Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (4): 1239-1252.doi: 10.19799/j.cnki.2095-4239.2024.0160
Previous Articles Next Articles
Xupeng XU1,2( ), Xuming XU1, Hongyan CHEN1, LIANGYaru1(
), Xuming XU1, Hongyan CHEN1, LIANGYaru1( ), Weixin LEI1(
), Weixin LEI1( ), Zengsheng MA1, Guoxin CHEN2, Peiling KE2
), Zengsheng MA1, Guoxin CHEN2, Peiling KE2
Received:2024-02-28
Revised:2024-03-06
Online:2024-04-26
Published:2024-04-22
Contact:
LIANGYaru, Weixin LEI
E-mail:cailiaopeng@smail.xtu.edu.cn;yaruliang@xtu.edu.cn;wxlei@xtu.edu.cn
CLC Number:
Xupeng XU, Xuming XU, Hongyan CHEN, LIANGYaru, Weixin LEI, Zengsheng MA, Guoxin CHEN, Peiling KE. Applications of in situ characterization techniques in the study of lithium-sulfur battery mechanisms[J]. Energy Storage Science and Technology, 2024, 13(4): 1239-1252.
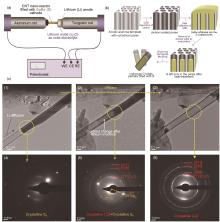
Fig. 4
(a) Schematic illustration of the electrochemical device set up for a real-time TEM observation of an electrochemical lithiation of nanoconfined S cathodes; (b) Diagram of sample preparation process for a single S-CNT reaction vessel; (c)Selected images of the sample evolution during a typical lithiation process: (1)—(3) TEM images captured during lithiation of S nanoconfined in a CNT reaction vessel and (4)—(6) their corresponding EDP patterns; (1) and (4) correspond to the sample before the initiation and (3) and (6) after the completion of the electrochemical reaction. The darker area appearing at the bottom left corner of the CNT in (3) is a contaminant[26]"
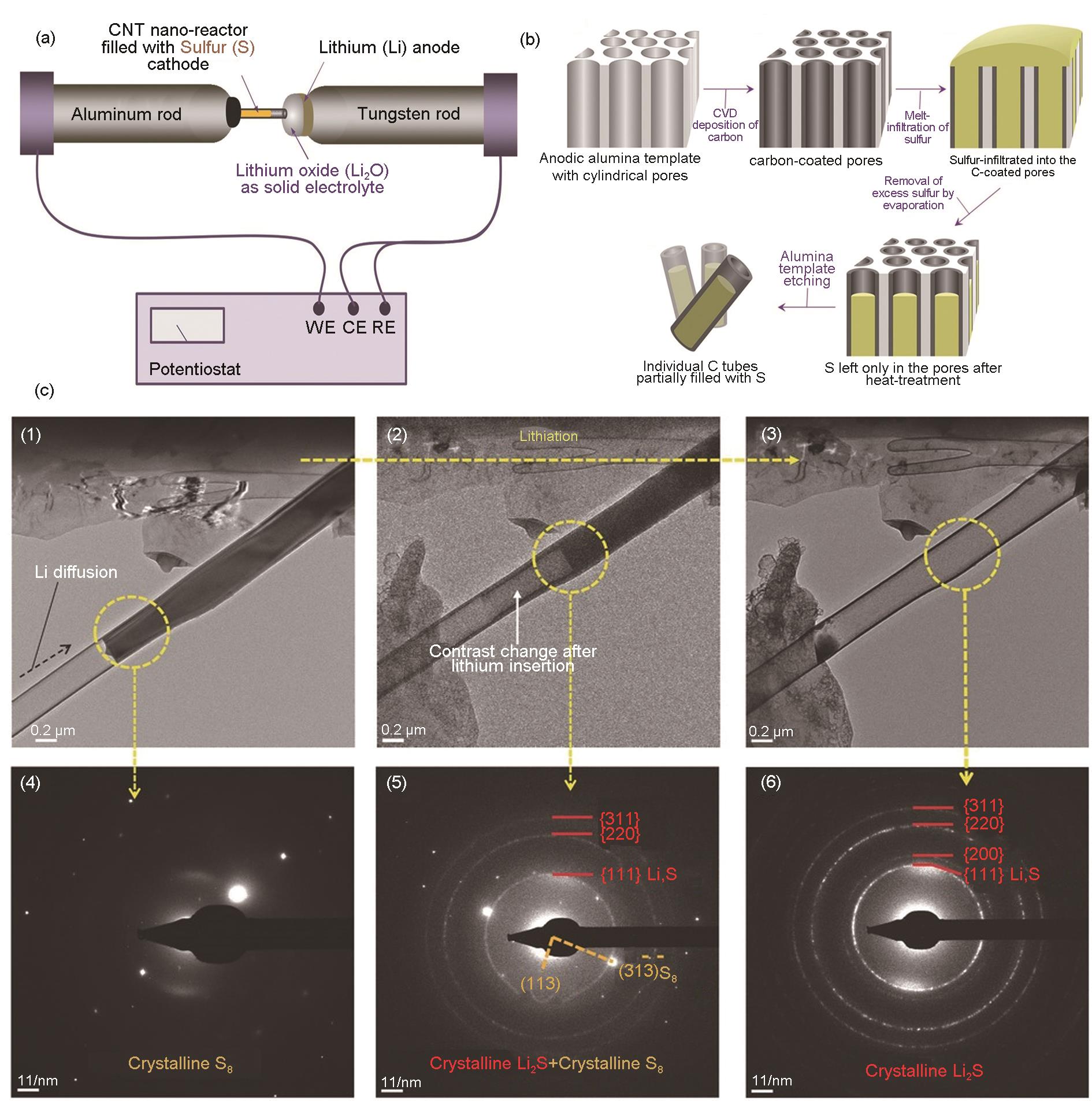
Fig. 5
(a) A scheme showing the setup of the solid cell implemented with a MEMS heating device for in situ TEM observation; (b)Scheme showing the reaction mechanism of the lithiation/delithiation of a S@CNT cathode, (1) Lithiation at 30 ℃, (2) lithiation at 100 ℃, (3) delithiation at 30 ℃, and (4) delithiation at 300 ℃[27]"

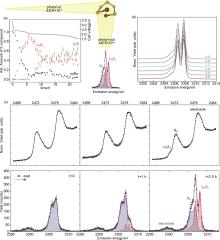
Fig. 6
