储能科学与技术 ›› 2022, Vol. 11 ›› Issue (9): 2944-2958.doi: 10.19799/j.cnki.2095-4239.2022.0295
收稿日期:2022-05-31
修回日期:2022-06-17
出版日期:2022-09-05
发布日期:2022-08-30
通讯作者:
李先锋
E-mail:yuanzhizhang@dicp.ac.cn;lixianfeng@dicp.ac.cn
作者简介:袁治章(1989—),男,博士,研究员,研究方向为锌基液流电池关键技术,E-mail:yuanzhizhang@dicp.ac.cn;
基金资助:
Zhizhang YUAN1( ), Zonghao LIU2, Xianfeng LI1(
), Zonghao LIU2, Xianfeng LI1( )
)
Received:2022-05-31
Revised:2022-06-17
Online:2022-09-05
Published:2022-08-30
Contact:
Xianfeng LI
E-mail:yuanzhizhang@dicp.ac.cn;lixianfeng@dicp.ac.cn
摘要:
储能技术是构建以新能源为主体的新型电力系统,实现双碳目标的关键支撑技术。液流电池储能技术具有安全可靠、寿命长、环境友好等优势,成为规模储能的首选技术之一。本文通过对传统液流电池储能技术包括铁铬液流电池储能技术、全钒液流电池储能技术、锌溴液流电池储能技术和液流电池新体系包括基于溴基氧化还原电对的液流电池新体系、醌基液流电池体系、吩嗪基液流电池体系、TEMPO类液流电池体系、紫精类液流电池体系的研究进展进行探讨,综述了各类液流电池储能技术的发展历程及其技术成熟度,着重介绍了各类液流电池储能技术的特点和进一步发展所面临的关键科学问题,重点分析了不同种类的液流电池储能技术实用化进程中的关键技术瓶颈。通过总结分析国内外液流电池储能技术的发展态势,对液流电池储能技术未来发展方向进行了展望。
中图分类号:
袁治章, 刘宗浩, 李先锋. 液流电池储能技术研究进展[J]. 储能科学与技术, 2022, 11(9): 2944-2958.
Zhizhang YUAN, Zonghao LIU, Xianfeng LI. Research progress of flow battery technologies[J]. Energy Storage Science and Technology, 2022, 11(9): 2944-2958.
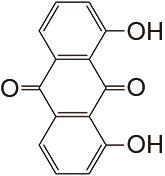
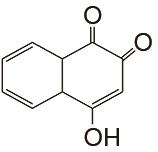
表1
负极以醌电对作为活性物质、正极以Fe(CN)64-/Fe(CN)63- 电对作为活性物质的液流电池体系"
| 负极活性物质 | 支持电解质 | 运行环境 | 电流密度/(mA/cm2) | 效率 | 循环 | 容量保持率 | 参考文献 |
|---|---|---|---|---|---|---|---|
 | 1 mol/L KOH | 高纯Ar | 100 | CE约99% EE约84% | 100 | 99.9% | [ |
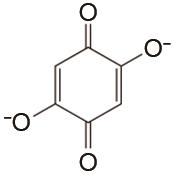 | 1 mol/L KOH | 高纯Ar | 100 | CE约99% EE约65% | 150 | 99.76% | [ |
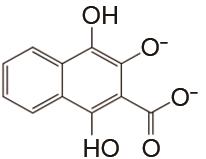 | 1 mol/L KOH | 高纯N2 | 100 | CE约100% EE约69% | 100 | 94.7% | [ |
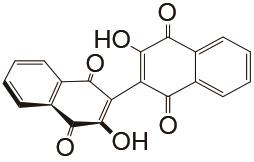 | 1 mol/L KOH | N2 | 100 | CE约99% EE约77% | <300 | 99.992% | [ |
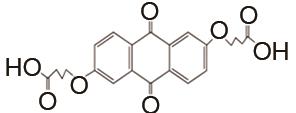 | 1 mol/L KOH | N2 | 100 | CE约99% | 250 | 99.999% | [ |
 | 1 mol/L KOH | N2 | 80 | CE约99% EE约77% | 100 | 99.88% | [ |
 | 1 mol/L KOH | 未提供 | 100 | CE约99% EE约55% | 200 | 99.994% | [ |
 | 1 mol/L KOH | 高纯N2 | 80 | CE约99% EE约76% | 140 | >99% | [ |
| 1 | THALLER L. Electrically rechargeable REDOX flow cell [M]. (NASA TM X-71540) 1974. |
| 2 | 李先锋, 张洪章, 郑琼, 等. 能源革命中的电化学储能技术[J]. 中国科学院院刊, 2019, 34(4): 443-449. |
| LI X F, ZHANG H Z, ZHENG Q, et al. Electrochemical energy storage technology in energy revolution[J]. Bulletin of Chinese Academy of Sciences, 2019, 34(4): 443-449. | |
| 3 | WANG F, HARINDINTWALI J D, YUAN Z Z, et al. Technologies and perspectives for achieving carbon neutrality[J]. The Innovation, 2021, 2(4): doi: 10.1016/j.xinn.2021.100180. |
| 4 | YUAN Z Z, YIN Y B, XIE C X, et al. Advanced materials for zinc-based flow battery: Development and challenge[J]. Advanced Materials, 2019, 31(50): doi: 10.1002/adma.201902025. |
| 5 | KANQRO W. Verfahren zur speicherung von elektrischer energie: DE914264C [P].1949-06-28. |
| 6 | ZHANG H, TAN Y, LI J Y, et al. Studies on properties of rayon- and polyacrylonitrile-based graphite felt electrodes affecting Fe/Cr redox flow battery performance[J]. Electrochimica Acta, 2017, 248: 603-613. |
