Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (6): 1693-1705.doi: 10.19799/j.cnki.2095-4239.2022.0098
Previous Articles Next Articles
ZHANG Yan1( ), WANG Hai1,2, LIU Zhaomeng1, ZHANG Deliu1, WANG Jiadong2, LI Jianzhong1, GAO Xuanwen1(
), WANG Hai1,2, LIU Zhaomeng1, ZHANG Deliu1, WANG Jiadong2, LI Jianzhong1, GAO Xuanwen1( ), LUO Wenbin1
), LUO Wenbin1
Received:2022-02-24
Revised:2022-03-19
Online:2022-06-05
Published:2022-06-13
Contact:
GAO Xuanwen
E-mail:1971478@stu.neu.edu.cn;gaoxuanwen@mail.neu.edu.cn
CLC Number:
ZHANG Yan, WANG Hai, LIU Zhaomeng, ZHANG Deliu, WANG Jiadong, LI Jianzhong, GAO Xuanwen, LUO Wenbin. Research progress of nickel-rich ternary cathode material ncm for lithium-ion batteries[J]. Energy Storage Science and Technology, 2022, 11(6): 1693-1705.

Fig. 4
(a) Schematic illustration of the preparation of NCM811@PANI-PVP;(b) HRTEM images of NCM811@PANI-PVP[38]; (c) Schematic illustration of the preparation procedure[39]; (d) Coating-plus-infusion’ microstructure for Co x B-infused NCM; (e) EELS line scan at a secondary-particle surface in cycled pristine NCM and Co x B-NCM[40]"
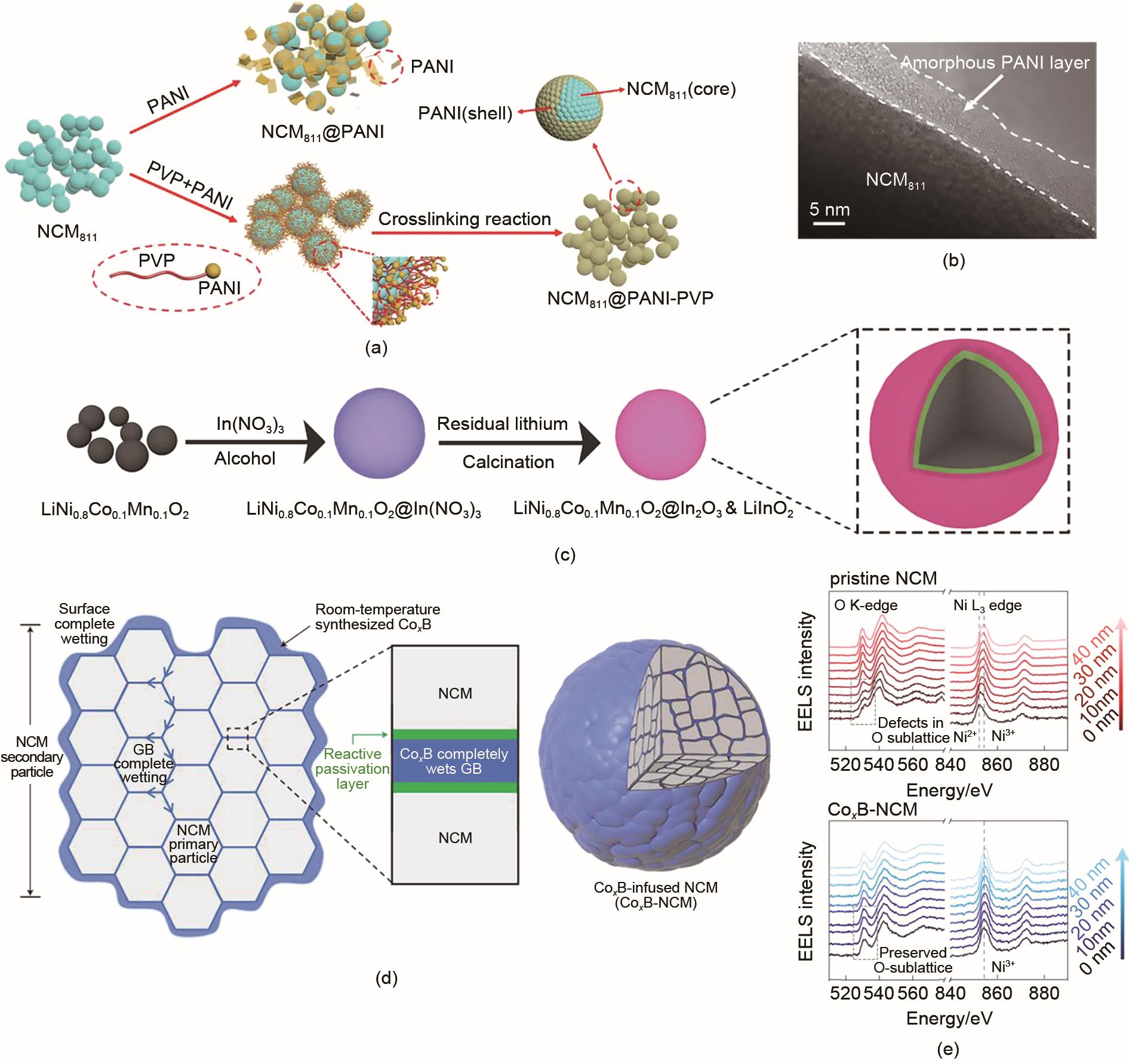

Fig. 6
(a) Summary of the three main approaches to single-crystal synthesis[50]; (b) TEM image of the S-NCM90 cathode particle charged to 4.3 V at 0.5 C and the c-axis lattice parameters at locations along the dashed yellow line[51]; (c) Schematic illustration of crack evolution and the internal morphological difference for N-NCM and SC-NCM cathodes during prolong cycling; (d) Cycling performances of N-NCM and SC-NCM electrodes in pouch-type full-cells [52]"

| 1 | 王嗣慧, 徐中领, 杜锐, 等. 高镍三元锂离子电池高温存储性能衰退机理[J]. 储能科学与技术, 2017, 6(4): 770-775. |
| WANG S H, XU Z L, DU R, et al. Degradation study of Ni-rich NCM batteries operated at high tempertures[J]. Energy Storage Science and Technology, 2017, 6(4): 770-775. | |
| 2 | MIZUSHIMA K, JONES P C, WISEMAN P J, et al. LixCoO2 (x≤1): A new cathode material for batteries of high energy density[J]. Solid State Ionics, 1981, 3/4: 171-174. |
| 3 | PADHI A K, NANJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries[J]. Journal of the Electrochemical Society, 1997, 144(4): 1188-1194. |
| 4 | JUNG S K, GWON H, HONG J, et al. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries[J]. Advanced Energy Materials, 2014, 4(1): doi:10.1002/aenm.201300787. |
| 5 | DING Y, WANG R, WANG L, et al. A short review on layered LiNi0.8Co0.1Mn0.1O2 positive electrode material for lithium-ion batteries[J]. Energy Procedia, 2017, 105: 2941-2952. |
| 6 | OHZUKU T, MAKIMURA Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries[J]. Chemistry Letters, 2001(7): 642-643. |
| 7 | ELLIS B L, LEE K T, NAZAR L F. Positive electrode materials for Li-ion and Li-batteries[J]. Chemistry of Materials, 2010, 22(3): 691-714. |
| 8 | CHO Y, OH P, CHO J. A new type of protective surface layer for high-capacity Ni-based cathode materials: Nanoscaled surface pillaring layer[J]. Nano Letters, 2013, 13(3): 1145-1152. |