(a) Relative intensity ratios of S8 and Li2S x compounds with the battery cathode during the discharge process together with the corresponding voltage diagram of the Li-S cell[28]; (b) RXES spectra of Li2S x standards excited at the pre-edge resonance; (c) Operando sulfur RXES spectra at 2470.7 eV excitation energy from a Li-S battery recorded at three points at the beginning of the discharge process. A linear combination fit using measured reference spectra is used to determine the relative amount of sulfur and Li2S x spectral components. The corresponding XANES spectra are added on top for comparison[28]"
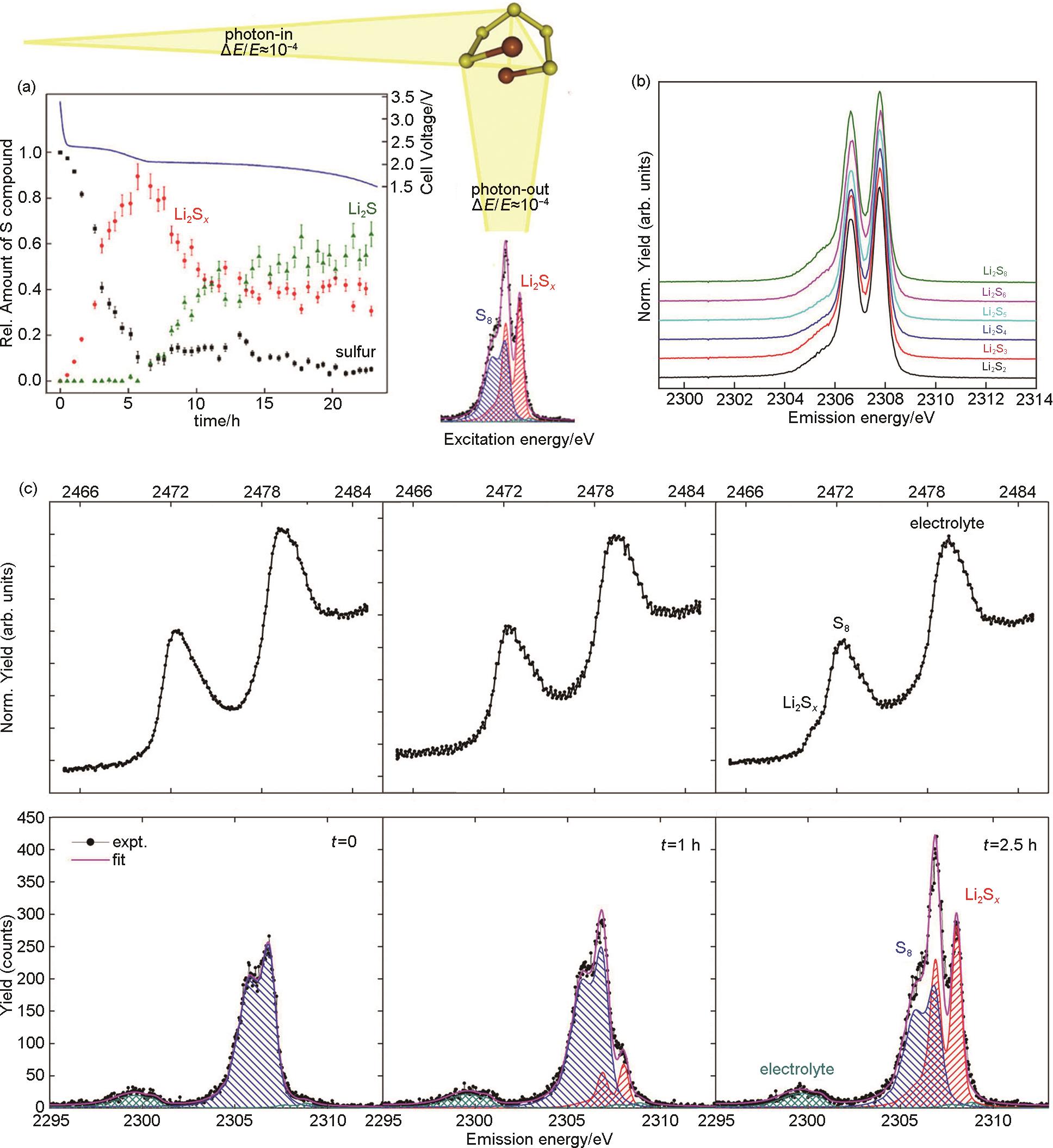
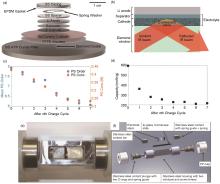
Fig. 7
(a) Schematic representation of the in operando ATR FTIR spectro-electrochemical cell; (b) Illustration of the IR beam reflected through the ATR crystal,absorbed by the electrolyte in a porous cathode; (c) The polysutfide order and concentratien after every charge cycle, and (d) capacity fading over 7 discharge/charge cycles[29]; (e) Battery window for Raman spectroscopy and schematic diagram thereof (f) [30]"
Fig. 8
(a) Schematic diagram and voltage distribution diagram of the battery using TFEE; (b)Molecular structures and electrochemical characterization[31]; (c) Schematic diagram of working mechanism of a typical lithium battery with gel polymer electrolyte; (d)Functional diagram of PVDF/PSPEG GPE in lithium sulfur battery[34]; (e) 7Li and 17O NMR spectra for different electrolytes Proposed solvent-dependent sulfur reduction reaction pathways[35]"
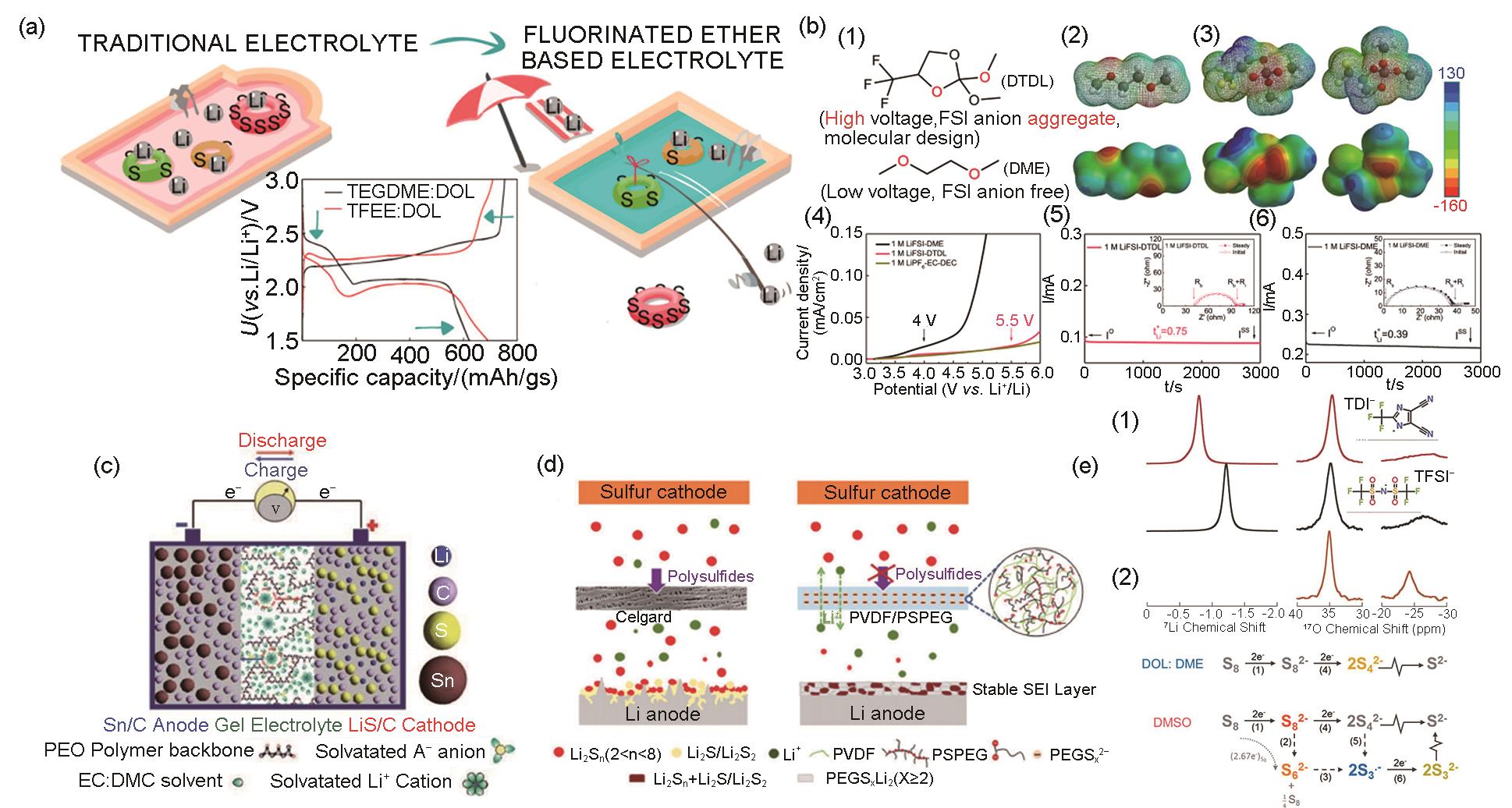

Fig. 9
(a)In situX ray photoelectron spectroscopy (XPS) and chemical imaging(left); ab initio molecular dynamics (AIMD) computational modeling(right); Cartoon representation of SEI layer growth mechanism based on the combined XPS and computational result (bottom); (b) Core level S 2p XPS spectra of the Li-electrolyte interfacial region with subsequent charge/discharge cycles; (c) Evolution of various sulfur based species over charge/discharge cycles based on atomic concentration derived from S 2p peak areas; (d) The ratio between terminal sulfide and bridging sulfur atoms (SB0/ST1–) along with the disulfide and sulfide ratio (S2–/S1–) derived from S 2p peak areas; (e) Molecular structure of lithium polysulfide Li2S6 (top) and TFSI anion (bottom) with chemical labels used in the XPS analysis[40]"