| 7 | ZHANG H, CHEN N, SUN C Y, et al. Investigations on physicochemical properties and electrochemical performance of graphite felt and carbon felt for iron-chromium redox flow battery[J]. International Journal of Energy Research, 2020, 44(5): 3839-3853. |
| 8 | INOUE M, TSUZUKI Y, IIZUKA Y, et al. Carbon fiber electrode for redox flow battery[J]. Journal of the Electrochemical Society, 1987, 134(3): 756-757. |
| 9 | WATERS S E, ROBB B H, MARSHAK M P. Effect of chelation on iron-chromium redox flow batteries[J]. ACS Energy Letters, 2020, 5(6): 1758-1762. |
| 10 | Rodes A, Feliu J M, Aldaz A, et al. The influence of polyoriented gold electrodes modified by reversibly and irreversibly adsorbed ad-atoms on the redox behaviour of the Cr(Ⅲ)/Cr(Ⅱ) [J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1989, 271(1): 127-139. |
| 11 | ZENG Y K, ZHOU X L, AN L, et al. A high-performance flow-field structured iron-chromium redox flow battery[J]. Journal of Power Sources, 2016, 324: 738-744. |
| 12 | Sum E, Skyllas-Kazacos M. A study of the V(Ⅱ)/V(Ⅲ) redox couple for redox flow cell applications [J]. Journal of Power Sources, 1985, 15(2): 179-190. |
| 13 | SKYLLAS-KAZACOS M, RYCHCIK M, ROBINS R G, et al. New all-vanadium redox flow cell[J]. Journal of the Electrochemical Society, 1986, 133(5): 1057-1058. |
| 14 | HUANG K L, LI X G, LIU S Q, et al. Research progress of vanadium redox flow battery for energy storage in China[J]. Renewable Energy, 2008, 33(2): 186-192. |
| 15 | YUAN Z Z, ZHANG H M, LI X F. Ion conducting membranes for aqueous flow battery systems[J]. Chemical Communications (Cambridge, England), 2018, 54(55): 7570-7588. |
| 16 | DAI Q, XING F, LIU X N, et al. High-performance PBI membranes for flow batteries: From the transport mechanism to the pilot plant[J]. Energy & Environmental Science, 2022, 15(4): 1594-1600. |
| 17 | LI T Y, XING F, LIU T, et al. Cost, performance prediction and optimization of a vanadium flow battery by machine-learning[J]. Energy & Environmental Science, 2020, 13(11): 4353-4361. |
| 18 | ZHANG J, JIANG G P, XU P, et al. An all-aqueous redox flow battery with unprecedented energy density[J]. Energy & Environmental Science, 2018, 11(8): 2010-2015. |
| 19 | GONG K, MA X Y, CONFORTI K M, et al. A zinc-iron redox-flow battery under $100 per kWh of system capital cost[J]. Energy & Environmental Science, 2015, 8(10): 2941-2945. |
| 20 | YIN Y B, WANG S N, ZHANG Q, et al. Dendrite-free zinc deposition induced by tin-modified multifunctional 3D host for stable zinc-based flow battery[J]. Advanced Materials, 2020, 32(6): doi:10.1002/adma.201906803. |
| 21 | WANG S N, YUAN C G, CHANG N N, et al. Act in contravention: A non-planar coupled electrode design utilizing "tip effect" for ultra-high areal capacity, long cycle life zinc-based batteries[J]. Science Bulletin, 2021, 66(9): 889-896. |
| 22 | WANG S N, WANG Z Y, YIN Y B, et al. A highly reversible zinc deposition for flow batteries regulated by critical concentration induced nucleation[J]. Energy & Environmental Science, 2021, 14(7): 4077-4084. |
