Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (3): 883-897.doi: 10.19799/j.cnki.2095-4239.2024.1121
• Emerging Investigator Issue of Energy Storage • Previous Articles Next Articles
Zhao CHEN1( ), Qinqin LIANG2, Yuting LI1,3, Fei XIE1(
), Qinqin LIANG2, Yuting LI1,3, Fei XIE1( ), Bin TANG2, Jianxin LI2, Yaxiang LU1, Aibing CHEN3, Yongsheng HU1(
), Bin TANG2, Jianxin LI2, Yaxiang LU1, Aibing CHEN3, Yongsheng HU1( )
)
Received:2024-11-27
Revised:2024-12-10
Online:2025-03-28
Published:2025-04-28
Contact:
Fei XIE, Yongsheng HU
E-mail:zchen@iphy.ac.cn;fxie@iphy.ac.cn;yshu@iphy.ac.cn
CLC Number:
Zhao CHEN, Qinqin LIANG, Yuting LI, Fei XIE, Bin TANG, Jianxin LI, Yaxiang LU, Aibing CHEN, Yongsheng HU. Recent progress of tin-based alloy-type anode materials in Na-ion batteries[J]. Energy Storage Science and Technology, 2025, 14(3): 883-897.
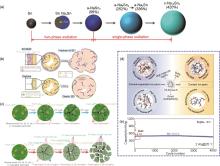
Fig. 2
(a) Schematic illustration of the structural evolution of tin particles during sodiation[12]; (b) Schematic comparison of the chemical composition, structure and mechanical response to volume expansion of the SEI layer formed from NaBF4/EC/DMC and NaBF4/diglyme[11]; (c) Modeling of the alloying reaction, comparison of the cycling behavior of the anode in a compatible electrolyte (top panel) and an incompatible electrolyte (bottom panel)[8]; (d) Basic mechanism of electrochemical performance enhancement by remodeling the solvated structure of 15-Crown-5[16]; (e) Comparative cycling plots of a standard electrolyte (BE) and a modified electrolyte (BE+15-C-5) with the addition of 15-Crown-5[16]"
Fig. 3
(a) Simplified description of the growth mechanism of tin nanofibres by cathodic electrodeposition[17], (b) Electrochemical properties of tin powder: 3 μm graphite∶PANa = 8∶1∶1 electrodes; the left panel shows the charge-discharge curves of the nano-Sn powder electrodes, and the right panel shows the charge-discharge curves of the micron-tin powder electrodes; the inset shows the corresponding scanning electron microscope (SEM) images of the powders[19], (c) Synthesis process of SnNGnP and charge-discharge curves[20], (d) Cycling comparison of α-Sn and β-Sn anode electrodes; the corresponding structures of the two tin crystals are shown in the inset[25], (e) Schematic illustration of the similarity between the high temperature sintering process and the self-healing of tin particles during electrochemical cycling[26], (f) Schematic representation of the cross-linking reaction of PAA and GLY to enhance the mechanical properties of the electrode[28], (g) Comparison of the cycling of micron tin anode electrodes at 0.01—1 V and 0.01—0.62 V, the crystal structures corresponding to different cycling voltage intervals are shown in the inset[29], (h) Volume capacity versus anode electrode volume expansion curves for different volume percentages of active materials in the anode electrode, where the vertical dashed lines indicate the maximum expansion rates of graphite (Gr), aluminum (Al) and silicon (Si), respectively[30], (i) Charge-discharge curves of tin foil anode for a representative number of cycles in 100 cycles of 0.1C cycling[31]"
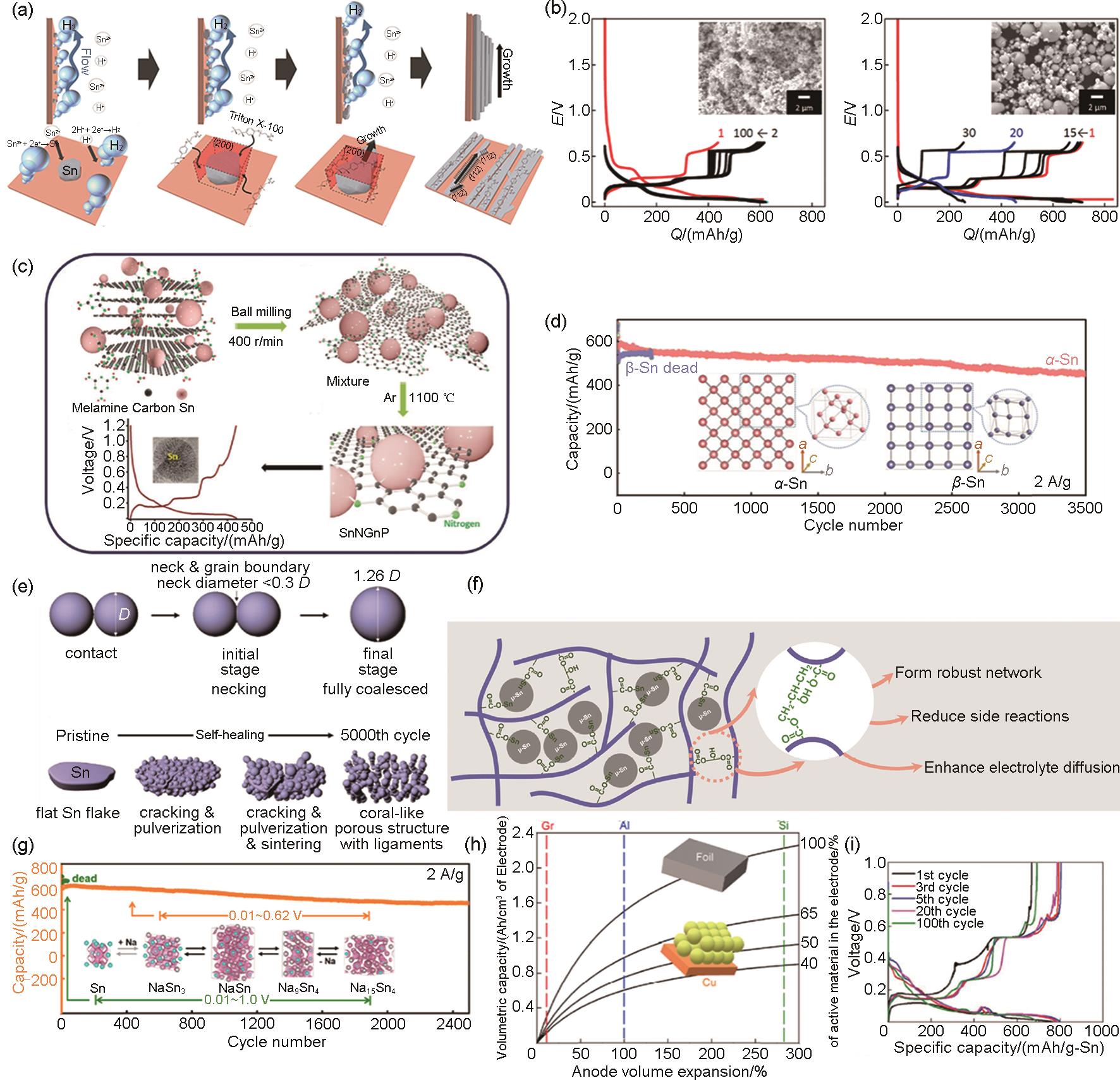
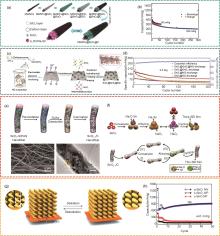
Fig. 4
(a) Schematic illustration of the preparation of MWNTs@SnO2@C composites[39]; (b) Cycling performance of MWNTs@SnO2@C composites at 50 mAh/g and 150 mAh/g current densities[39]; (c) Schematic flow illustration of the preparation of SnO2@SnS2@NG[41]; (d) Cycling performance of SnO2@SnS2@NG, SnO2@SnS2, SnO2@NG and SnS2@NG at 3 A/g[41]; (e) Schematic illustration of the synthesis process of SnO2-x /C nanofibres (upper panel) and SEM images of the morphology (lower panel)[37]; (f) Schematic of the reaction mechanism of SnO2 (upper panel) and SnO2-x /C (lower panel) electrodes