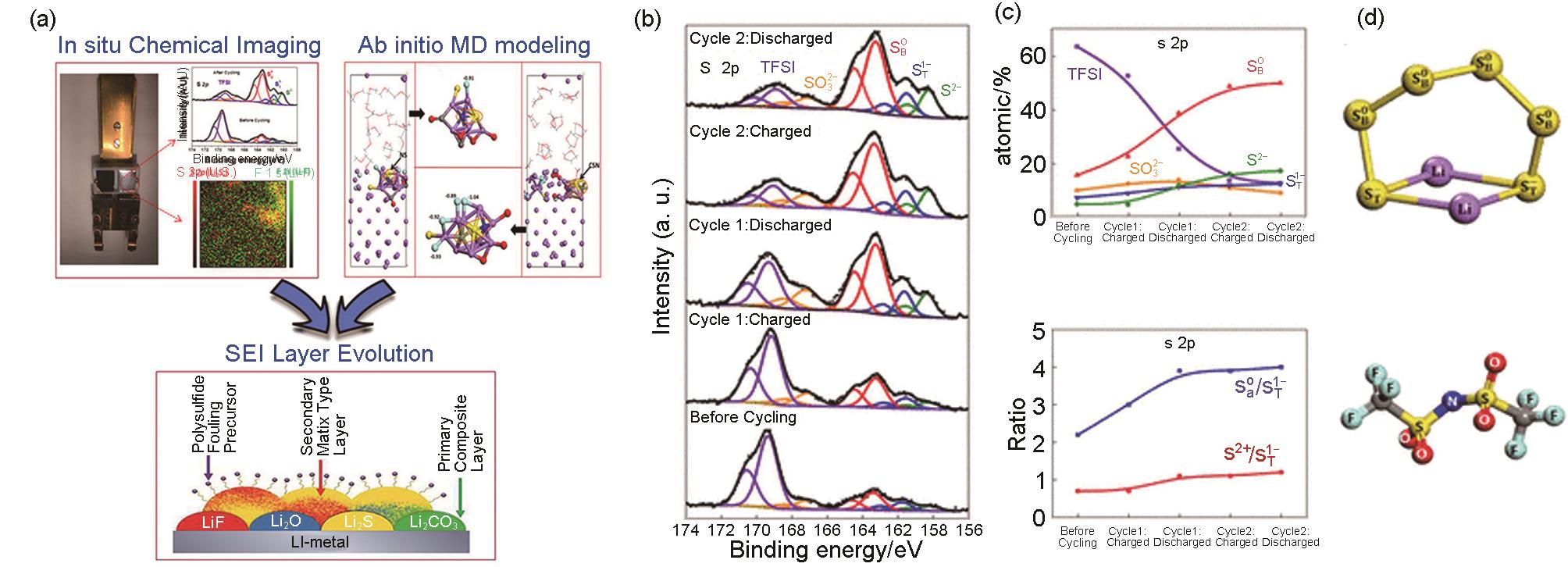

Fig. 10
(a) Cylindrical micro battery, fitted into the in situ NMR probe; (b) Stacked plot of the in situ7Li NMR spectra in a functioning Li-S cell vs time during discharge/charge; (c) Major peaks of interest within the range of -260 to 300 ppm extracted at different times by fitting the spectra[41]; (d) Solid species formation upon cycling;(e) Soluble species at around -0.16 ppm evolution with time[42]"
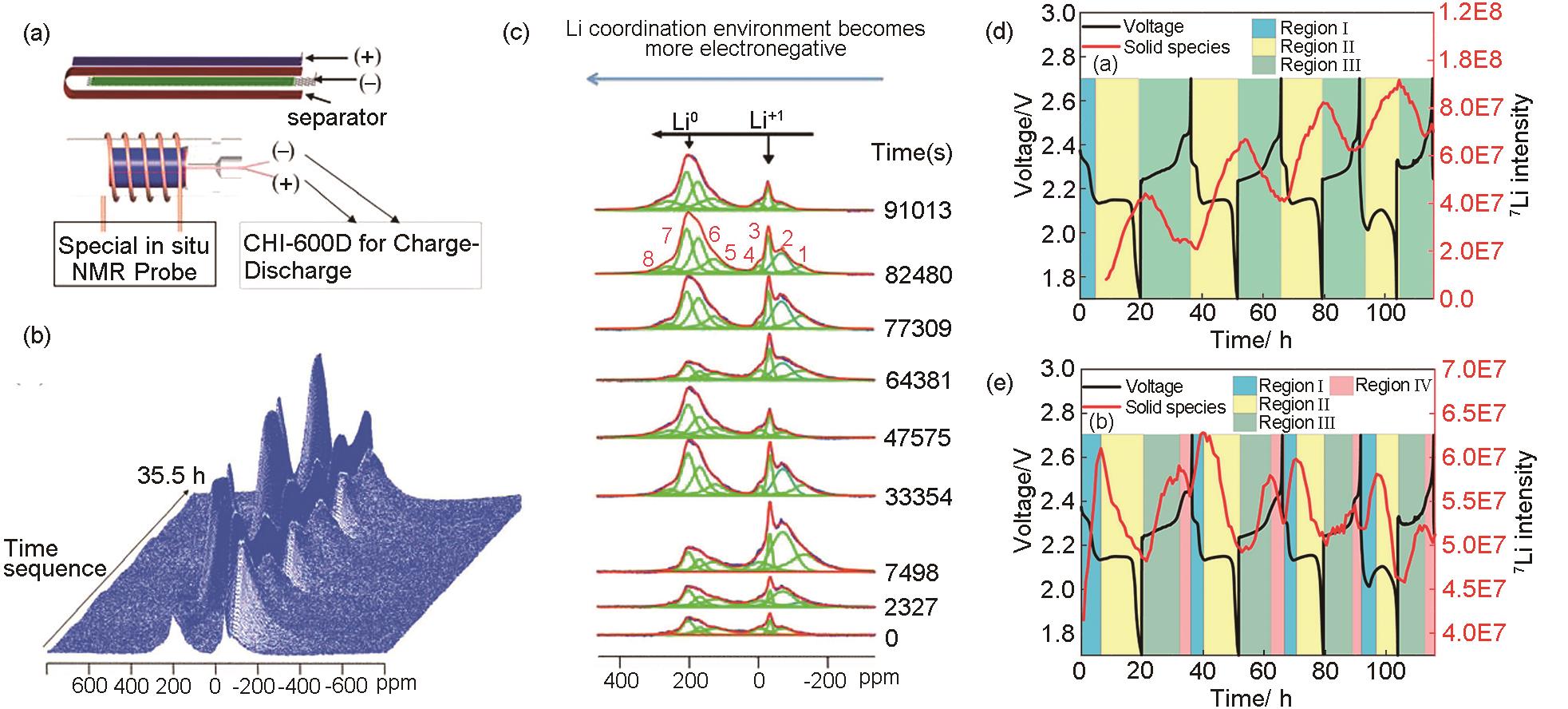
Table 1
Advantages and limitations of in situ characterization techniques"
| 原位表征技术 | 优势 | 局限性 |
|---|---|---|
| 原位TEM | 晶体转化可视化 | 只能观测局部区域 |
| 原位SEM | 高分辨率,三维成像 | 表面充电,影响成像效果 |
| 原位XPS | 实时观测材料价态和化学反应动力学 | 只能分析材料表面 |
| 原位Raman | 实时观测材料化学成分的变化 | 对硫化物等化合物散射信号较弱 |
| 原位XRD | 可进行高温检测 | 需要特殊的装置和X射线探测器 |
| 原位NMR | 无需真空环境 | 信号强度较弱 |
| 原位Vis-UV | 不对电池系统造成损伤 | 只能表征材料表面光学特性变化 |
| 原位XANES | 实时监测电极材料中多种化学物种的变化 | 实验条件复杂 |
| 35 | CHEN J, HAN K S, HENDERSON W A, et al. Restricting the solubility of polysulfides in Li-S batteries via electrolyte salt selection[J]. Advanced Energy Materials, 2016, 6(11): 1600160.. |
| 36 | ZOU Q L, LU Y C. Solvent-dictated lithium sulfur redox reactions: An operando UV-vis spectroscopic study[J]. The Journal of Physical Chemistry Letters, 2016, 7(8): 1518-1525. |
| 37 | MU W Y, LIU X L, WEN Z, et al. Numerical simulation of the factors affecting the growth of lithium dendrites[J]. Journal of Energy Storage, 2019, 26: 100921. |
| 38 | ZHANG Y J, LIU S F, WANG X L, et al. Composite Li metal anode with vertical graphene host for high performance Li-S batteries[J]. Journal of Power Sources, 2018, 374: 205-210. |
| 39 | PU J, SHEN Z, ZHENG J, et al. Co3S4@sulfur nanotubes for enhanced lithium-sulfur battery performance[J]. Nano Energy, 2017, 37: 7-14. |
| 40 | NANDASIRI MANJULA I, CAMACHO FORERO LUIS E, SCHWARZ ASHLEIGH M, et al. in situ chemical imaging of solid-electrolyte interphase layer evolution in Li-S batteries[J]. Chemistry of Materials, 2017, 29(11): 4728-4737. |