| 23 | LI X J, LI T Y, XU P C, et al. A complexing agent to enable a wide-temperature range bromine-based flow battery for stationary energy storage[J]. Advanced Functional Materials, 2021, 31(22): doi: 10.1002/adfm.202100133. |
| 24 | LI X J, XIE C X, LI T Y, et al. Low-cost titanium-bromine flow battery with ultrahigh cycle stability for grid-scale energy storage[J]. Advanced Materials (Deerfield Beach, Fla), 2020, 32(49): doi: 10.1002/adma.202005036. |
| 25 | LU W J, XU P C, SHAO S Y, et al. Multifunctional carbon felt electrode with N-rich defects enables a long-cycle zinc-bromine flow battery with ultrahigh power density[J]. Advanced Functional Materials, 2021, 31(30): doi: 10.1002/adfm.202102913. |
| 26 | HUA L, LU W, LI T, et al. A highly selective porous composite membrane with bromine capturing ability for a bromine-based flow battery[J]. Materials Today Energy, 2021, 21: doi:10.1016/j.mtener.2021.100763. |
| 27 | ADAMS G B. Electrically rechargeable battery: US4180623[P]. 1979-12-25. |
| 28 | HU J, YUE M, ZHANG H M, et al. A boron nitride nanosheets composite membrane for a long-life zinc-based flow battery[J]. Angewandte Chemie (International Ed in English), 2020, 59(17): 6715-6719. |
| 29 | WU J E, YUAN C G, LI T Y, et al. Dendrite-free zinc-based battery with high areal capacity via the region-induced deposition effect of turing membrane[J]. Journal of the American Chemical Society, 2021, 143(33): 13135-13144. |
| 30 | YUAN Z Z, LIU X Q, XU W B, et al. Negatively charged nanoporous membrane for a dendrite-free alkaline zinc-based flow battery with long cycle life[J]. Nature Communications, 2018, 9: 3731. |
| 31 | HU J, ZHANG H M, XU W B, et al. Mechanism and transfer behavior of ions in Nafion membranes under alkaline media[J]. Journal of Membrane Science, 2018, 566: 8-14. |
| 32 | HU J, TANG X M, DAI Q, et al. Layered double hydroxide membrane with high hydroxide conductivity and ion selectivity for energy storage device[J]. Nature Communications, 2021, 12: 3409. |
| 33 | YUAN Z Z, LIANG L X, DAI Q, et al. Low-cost hydrocarbon membrane enables commercial-scale flow batteries for long-duration energy storage[J]. Joule, 2022, 6(4): 884-905. |
| 34 | WINSBERG J, STOLZE C, SCHWENKE A, et al. Aqueous 2, 2, 6, 6-tetramethylpiperidine-N-oxyl catholytes for a high-capacity and high current density oxygen-insensitive hybrid-flow battery[J]. ACS Energy Letters, 2017, 2(2): 411-416. |
| 35 | ULAGANATHAN M, SURESH S, MARIYAPPAN K, et al. New zinc-vanadium (Zn-V) hybrid redox flow battery: High-voltage and energy-efficient advanced energy storage system[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(6): 6053-6060. |
| 36 | XIE C X, LI T Y, DENG C Z, et al. A highly reversible neutral zinc/manganese battery for stationary energy storage[J]. Energy & Environmental Science, 2020, 13(1): 135-143. |
| 37 | XIE C X, DUAN Y Q, XU W B, et al. A low-cost neutral zinc-iron flow battery with high energy density for stationary energy storage[J]. Angewandte Chemie (International Ed in English), 2017, 56(47): 14953-14957. |