during charging/discharging[37]; (g) Schematic illustration of the structural changes that occur in tin oxide nano spiral arrays fabricated directly on copper substrates after discharging and charging processes[46]; (h) Comparison of the cycling performance of electrodes made of amorphous SnO x nano spiral arrays, crystalline SnO2 nanoparticles and crystalline SnO nanoparticles[46]"
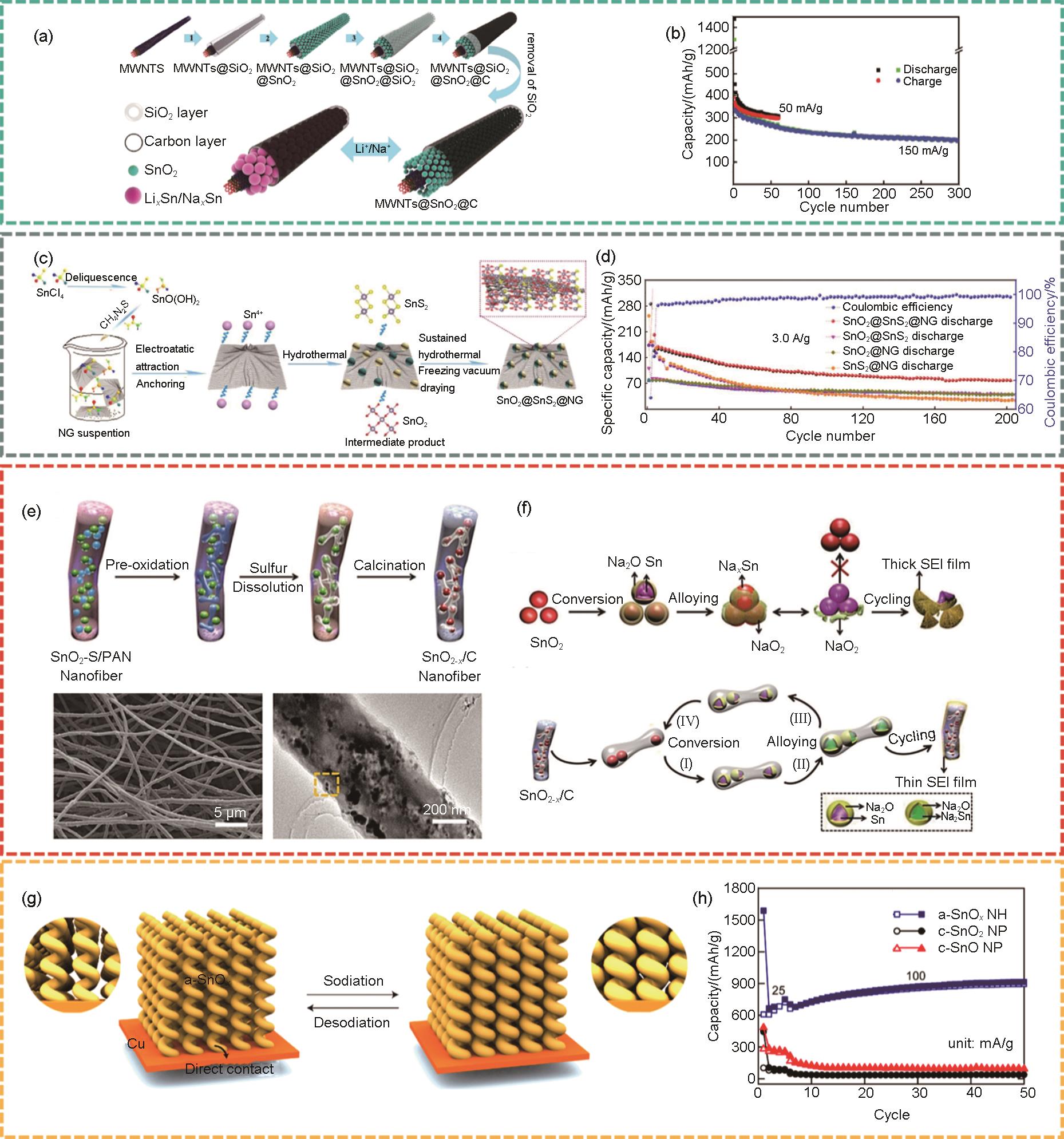
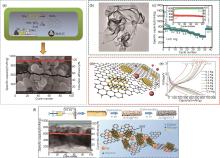
Fig. 5
(a) Schematic of the synthesis steps of SnS2/C nanospheres, nanosphere morphology, and cycling performance at 50 mA/g current density[47], (b) SEM images of SnS2 ultrathin nanosheets[48], (c) Rate performance of SnS2 ultrathin nanosheets.[48], (d) Schematic diagram of SnS2/rGO[49], (e) Rate performance of SnS2/rGO[49], (f) The upper panel shows the schematic of the synthesis of SnS@SnCF, the lower left figure shows the cycling performance of SnS@SnCF at 100 mA/g current density, the lower right figure shows the microstructure of SnS@SnCF[50]"
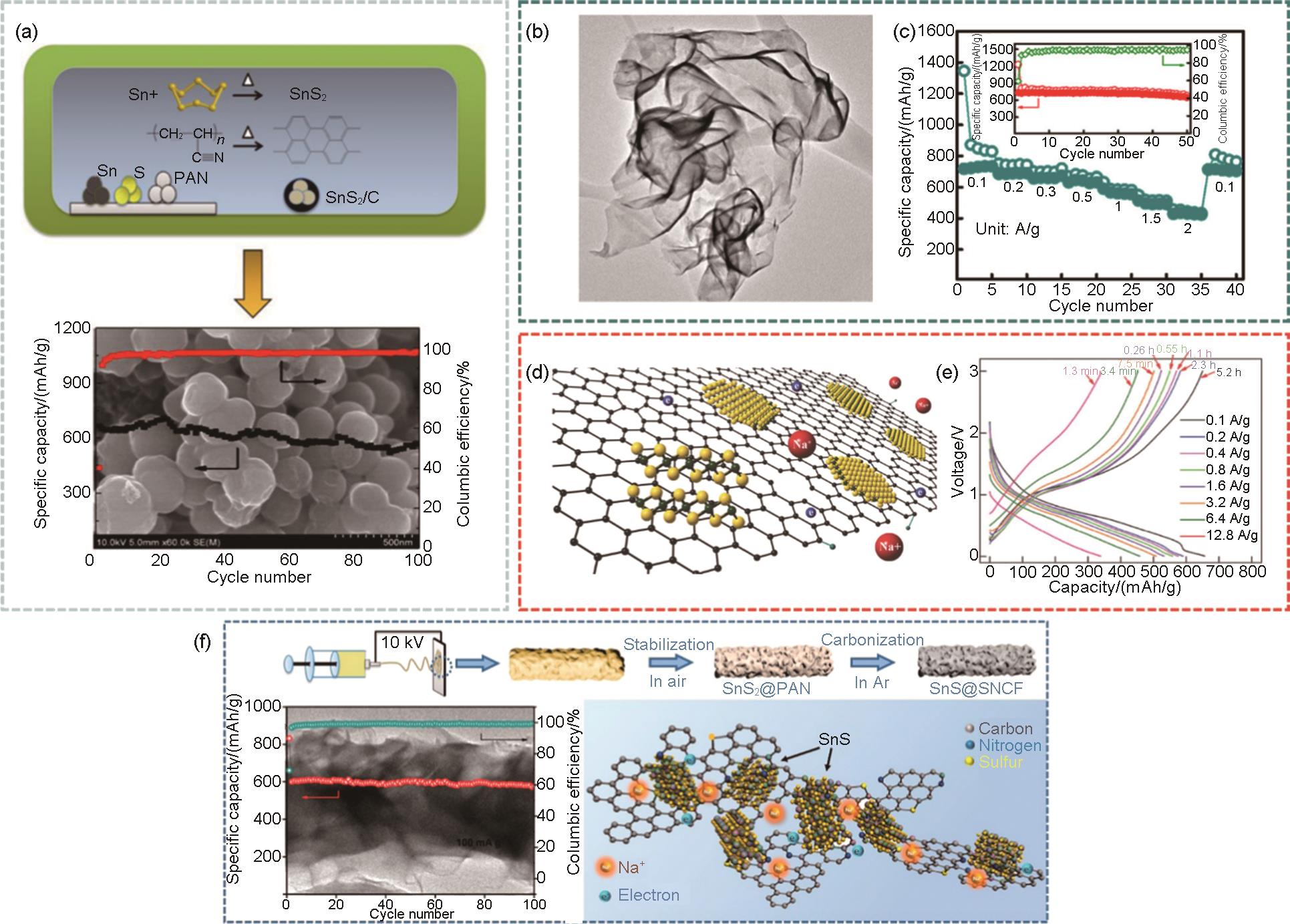
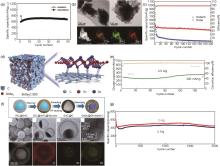
Fig. 6