| 41 | XIAO J, HU J Z, CHEN H H, et al. Following the transient reactions in lithium-sulfur batteries using an in situ nuclear magnetic resonance technique[J]. Nano Letters, 2015, 15(5): 3309-3316. |
| 42 | WANG H, SA N Y, HE M N, et al. In situ NMR observation of the temporal speciation of lithium sulfur batteries during electrochemical cycling[J]. The Journal of Physical Chemistry C, 2017, 121(11): 6011-6017. |
| 1 | LI G X, SUN J H, HOU W P, et al. Three-dimensional porous carbon composites containing high sulfur nanoparticle content for high-performance lithium-sulfur batteries[J]. Nature Communications, 2016, 7: 10601. |
| 2 | GAO X, ZHENG X L, TSAO Y, et al. All-solid-state lithium-sulfur batteries enhanced by redox mediators[J]. Journal of the American Chemical Society, 2021, 143(43): 18188-18195. |
| 3 | JIN Z S, ZHAO M, LIN T N, et al. Ordered micro-mesoporous carbon spheres embedded with well-dispersed ultrafine Fe3C nanocrystals as cathode material for high-performance lithium-sulfur batteries[J]. Chemical Engineering Journal, 2020, 388: 124315. |
| 4 | CHEN R, LUO R, HUANG Y, et al. Advanced high energy density secondary batteries with multi-electron reaction materials[J]. Advanced Science, 2016, 3(10): 1600051. |
| 5 | DENG Z F, ZHANG Z A, LAI Y Q, et al. Electrochemical impedance spectroscopy study of a lithium/sulfur battery: Modeling and analysis of capacity fading[J]. Journal of the Electrochemical Society, 2013, 160(4): A553-A558. |
| 6 | KOH J Y, PARK M S, KIM E H, et al. Electrochemical reduction mechanism of sulfur particles electrically isolated from carbon cathodes of lithium-sulfur cells[J]. Journal of the Electrochemical Society, 2014, 161(14): A2117-A2120. |
| 7 | RISSE S, ANGIOLETTI-UBERTI S, DZUBIELLA J, et al. Capacity fading in lithium/sulfur batteries: A linear four-state model[J]. Journal of Power Sources, 2014, 267: 648-654. |
| 8 | SONG M K, CAIRNS E J, ZHANG Y G. Lithium/sulfur batteries with high specific energy: Old challenges and new opportunities[J]. Nanoscale, 2013, 5(6): 2186-2204. |
| 9 | HUANG L, LI J J, LIU B, et al. Electrode design for lithium-sulfur batteries: Problems and solutions[J]. Advanced Functional Materials, 2020, 30(22): 1910375. |
