): 938-944. | |
| 10 | WANG D H, LV H, HUSSAIN T, et al. A manganese hexacyanoferrate framework with enlarged ion tunnels and two-species redox reaction for aqueous Al-ion batteries[J]. Nano Energy, 2021, 84: doi:10. 1016/j.nanoen.2021.105945. |
| 11 | WESSELLS C D, PEDDADA S V, MCDOWELL M T, et al. The effect of insertion species on nanostructured open framework hexacyanoferrate battery electrodes[J]. Journal of the Electrochemical Society, 2011, 159(2): A98-A103. |
| 12 | LI C Y, YAN W Q, LIANG S S, et al. Achieving a high-performance Prussian blue analogue cathode with an ultra-stable redox reaction for ammonium ion storage[J]. Nanoscale Horizons, 2019, 4(4): 991-998. |
| 13 | LI C Y, WU J H, MA F X, et al. High-rate and high-voltage aqueous rechargeable zinc ammonium hybrid battery from selective cation intercalation cathode[J]. ACS Applied Energy Materials, 2019, 2(10): 6984-6989. |
| 14 | WU X Y, XU Y K, JIANG H, et al. NH4 + topotactic insertion in berlin green: An exceptionally long-cycling cathode in aqueous ammonium-ion batteries[J]. ACS Applied Energy Materials, 2018, 1(7): 3077-3083. |
| 15 | LUKATSKAYA M R, MASHTALIR O, REN C E, et al. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide[J]. Science, 2013, 341(6153): 1502-1505. |
| 16 | QIU S, XU Y K, LI X, et al. Reinforced potassium and ammonium storage of the polyimide anode in acetate-based water-in-salt electrolytes[J]. Electrochemistry Communications, 2021, 122: doi:10.1016/j.elecom.2020.106880. |
| 17 | ZHOU G Y, AN X Y, ZHOU C Y, et al. Highly porous electroactive polyimide-based nanofibrous composite anode for all-organic aqueous ammonium dual-ion batteries[J]. Composites Communications, 2020, 22: doi: 10.1016/j.coco.2020.100519. |
| 18 | KUCHENA S F, WANG Y. Superior polyaniline cathode material with enhanced capacity for ammonium ion storage[J]. ACS Applied Energy Materials, 2020, 3(12): 11690-11698. |
| 19 | HOLOUBEK J J, JIANG H, LEONARD D, et al. Amorphous titanic acid electrode: Its electrochemical storage of ammonium in a new water-in-salt electrolyte[J]. Chemical Communications (Cambridge, England), 2018, 54(70): 9805-9808. |
| 20 | DONG S Y, SHIN W, JIANG H, et al. Ultra-fast NH4 + storage: Strong H bonding between NH4 + and Bi-layered V2O5[J]. Chem, 2019, 5(6): 1537-1551. |
| 21 | LI H, YANG J, CHENG J L, et al. Flexible aqueous ammonium-ion full cell with high rate capability and long cycle life[J]. Nano Energy, 2020, 68: doi: 10.1016/j.nanoen.2019.104369. |
| 22 | KUMAR V, KUMAR V, WANG X, et al. Formation of hexagonal-molybdenum trioxide (h-MoO3) nanostructures and their pseudocapacitive behavior[J]. Nanoscale, 2015, 7(27): 11777-11786. |
| 23 | CHAO D L, FAN H J. Intercalation pseudocapacitive behavior Powers aqueous batteries[J]. Chem, 2019, 5(6): 1359-1361. |
| 24 | KOMABA S, KUMAGAI N, CHIBA S. Synthesis of layered MnO2 by calcination of KMnO4 for rechargeable lithium battery cathode[J]. Electrochimica Acta, 2000, 46(1): 31-37. |
| 25 | WEI C G, XU C J, LI B H, et al. Preparation and characterization of manganese dioxides with nano-sized tunnel structures for zinc ion storage[J]. Journal of Physics and Chemistry of Solids, 2012, 73(12): 1487-1491. |
| 26 | XU J, LI J Q, YANG Q L, et al. In-situ synthesis of MnO2@graphdiyne oxides nanocomposite with enhanced performance of supercapacitors[J]. Electrochimica Acta, 2017, 251: 672-680. |