| 38 | XIE C X, LIU Y, LU W J, et al. Highly stable zinc-iodine single flow batteries with super high energy density for stationary energy storage[J]. Energy & Environmental Science, 2019, 12(6): 1834-1839. |
| 39 | WENG G M, LI Z J, CONG G T, et al. Unlocking the capacity of iodide for high-energy-density zinc/polyiodide and lithium/polyiodide redox flow batteries[J]. Energy & Environmental Science, 2017, 10(3): 735-741. |
| 40 | LIU Z, CUI T, PULLETIKURTHI G, et al. Dendrite-free nanocrystalline zinc electrodeposition from an ionic liquid containing nickel triflate for rechargeable Zn-based batteries[J]. Angewandte Chemie (International Ed in English), 2016, 55(8): 2889-2893. |
| 41 | PARKER J F, CHERVIN C N, NELSON E S, et al. Wiring zinc in three dimensions re-writes battery performance-dendrite-free cycling[J]. Energy Environ Sci, 2014, 7(3): 1117-1124. |
| 42 | BANIK S J, AKOLKAR R. Suppressing dendrite growth during zinc electrodeposition by PEG-200 additive[J]. Journal of the Electrochemical Society, 2013, 160(11): D519-D523. |
| 43 | FU J, CANO Z P, PARK M G, et al. Electrically rechargeable zinc-air batteries: Progress, challenges, and perspectives[J]. Advanced Materials, 2017, 29(7): doi: 10.1002/adma.201604685. |
| 44 | PARKER J F, CHERVIN C N, PALA I R, et al. Rechargeable nickel-3D zinc batteries: An energy-dense, safer alternative to lithium-ion[J]. Science, 2017, 356(6336): 415-418. |
| 45 | KNEHR K W, BISWAS S, STEINGART D A. Quantification of the voltage losses in the minimal architecture zinc-bromine battery using GITT and EIS[J]. Journal of the Electrochemical Society, 2017, 164(13): A3101-A3108. |
| 46 | HUSKINSON B, MARSHAK M P, SUH C, et al. A metal-free organic-inorganic aqueous flow battery[J]. Nature, 2014, 505(7482): 195-198. |
| 47 | KHATAEE A, WEDEGE K, DRAŽEVIĆ E, et al. Differential pH as a method for increasing cell potential in organic aqueous flow batteries[J]. J Mater Chem A, 2017, 5(41): 21875-21882. |
| 48 | LIN K X, CHEN Q, GERHARDT M R, et al. Alkaline quinone flow battery[J]. Science, 2015, 349(6255): 1529-1532. |
| 49 | YANG Z J, TONG L C, TABOR D P, et al. Alkaline benzoquinone aqueous flow battery for large-scale storage of electrical energy[J]. Advanced Energy Materials, 2018, 8(8): doi: 10.1002/aenm.201870034. |
| 50 | LIU W Q, ZHAO Z M, LI T Y, et al. A high potential biphenol derivative cathode: Toward a highly stable air-insensitive aqueous organic flow battery[J]. Science Bulletin, 2021, 66(5): 457-463. |
| 51 | WANG C X, YANG Z, WANG Y R, et al. High-performance alkaline organic redox flow batteries based on 2-hydroxy-3-carboxy-1, 4-naphthoquinone[J]. ACS Energy Letters, 2018, 3(10): 2404-2409. |
| 52 | TONG L C, GOULET M A, TABOR D P, et al. Molecular engineering of an alkaline naphthoquinone flow battery[J]. ACS Energy Letters, 2019, 4(8): 1880-1887. |
| 53 | KWABI D G, LIN K X, JI Y L, et al. Alkaline quinone flow battery with long lifetime at pH 12[J]. Joule, 2018, 2(9): 1894-1906. |