| 10 | CHEN W, QIAN T, XIONG J, et al. A new type of multifunctional polar binder: Toward practical application of high energy lithium sulfur batteries[J]. Advanced Materials, 2017, 29(12): 1605160. |
| 11 | FENG S, FU Z H, CHEN X, ZHANG Q, et al. A review on theoretical models for lithium-sulfur battery cathodes[J]. InfoMat, 2022, 4(3): e12304. |
| 12 | YEON J S, KO Y H, PARK T H, et al. Multidimensional hybrid architecture encapsulating cobalt oxide nanoparticles into carbon nanotube branched nitrogen-doped reduced graphene oxide networks for lithium-sulfur batteries[J]. Energy & Environmental Materials, 2022, 5(2): 555-564. |
| 13 | LIU J, XIAOA S, CHANG L, et al. Regulating the d band in WS2@NC hierarchical nanospheres for efficient lithium polysulfide conversion in lithium-sulfur batteries[J]. Journal of Energy Chemistry, 2021, 30(5): 343-352. |
| 14 | ZHAO E, NIE K, YU X, et al. Advanced characterization techniques in promoting mechanism understanding for lithium–sulfur batteries[J]. Advanced Functional Materials, 2018, 28(38): 1707543. |
| 15 | REHMAN S, POPE M, TAO S W, et al. Evaluating the effectiveness of in situ characterization techniques in overcoming mechanistic limitations in lithium-sulfur batteries[J]. Energy & Environmental Science, 2022, 15(4): 1423-1460. |
| 16 | CUISINIER M, CABELGUEN P E, EVERS S, et al. Sulfur speciation in Li-S batteries determined by operando X-ray absorption spectroscopy[J]. The Journal of Physical Chemistry Letters, 2013, 4(19): 3227-3232. |
| 17 | WUJCIK K H, PASCAL T A, PEMMARAJU C D, et al. Characterization of polysulfide radicals present in an ether‐based electrolyte of a lithium-sulfur battery during initial discharge using in situ X-ray absorption spectroscopy experiments and first‐principles calculations[J]. Advanced Energy Materials, 2015, 5(16): 1500285. |
| 18 | SUN Y, SEH Z W, LI W, et al. In-operando optical imaging of temporal and spatial distribution of polysulfides in lithium-sulfur batteries[J]. Nano Energy, 2015: 579-586. |
| 19 | LANG S Y, SHI Y, GUO Y G, et al. Insight into the interfacial process and mechanism in lithium-sulfur batteries: An in situ AFM study[J]. Angewandte Chemie, 2016, 55(51): 15835-15839. |