| 9 | BIE X F, LIU L N, EHRENBERG H, et al. Revisiting the layered LiNi0.4Mn0.4Co0.2O2: A magnetic approach[J]. RSC Advances, 2012, 2(26): 9986. |
| 10 | ZENG D L, CABANA J, BRÉGER J, et al. Cation ordering in Li[NixMnxCo(1–2 x)]O2-layered cathode materials: A nuclear magnetic resonance (NMR), pair distribution function, X-ray absorption spectroscopy, and electrochemical study[J]. Chemistry of Materials, 2007, 19(25): 6277-6289. |
| 11 | KONDRAKOV A O, SCHMIDT A, XU J, et al. Anisotropic lattice strain and mechanical degradation of high-and low-nickel NCM cathode materials for Li-ion batteries[J]. The Journal of Physical Chemistry C, 2017, 121(6): 3286-3294. |
| 12 | ZHENG J M, KAN W H, MANTHIRAM A. Role of Mn content on the electrochemical properties of nickel-rich layered LiNi0.8- xCo0.1Mn0.1+ xO2 (0.0≤x≤0.08) cathodes for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2015, 7(12): 6926-6934. |
| 13 | GONG J Q, WANG Q S, SUN J H. Thermal analysis of nickel cobalt lithium manganese with varying nickel content used for lithium ion batteries[J]. Thermochimica Acta, 2017, 655: 176-180. |
| 14 | ZHANG N, LI J, LI H Y, et al. Structural, electrochemical, and thermal properties of nickel-rich LiNixMnyCozO2 materials[J]. Chemistry of Materials, 2018, 30(24): 8852-8860. |
| 15 | YU H J, QIAN Y M, OTANI M, et al. Study of the lithium/nickel ions exchange in the layered LiNi0.42Mn0.42Co0.16O2 cathode material for lithium ion batteries: Experimental and first-principles calculations[J]. Energy & Environmental Science, 2014, 7(3): 1068. |
| 16 | RYU H H, PARK K J, YOON C S, et al. Capacity fading of Ni-rich Li[NixCoyMn1- x- y]O2 (0.6≤x≤0.95) cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation?[J]. Chemistry of Materials, 2018, 30(3): 1155-1163. |
| 17 | PARK J H, CHOI B, KANG Y S, et al. Effect of residual lithium rearrangement on Ni-rich layered oxide cathodes for lithium-ion batteries[J]. Energy Technology, 2018, 6(7): 1361-1369. |
| 18 | LIU W, OH P, LIU X E, et al. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries[J]. Angewandte Chemie International Edition, 2015, 54(15): 4440-4457. |
| 19 | WU F, TIAN J, SU Y F, et al. Effect of Ni2+ content on lithium/nickel disorder for Ni-rich cathode materials[J]. ACS Applied Materials & Interfaces, 2015, 7(14): 7702-7708. |
| 20 | NAM G W, PARK N Y, PARK K J, et al. Capacity fading of Ni-rich NCA cathodes: Effect of microcracking extent[J]. ACS Energy Letters, 2019, 4(12): 2995-3001. |
| 21 | RYU H H, PARK G T, YOON C S, et al. Microstructural degradation of Ni-rich Li[NixCoyMn1- x- y]O2 cathodes during accelerated calendar aging[J]. Small, 2018, 14(45): doi:10.1002/smll.201803179. |
| 22 | YAN P F, ZHENG J M, GU M, et al. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries[J]. Nature Communications, 2017, 8: 14101. |
| 23 | CHEN H, DAWSON J A, HARDING J H. Effects of cationic substitution on structural defects in layered cathode materials LiNiO2[J]. Journal of Materials Chemistry A, 2014, 2(21): 7988. |