(a) Cycling performance diagram of SnSe/C[51], (b) Transmission electron microscope (TEM) images of SnSe/C nanocomposites with corresponding as well as EDS elemental mapping of carbon, tin and selenium in the materials[52], (c) Comparison of the cycling performance of SnSe/C and unmodified SnSe at 0.5 A/g current density[52], (d) Schematic diagram of SnSe2 ⊂3DC buffered porous structure[53], (e) Cycling test of SnSe2 ⊂3DC at 2.5 A/g, where the initial activation was 0.13 A/g for 2 cycles[53], (f) Schematic diagram of the synthesis process of SnSe2@Se-C and SEM, TEM, high-Aangle annular dark field scanning transmission electron microscopy (HAADF-STEM) images and corresponding elemental mapping of SnSe2@Se-C[54], (g) Cycling performance of SnSe2@Se-C[54]"
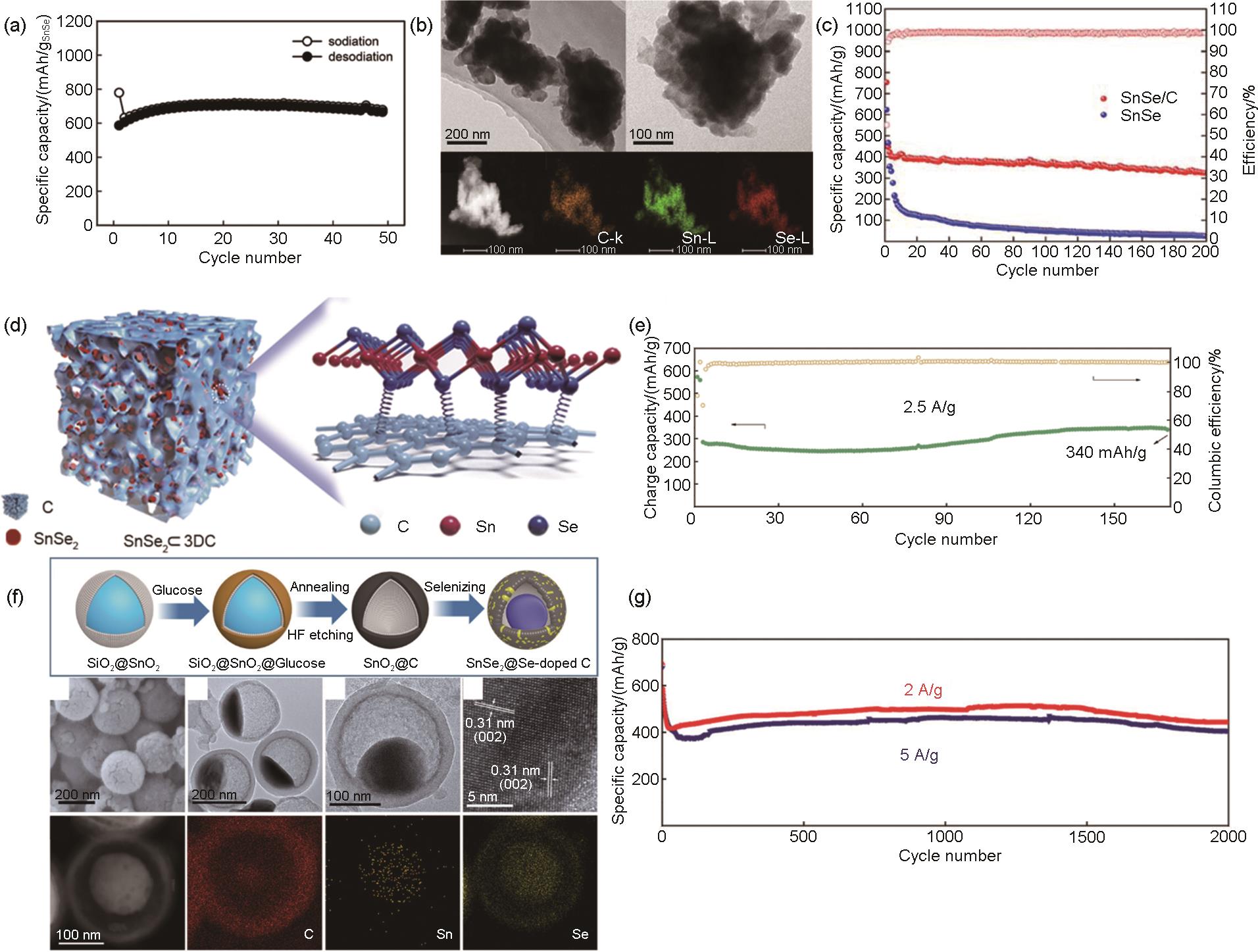

Fig. 7
(a) Comparison of cycling performance of Sn4P3 and Sn anode. TEM image of Sn4P3 in the background[56], (b) Synthesis of the Sn4P3@C yolk-shell nanocube and its structural advantages over other anode materials. Schematic diagram of the synthesis of Sn4P3@C yolk-shell nanocube. Structural advantages of the Sn4P3@C yolk-shell nanocube over Sn4P3/C and bare Sn4P3 in the process of sodation/desodation[57], (c) Long-term cycling performance of the Sn4P3@C yolk-shell nanocube electrode at 1.0 A/g and 2.0 A/g and the corresponding Coulombic efficiency[57], the long-term cycling performance of Sn4P3@HC at NaClO4/EC:PC (orange inverted