| 20 | LANG S Y, SHI Y, GUO Y G, et al. High-temperature formation of a functional film at the cathode/electrolyte interface in lithium-sulfur batteries: An in situ AFM study[J]. Angewandte Chemie, 2017, 56(46): 14433-14437. |
| 21 | YIM T, PARK M S, YU J S, et al. Effect of chemical reactivity of polysulfide toward carbonate-based electrolyte on the electrochemical performance of Li-S batteries[J]. Electrochimica Acta, 2013, 107: 454-460. |
| 22 | LIU X, HUANG J Q, ZHANG Q, et al. Nanostructured metal oxides and sulfides for lithium-sulfur batteries[J]. Advanced Materials, 2017, 29(20): 1601759. |
| 23 | ZHU X, ZHANG F, ZHANG L, et al. A highly stretchable cross‐linked polyacrylamide hydrogel as an effective binder for silicon and sulfur electrodes toward durable lithium‐ion storage[J]. Advanced Functional Materials, 2018, 28(11): 1705015. |
| 24 | FANG R P, ZHAO S Y, SUN Z H, et al. More reliable lithium-sulfur batteries: Status, solutions and prospects[J]. Advanced Materials, 2017, 29(48): 1606823. |
| 25 | LIU R L, WEI Z Y, PENG L L, et al. Establishing reaction networks in the 16-electron sulfur reduction reaction[J]. Nature, 2024, 626(7997): 98-104. |
| 26 | KIM H, LEE J T, MAGASINSKI A, et al. in situ TEM observation of electrochemical lithiation of sulfur confined within inner cylindrical pores of carbon nanotubes[J]. Advanced Energy Materials, 2015, 5(24): 1501306. |
| 27 | WANG Z F, TANG Y F, ZHANG L Q, et al. in situ TEM observations of discharging/charging of solid-state lithium-sulfur batteries at high temperatures[J]. Small, 2020, 16(28): e2001899. |
| 28 | KAVCIC M, BUCAR K, PETRIC M, et al. Operando resonant inelastic X-ray scattering: An appropriate tool to characterize sulfur in Li-S batteries[J]. The Journal of Physical Chemistry C, 2016, 120(43): 24568-24576. |
| 29 | SAQIB N, OHLHAUSEN G M, PORTER J M. In operando infrared spectroscopy of lithium polysulfides using a novel spectro-electrochemical cell[J]. Journal of Power Sources, 2017, 364: 266-271. |