| 24 | DU K, GAO A, GAO L F, et al. Enhancing the structure stability of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode via encapsulating in negative thermal expansion nanocrystalline shell[J]. Nano Energy, 2021, 83: doi:10.1016/j.nanoen.2021.105775. |
| 25 | KIM Y, PARK H, SHIN K, et al. Rational design of coating ions via advantageous surface reconstruction in high-nickel layered oxide cathodes for lithium-ion batteries[J]. Advanced Energy Materials, 2021, 11(38): doi:10.1002/aenm.202101112. |
| 26 | HATSUKADE T, SCHIELE A, HARTMANN P, et al. Origin of carbon dioxide evolved during cycling of nickel-rich layered NCM cathodes[J]. ACS Applied Materials & Interfaces, 2018, 10(45): 38892-38899. |
| 27 | MALEKI KHEIMEH SARI H, LI X F. Controllable cathode-electrolyte interface of Li[Ni0.8Co0.1Mn0.1]O2 for lithium ion batteries: A review[J]. Advanced Energy Materials, 2019, 9(39): doi:10.1002/aenm.201901597. |
| 28 | XU J J, HU Y Y, LIU T, et al. Improvement of cycle stability for high-voltage lithium-ion batteries by in situ growth of SEI film on cathode[J]. Nano Energy, 2014, 5: 67-73. |
| 29 | 马爱军, 曹征领, 陈永炜, 等. 三元层状正极材料失效机理及改性研究进展[J]. 浙江电力, 2021, 40(1): 106-115. |
| MA A J, CAO Z L, CHEN Y W, et al. Degradation mechanisms and modification research progress of Li[Ni1- xMx]O2 layered cathode materials[J]. Zhejiang Electric Power, 2021, 40(1): 106-115. | |
| 30 | 刘浩文, 乐琦, 吴瑞, 等. Ca掺杂的LiNi1/3Co1/3Mn1/3O2正极材料及其电化学性能研究[J]. 中南民族大学学报(自然科学版), 2018, 37(3): 1-4, 27. |
| LIU H W, LE Q, WU R, et al. Ca doping of LiNi1/3Co1/3Mn1/3O2 anode material and its electrochemical performance[J]. Journal of South-Central University for Nationalities (Natural Science Edition), 2018, 37(3): 1-4, 27. | |
| 31 | LI J, ZHANG M L, ZHANG D Y, et al. An effective doping strategy to improve the cyclic stability and rate capability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode[J]. Chemical Engineering Journal, 2020, 402: 126195. |
| 32 | SUSAI F A, KOVACHEVA D, CHAKRABORTY A, et al. Improving performance of LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium-ion batteries by doping with molybdenum-ions: Theoretical and experimental studies[J]. ACS Applied Energy Materials, 2019, 2(6): 4521-4534. |
| 33 | LIU X L, WANG S, WANG L, et al. Stabilizing the high-voltage cycle performance of LiNi0.8Co0.1Mn0.1O2 cathode material by Mg doping[J]. Journal of Power Sources, 2019, 438: doi:10.1016/j.jpowsour.2019.227017. |
| 34 | HE T, LU Y, SU Y F, et al. Sufficient utilization of zirconium ions to improve the structure and surface properties of nickel-rich cathode materials for lithium-ion batteries[J]. ChemSusChem, 2018, 11(10): 1639-1648. |
| 35 | LI J W, LI Y, YI W T, et al. Improved electrochemical performance of cathode material LiNi0.8Co0.1Mn0.1O2 by doping magnesium via co-precipitation method[J]. Journal of Materials Science: Materials in Electronics, 2019, 30(8): 7490-7496. |
| 36 | LI J Y, LI W D, YOU Y, et al. Extending the service life of high-Ni layered oxides by tuning the electrode-electrolyte interphase[J]. Advanced Energy Materials, 2018, 8(29): doi:10.1002/aenm.201801957. |