| 54 | CAO J Y, TAO M, CHEN H P, et al. A highly reversible anthraquinone-based anolyte for alkaline aqueous redox flow batteries[J]. Journal of Power Sources, 2018, 386: 40-46. |
| 55 | LEE W, PARK G, KWON Y. Alkaline aqueous organic redox flow batteries of high energy and power densities using mixed naphthoquinone derivatives[J]. Chemical Engineering Journal, 2020, 386: doi: 10.1016/j.cej.2019.123985. |
| 56 | CHEN D J, DUAN W Q, HE Y Y, et al. Porous membrane with high selectivity for alkaline quinone-based flow batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(43): 48533-48541. |
| 57 | LIN K X, GÓMEZ-BOMBARELLI R, BEH E S, et al. A redox-flow battery with an alloxazine-based organic electrolyte[J]. Nature Energy, 2016, 1: 16102. |
| 58 | ORITA A, VERDE M G, SAKAI M, et al. A biomimetic redox flow battery based on flavin mononucleotide[J]. Nature Communications, 2016, 7: 13230. |
| 59 | HOLLAS A, WEI X L, MURUGESAN V, et al. A biomimetic high-capacity phenazine-based anolyte for aqueous organic redox flow batteries[J]. Nature Energy, 2018, 3(6): 508-514. |
| 60 | WANG C, LI X, YU B, et al. Molecular disign of fused-ring phenazine derivatives for long-cycling alkaline redox flow batteries [J]. ACS Energy Letters, 2020, (2): 411-417. |
| 61 | PANG S, WANG X Y, WANG P, et al. Biomimetic amino acid functionalized phenazine flow batteries with long lifetime at near-neutral pH[J]. Angewandte Chemie (International Ed in English), 2021, 60(10): 5289-5298. |
| 62 | XU J C, PANG S, WANG X Y, et al. Ultrastable aqueous phenazine flow batteries with high capacity operated at elevated temperatures[J]. Joule, 2021, 5(9): 2437-2449. |
| 63 | ZHANG C K, NIU Z H, PENG S S, et al. Phenothiazine-based organic catholyte for high-capacity and long-life aqueous redox flow batteries[J]. Advanced Materials, 2019, 31(24): doi: 10.1002/adma.201901052. |
| 64 | LIU T B, WEI X L, NIE Z M, et al. A total organic aqueous redox flow battery employing a low cost and sustainable methyl viologen anolyte and 4-HO-TEMPO catholyte[J]. Advanced Energy Materials, 2016, 6(3): doi: 10.1002/aenm.201501449. |
| 65 | JANOSCHKA T, MARTIN N, HAGER M D, et al. An aqueous redox-flow battery with high capacity and power: The TEMPTMA/MV system[J]. Angewandte Chemie (International Ed in English), 2016, 55(46): 14427-14430. |
| 66 | WINSBERG J, STOLZE C, MUENCH S, et al. TEMPO/phenazine combi-molecule: A redox-active material for symmetric aqueous redox-flow batteries[J]. ACS Energy Letters, 2016, 1(5): 976-980. |
| 67 | JANOSCHKA T, MARTIN N, MARTIN U, et al. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials[J]. Nature, 2015, 527(7576): 78-81. |
| 68 | HU B, FAN H, LI H B, et al. Five-membered ring nitroxide radical: A new class of high-potential, stable catholytes for neutral aqueous organic redox flow batteries[J]. Advanced Functional Materials, 2021, 31(35): doi: 10.1002/adfm.202102734. |
| 69 | FAN H, HU B, LI H B, et al. Conjugate-driven electron density delocalization of piperidine nitroxyl radical for stable aqueous zinc hybrid flow batteries[J]. Angewandte Chemie (International Ed in English), 2022, 61(17): doi: 10.1002/anie.202115908. |