| 30 | HAGEN M, SCHIFFELS P, HAMMER M, et al. In-situ Raman investigation of polysulfide formation in Li-S cells[J]. Journal of the Electrochemical Society, 2013, 160(8): a1205-a1214. |
| 31 | DRVARIČ TALIAN S, JESCHKE S, VIZINTIN A, et al. Fluorinated ether based electrolyte for high-energy lithium-sulfur batteries: Li+ solvation role behind reduced polysulfide solubility[J]. Chemistry of Materials, 2017, 29(23): 10037-10044. |
| 32 | ZHAO Y, ZHOU T H, ASHIROV T, et al. Fluorinated ether electrolyte with controlled solvation structure for high voltage lithium metal batteries[J]. Nature Communications, 2022, 13(1): 2575. |
| 33 | BALOCH M, VIZINTIN A, CHELLAPPAN R K, et al. Application of gel polymer electrolytes based on ionic liquids in lithium-sulfur batteries[J]. Journal of The Electrochemical Society, 2016, 163(10): A2390. |
| 34 | LI W L, XING Y J, WU Y H, et al. Study the effect of ion-complex on the properties of composite gel polymer electrolyte based on Electrospun PVdF nanofibrous membrane[J]. Electrochimica Acta, 2015, 151: 289-296. |
| [1] | Mingxun JIA, Tong WU, Daotong YANG, Xiaoxi QIN, Jinghai LIU, Limei DUAN. Electrolyte multifunctional additives of lithium-sulfur battery: Mechanism of action and advanced characterization [J]. Energy Storage Science and Technology, 2024, 13(1): 36-47. |
| [2] | Shun ZHANG, Fanglei ZENG, Ning LI, Ningyi YUAN. Study on the preparation and properties of high-flame retardant sulfur cathode [J]. Energy Storage Science and Technology, 2023, 12(4): 1018-1024. |
| [3] | Liyuan SHEN, Guixin ZHANG, Zhaoling MA. Catalytic conversion performance study of O-doped NiCo2S4/CNT composites for Li polysulfides [J]. Energy Storage Science and Technology, 2023, 12(11): 3318-3329. |
| [4] | Yuqi SUN, Feng WEI, Hong ZHOU, Chaofeng ZHOU. Analysis of global lithium-sulfur battery technology competition from the perspective of patent [J]. Energy Storage Science and Technology, 2022, 11(5): 1657-1666. |
| [5] | Bin XIE, Jia'nan SUN. Development of high specific energy lithium-sulfur cell module based on mechanical simulations [J]. Energy Storage Science and Technology, 2021, 10(2): 586-597. |