| 37 | BI Y J, LIU M, XIAO B W, et al. Highly stable Ni-rich layered oxide cathode enabled by a thick protective layer with bio-tissue structure[J]. Energy Storage Materials, 2020, 24: 291-296. |
| 38 | GAN Q M, QIN N, ZHU Y H, et al. Polyvinylpyrrolidone-induced uniform surface-conductive polymer coating endows Ni-rich LiNi0.8Co0.1Mn0.1O2 with enhanced cyclability for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(13): 12594-12604. |
| 39 | LIU Y, TANG L B, WEI H X, et al. Enhancement on structural stability of Ni-rich cathode materials by in situ fabricating dual-modified layer for lithium-ion batteries[J]. Nano Energy, 2019, 65: doi:10.1016/j.nanoen.2019.104043. |
| 40 | YOON M, DONG Y H, HWANG J, et al. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries[J]. Nature Energy, 2021, 6(4): 362-371. |
| 41 | HUANG X, ZHU W C, YAO J Y, et al. Suppressing structural degradation of Ni-rich cathode materials towards improved cycling stability enabled by a Li2MnO3 coating[J]. Journal of Materials Chemistry A, 2020, 8(34): 17429-17441. |
| 42 | NEUDECK S, STRAUSS F, GARCIA G, et al. Room temperature, liquid-phase Al2O3 surface coating approach for Ni-rich layered oxide cathode material[J]. Chemical Communications, 2019, 55(15): 2174-2177. |
| 43 | ZHU W C, HUANG X, LIU T T, et al. Ultrathin Al2O3 coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced cycleability at extended voltage ranges[J]. Coatings, 2019, 9(2): 92. |
| 44 | BAO W D, QIAN G N, ZHAO L Q, et al. Simultaneous enhancement of interfacial stability and kinetics of single-crystal LiNi 0.6 Mn0.2Co0.2O2 through optimized surface coating and doping[J]. Nano Letters, 2020, 20(12): 8832-8840. |
| 45 | XIN F X, ZHOU H, ZONG Y X, et al. What is the role of Nb in nickel-rich layered oxide cathodes for lithium-ion batteries?[J]. ACS Energy Letters, 2021, 6(4): 1377-1382. |
| 46 | FENG Z, RAJAGOPALAN R, ZHANG S, et al. A three in one strategy to achieve zirconium doping, boron doping, and interfacial coating for stable LiNi0.8Co0.1Mn0.1O2 cathode[J]. Advanced Science, 2021, 8(2): doi:10.1002/advs.202001809. |
| 47 | SCHIPPER F, BOUZAGLO H, DIXIT M, et al. From surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium ion batteries[J]. Advanced Energy Materials, 2018, 8(4): doi:10.1002/aenm.201701682. |
| 48 | LIU A, ZHANG N, STARK J E, et al. Synthesis of co-free Ni-rich single crystal positive electrode materials for lithium ion batteries (I): Two-step lithiation method for Al-or Mg-doped LiNiO2[J]. Journal of the Electrochemical Society, 2021, 168(4): 040531. |
| 49 | LIU G L, LI M L, WU N T, et al. Single-crystalline particles: An effective way to ameliorate the intragranular cracking, thermal stability, and capacity fading of the LiNi0.6Co0.2Mn0.2O2 electrodes[J]. Journal of the Electrochemical Society, 2018, 165(13): A3040-A3047. |
| 50 | LANGDON J, MANTHIRAM A. A perspective on single-crystal layered oxide cathodes for lithium-ion batteries[J]. Energy Storage Materials, 2021, 37: 143-160. |