| 27 | MA Z C, WEI X Y, XING S T, et al. Hydrothermal synthesis and characterization of surface-modified δ-MnO2 with high fenton-like catalytic activity[J]. Catalysis Communications, 2015, 67: 68-71. |
| 28 | KAABI N, CHOUCHENE B, MABROUK W, et al. Electrochemical properties of a modified electrode with δ-MnO2-based new nanocomposites[J]. Solid State Ionics, 2018, 325: 74-79. |
| 29 | SHAH H U, WANG F P, JAVED M S, et al. In-situ growth of MnO2 nanorods forest on carbon textile as efficient electrode material for supercapacitors[J]. Journal of Energy Storage, 2018, 17: 318-326. |
| 30 | ZHAO Q, HUANG X J, ZHOU M M, et al. Proton insertion promoted a polyfurfural/MnO2 nanocomposite cathode for a rechargeable aqueous Zn-MnO2 battery[J]. ACS Applied Materials & Interfaces, 2020, 12(32): 36072-36081. |
| 31 | WANG D H, WANG L F, LIANG G J, et al. A superior δ-MnO2 cathode and a self-healing Zn-δ-MnO2 battery[J]. ACS Nano, 2019, 13(9): 10643-10652. |
| 32 | CHEN W, LI G D, PEI A, et al. A manganese-hydrogen battery with potential for grid-scale energy storage[J]. Nature Energy, 2018, 3(5): 428-435. |
| 33 | TANG Q W, JIANG L H, LIU J, et al. Effect of surface manganese valence of manganese oxides on the activity of the oxygen reduction reaction in alkaline media[J]. ACS Catalysis, 2014, 4(2): 457-463. |
| 34 | JUNTA J L, HOCHELLA M F Jr. Manganese (II) oxidation at mineral surfaces: A microscopic and spectroscopic study[J]. Geochimica et Cosmochimica Acta, 1994, 58(22): 4985-4999. |
| 35 | CHAO D, ZHU C, YANG P, et al. Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance[J]. Nature Communications, 2016, 7: doi: 10. 1038/ncomms12122. |
| 36 | WAN F, ZHANG L, DAI X, et al. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers[J]. Nat Commun, 2018, 9(1): 1656. |
| 37 | CHAO D, LIANG P, CHEN Z, et al. Pseudocapacitive Na-ion storage boosts high rate and areal capacity of self-branched 2D layered metal chalcogenide nanoarrays[J]. ACS Nano, 2016, 10(11): 10211-10219. |
| 38 | SONG Y, PAN Q, LV H, et al. Ammonium-ion storage using electrodeposited manganese oxides[J]. Angewandte Chemie (International Ed in English), 2021, 60(11): 5718-5722. |
| 39 | DELGADO M C, KIM E G, DA SILVA FILHO D A, et al. Tuning the charge-transport parameters of perylene diimide single crystals via end and/or core functionalization: A density functional theory investigation[J]. Journal of the American Chemical Society, 2010, 132(10): 3375-3387. |
| 40 | HAN X, CHANG C, YUAN L, et al. Aromatic carbonyl derivative polymers as high-performance Li-ion storage materials[J]. Advanced Materials, 2007, 19(12): 1616-1621. |
| 41 | DENG W, SHEN Y, QIAN J, et al. A perylene diimide crystal with high capacity and stable cyclability for Na-ion batteries[J]. ACS Applied Materials & Interfaces, 2015, 7(38): 21095-21099. |
| 42 | LESEL B K, KO J S, DUNN B, et al. Mesoporous LixMn2O4 thin film cathodes for lithium-ion pseudocapacitors[J]. ACS Nano, 2016, 10(8): 7572-7581. |
| [1] | LUO Lirong, CAI Ying, HU Feng. Electrochemical and kinetic properties of as-cast and quenched CeMg11Ni hydrogen storage alloys [J]. Energy Storage Science and Technology, 2019, 8(5): 904-910. |
| [2] | LI Yu, ZHAO Huichun, BAI Ying, WU Feng, WU Chuan. Progress in the modification of lithium-rich manganese-based layered cathode material [J]. Energy Storage Science and Technology, 2018, 7(3): 394-403. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