| [6] | YE Ge, YUAN Hong, ZHAO Chenzi, ZHU Gaolong, XU Lei, HOU Lipeng, CHENG Xinbing, HE Chuanxin, NAN Haoxiong, LIU Quanbin, HUANG Jiaqi, ZHANG Qiang. Balance between ion migration and electron transport in composite cathodes for all-solid-state lithium-sulfur batteries [J]. Energy Storage Science and Technology, 2020, 9(2): 339-345. |
| [7] | YAO Lin, ZHOU Ling, LI Shixiong, LI Xiaomin, HE Kai, HE Qingquan, ZAI Jiantao, REN Qizhi, QIAN Xuefeng. Edge-rich MoS2 nanosheets for high performance self-supporting Li-S batteries [J]. Energy Storage Science and Technology, 2019, 8(3): 523-531. |
| [8] | HU Cejun, YANG Jijin, WANG Hangchao, CHEN Yifan, ZHANG Rongrong, LIU Wen, SUN Xiaoming. Research progress of safe lithium sulfur batteries [J]. Energy Storage Science and Technology, 2018, 7(6): 1082-1093. |
| [9] | PEI Haijuan, GUO Rui, LI Yong, LIU Wen, CHEN Zhujun, WANG Yong, XIE Jingying. Conductive carbon-coated separator for high sulfur-loading lithium sulfur batteries [J]. Energy Storage Science and Technology, 2018, 7(1): 56-. |
| [10] | YAN Changqing, CAO Yong. Patent analysis of lithium-sulfur battery technology in China [J]. Energy Storage Science and Technology, 2017, 6(S1): 74-. |
| [11] | ZHAO Meng, XU Rui, HUANG Jiaqi, ZHANG Qiang. Flexible cathodes for lithium sulfur battery: A review [J]. Energy Storage Science and Technology, 2017, 6(3): 360-379. |
| [12] | SHANG Yongliang1, WANG Chengwen1, LIU Bin1, LIU Jun1,2, KE Xi1,2, LIU Liying1,2, SHI Zhicong1,2. Preparation and properties of manganese dioxide coated carbon nanotubes-sulfur composite cathode material [J]. Energy Storage Science and Technology, 2017, 6(3): 411-417. |
| [13] | XU Rui1, ZHAO Meng1, HUANG Jiaqi1,2. Progress in composite separators for lithium sulfur batteries [J]. Energy Storage Science and Technology, 2017, 6(3): 433-450. |
| [14] | TANG Zhen, XIONG Chuanxi. Agar as water soluble binder for lithium-sulfur battery [J]. Energy Storage Science and Technology, 2017, 6(3): 493-499. |
| [15] | XU Na, WANG Mengfan, QIAN Tao, YAN Chenglin. Application of in-situ UV/vis analysis in lithium-sulfur batteries [J]. Energy Storage Science and Technology, 2017, 6(3): 522-528. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