| 51 | RYU H H, NAMKOONG B, KIM J H, et al. Capacity fading mechanisms in Ni-rich single-crystal NCM cathodes[J]. ACS Energy Letters, 2021, 6(8): 2726-2734. |
| 52 | FAN X M, HU G R, ZHANG B, et al. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries[J]. Nano Energy, 2020, 70: doi:10.1016/j.nanoen.2020.104450. |
| 53 | ZHAO Z Y, HUANG B, WANG M, et al. Facile synthesis of fluorine doped single crystal Ni-rich cathode material for lithium-ion batteries[J]. Solid State Ionics, 2019, 342: doi:10.1016/j.ssi.2019.115065. |
| 54 | HUANG B, WANG M, ZUO Y X, et al. The effects of reheating process on the electrochemical properties of single crystal LiNi0.6Mn0.2Co0.2O2[J]. Solid State Ionics, 2020, 345: doi:10.1016/j.ssi.2020.115200. |
| 55 | LI F, KONG L L, SUN Y Y, et al. Micron-sized monocrystalline LiNi1/3Co1/3Mn1/3O2 as high-volumetric-energy-density cathode for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(26): 12344-12352. |
| 56 | LI H Y, LI J, ZAKER N, et al. Synthesis of single crystal LiNi0.88Co0.09Al0.03O2 with a two-step lithiation method[J]. Journal of the Electrochemical Society, 2019, 166(10): A1956-A1963. |
| 57 | LIANG R, WU Z Y, YANG W M, et al. A simple one-step molten salt method for synthesis of micron-sized single primary particle LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries[J]. Ionics, 2020, 26(4): 1635-1643. |
| 58 | QIAN G N, ZHANG Y T, LI L S, et al. Single-crystal nickel-rich layered-oxide battery cathode materials: Synthesis, electrochemistry, and intra-granular fracture[J]. Energy Storage Materials, 2020, 27: 140-149. |
| 59 | TREVISANELLO E, RUESS R, CONFORTO G, et al. Polycrystalline and single crystalline NCM cathode materials— quantifying particle cracking, active surface area, and lithium diffusion[J]. Advanced Energy Materials, 2021, 11(18): doi:10.1002/aenm.2020.03400. |
| 60 | KONG X B, ZHANG Y G, PENG S Y, et al. Superiority of single-crystal to polycrystalline LiNixCoyMn1- x- yO2 cathode materials in storage behaviors for lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(39): 14938-14948. |
| 61 | SUN H L, ZHANG Y F, LI W, et al. Effects of Ag coating on the structural and electrochemical properties of LiNi0.8Co0.1Mn0.1O2 as cathode material for lithium ion batteries[J]. Electrochimica Acta, 2019, 327: doi:10.1016/j.electacta.2019.135054. |
| 62 | MA F, WU Y H, WEI G Y, et al. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode via wet-chemical coating of MgO[J]. Journal of Solid State Electrochemistry, 2019, 23(7): 2213-2224. |
| 63 | LEE S H, PARK G J, SIM S J, et al. Improved electrochemical performances of LiNi0.8Co0.1Mn0.1O2 cathode via SiO2 coating[J]. Journal of Alloys and Compounds, 2019, 791: 193-199. |
| 64 | WU K, LI Q, DANG R B, et al. A novel synthesis strategy to improve cycle stability of LiNi0.8Mn0.1Co0.1O2 at high cut-off voltages through core-shell structuring[J]. Nano Research, 2019, 12(10): 2460-2467. |
| 65 | SU Y F, CHEN G, CHEN L, et al. High-rate structure-gradient Ni-rich cathode material for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(40): 36697-36704. |