| 70 | DEBRULER C, HU B, MOSS J, et al. Designer two-electron storage viologen anolyte materials for neutral aqueous organic redox flow batteries[J]. Chem, 2017, 3(6): 961-978. |
| 71 | HU B, DEBRULER C, RHODES Z, et al. Long-cycling aqueous organic redox flow battery (AORFB) toward sustainable and safe energy storage[J]. Journal of the American Chemical Society, 2017, 139(3): 1207-1214. |
| 72 | BEH E S, DE PORCELLINIS D, GRACIA R L, et al. A neutral pH aqueous organic-organometallic redox flow battery with extremely high capacity retention[J]. ACS Energy Letters, 2017, 2(3): 639-644. |
| 73 | LUO J, HU B, DEBRULER C, et al. A π-conjugation extended viologen as a two-electron storage anolyte for total organic aqueous redox flow batteries[J]. Angewandte Chemie, 2018, 57(1): 231-235. |
| 74 | HU S Z, LI T Y, HUANG M B, et al. Phenylene-bridged bispyridinium with high capacity and stability for aqueous flow batteries[J]. Advanced Materials, 2021, 33(7): doi: 10.1002/adma.202005839. |
| 75 | LIU W Q, LIU Y, ZHANG H M, et al. A highly stable neutral viologen/bromine aqueous flow battery with high energy and power density[J]. Chemical Communications, 2019, 55(33): 4801-4804. |
| 76 | HUANG J H, HU S Z, YUAN X Z, et al. Radical stabilization of a tripyridinium-triazine molecule enables reversible storage of multiple electrons[J]. Angewandte Chemie, 2021, 60(38): 20921-20925. |
| 77 | HUANG M B, HU S Z, YUAN X Z, et al. Five-membered-heterocycle bridged viologen with high voltage and superior stability for flow battery[J]. Advanced Functional Materials, 2022, 32(16): doi: 10.1002/adfm.202111744. |
| [1] | 张华民. 全钒液流电池的技术进展、不同储能时长系统的价格分析及展望[J]. 储能科学与技术, 2022, 11(9): 2772-2780. |
| [2] | 唐奡, 严川伟. 液流电池模拟仿真研究现状与展望[J]. 储能科学与技术, 2022, 11(9): 2866-2878. |
| [3] | 李洪涛, 张帅, 李旭东, 纪运广, 孙明旭, 李欣. 单罐式储能换热系统在热风无纺布工艺中的应用[J]. 储能科学与技术, 2022, 11(7): 2250-2257. |
| [4] | 姚祯, 张琦, 王锐, 刘庆华, 王保国, 缪平. 生物质衍生碳材料在全钒液流电池电极方面的应用[J]. 储能科学与技术, 2022, 11(7): 2083-2091. |
| [5] | 鲁志颖, 江杉, 李全龙, 马可心, 傅腾, 郑志刚, 刘志成, 李淼, 梁永胜, 董知非. 全钒液流电池在充电结束搁置阶段的开路电压变化[J]. 储能科学与技术, 2022, 11(7): 2046-2050. |
| [6] | 王瑄, 叶强. 全钒液流电池电堆局部供液不足导致副反应加剧的现象[J]. 储能科学与技术, 2022, 11(5): 1455-1467. |
| [7] | 房茂霖, 张英, 乔琳, 刘淑敏, 曹中琦, 张华民, 马相坤. 铁铬液流电池技术的研究进展[J]. 储能科学与技术, 2022, 11(5): 1358-1367. |
| [8] | 彭康, 刘俊敏, 唐珙根, 杨正金, 徐铜文. 水系有机液流电池电化学活性分子研究现状及展望[J]. 储能科学与技术, 2022, 11(4): 1246-1263. |
| [9] | 王振宇, 郭子啸, 范新庄, 赵天寿. 全钒液流电池中蛇型和插指型流道的对比[J]. 储能科学与技术, 2022, 11(4): 1121-1130. |
| [10] | 赵志伟, 杨智, 彭章泉. 飞行时间二次离子质谱在锂基二次电池中的应用[J]. 储能科学与技术, 2022, 11(3): 781-794. |
| [11] | 李昂, 李晓蒙, 杨林, 王含, 项俊帆, 刘雨涵. 液流电池封装压力计算[J]. 储能科学与技术, 2022, 11(2): 609-614. |
| [12] | 汤匀, 岳芳, 郭楷模, 李岚春, 陈伟. 下一代电化学储能技术国际发展态势分析[J]. 储能科学与技术, 2022, 11(1): 89-97. |
| [13] | 张子岩, 张俊艳. 基于高质量专利的储能关键技术国际竞争态势[J]. 储能科学与技术, 2022, 11(1): 321-334. |
| [14] | 姚祯, 王锐, 阳雪, 张琦, 刘庆华, 王保国, 缪平. 锌铁液流电池研究现状及展望[J]. 储能科学与技术, 2022, 11(1): 78-88. |
| [15] | 谢克桓, 李传常, 陈荐, 余龙海, 谭准, 秦位海. 全钒液流电池储能仿真模型及荷电状态监测方法研究[J]. 储能科学与技术, 2021, 10(6): 2363-2372. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