| 66 | SUN Y K, MYUNG S T, PARK B C, et al. High-energy cathode material for long-life and safe lithium batteries[J]. Nature Materials, 2009, 8(4): 320-324. |
| 67 | SUN Y K, CHEN Z H, NOH H J, et al. Nanostructured high-energy cathode materials for advanced lithium batteries[J]. Nature Materials, 2012, 11(11): 942-947. |
| 68 | LI W M, TANG W J, QIU M Q, et al. Effects of gradient concentration on the microstructure and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials[J]. Frontiers of Chemical Science and Engineering, 2020, 14(6): 988-996. |
| [1] | Xiaohan FENG, Jie SUN, Jianhao HE, Yihua WEI, Chenggang ZHOU, Ruimin SUN. Research progress in LiFePO4 cathode material modification [J]. Energy Storage Science and Technology, 2022, 11(2): 467-486. |
| [2] | Can WANG, Pan MA, Guoliang ZHU, Yongchao MA, Pengcheng JI, Shuimiao WEI, Jian ZHAO, Zhishui YU. LIB long life graphite electrode: State-of-art development and perspective [J]. Energy Storage Science and Technology, 2021, 10(1): 59-67. |
| [3] | Jixian WANG, Sikan PENG, Wenzheng NAN, Xiang CHEN, Chen WANG, Shaojiu YAN, Shenglong DAI. Preparation of graphene-coated Li1.22Mn0.52Ni0.26O2 using a spray drying method for lithium-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(1): 111-117. |
| [4] | Yueyuan GU, Jucai WEI, Jindong LI, Luyang WANG, Xu WU. Overview and prospect of studies on electrochemical reduction of carbon dioxide electrolyzers [J]. Energy Storage Science and Technology, 2020, 9(6): 1691-1701. |
| [5] | Sijia REN, Leiwu TIAN, Qinjun SHAO, Jian CHEN. Synthesis of single-crystal LiNi0.8Co0.1Mn0.1O2 by flux method [J]. Energy Storage Science and Technology, 2020, 9(6): 1702-1713. |
| [6] | ZHANG Xin, KONG Lingli, GAO Tengyue, LI Haitao, YAO Xiaohui, LI Fuxuan. Analysis and improvement of cycle performance for Ni-rich lithium ion battery [J]. Energy Storage Science and Technology, 2020, 9(3): 813-817. |
| [7] | SUN Xingwei, WANG Longlong, JIANG Feng, MA Jun, ZHOU Xinhong, CUI Guanglei. Failure mechanisms and characterization techniques for solid state polymer lithium batteries [J]. Energy Storage Science and Technology, 2019, 8(6): 1024-1032. |
| [8] | LIANG Dayu, BAO Tingting, GAO Tianhui, ZHANG Jian. Analysis of cycling performance failure of NMC811/SiO-C pouch cells with high specific energy [J]. Energy Storage Science and Technology, 2018, 7(3): 459-464. |
| [9] | WU Minchang1, YU Ningbo1, QIAO Yongmin1, SUN Fangjing2, ZHANG Jie1. The evaluation of fast-charging performance of hard carbon coating artificial graphite for lithium-ion batteries [J]. Energy Storage Science and Technology, 2017, 6(S1): 15-. |
| [10] | WANG Sihui, XU Zhongling, DU Rui, MENG Huanping, LIU Yong, LIU Na, LIANG Chengdu. Degradation study of Ni-rich NCM batteries operated at high tempertures [J]. Energy Storage Science and Technology, 2017, 6(4): 770-775. |
| [11] | XIE Jia, PENG Wen, YANG Xulai. The cycle life investigation for spinel LiNi0.5Mn1.5O4 full cells [J]. Energy Storage Science and Technology, 2014, 3(6): 624-628. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