triangles), NaClO4/EC:PC:FEC (red diamonds), NaPF6/EC:PC (dark cyan hexagons), NaPF6/EC:PC:FEC (green circles), NaPF6/DEGDME (black squares) and NaPF6/DEGDME:FEC (dark gray star) electrolytes in (d) cycling and (e) rate performance[58], (f) Schematic of the synthetic pathway of Sn4P3/NHC[59], (g) cycling performance of Sn4P3@HC at 1 A/g current density[60]"
| 1 | LI M H, WANG H F, LUO W, et al. Heterogeneous single-atom catalysts for electrochemical CO2 reduction reaction[J]. Advanced Materials, 2020, 32(34): e2001848. DOI:10.1002/adma.202001848. |
| 2 | CHU S, CUI Y, LIU N. The path towards sustainable energy[J]. Nature Materials, 2016, 16(1): 16-22. DOI:10.1038/nmat4834. |
| 3 | LARCHER D, TARASCON J M. Towards greener and more sustainable batteries for electrical energy storage[J]. Nature Chemistry, 2015, 7(1): 19-29. DOI:10.1038/nchem.2085. |
| 4 | USISKIN R, LU Y X, POPOVIC J, et al. Fundamentals, status and promise of sodium-based batteries[J]. Nature Reviews Materials, 2021, 6: 1020-1035. DOI:10.1038/s41578-021-00324-w. |
| 5 | HU Y S, LU Y X. 2019 Nobel prize for the Li-ion batteries and new opportunities and challenges in Na-ion batteries[J]. ACS Energy Letters, 2019, 4(11): 2689-2690. DOI:10.1021/acsenergylett. 9b02190. |
| 6 | LAO M M, ZHANG Y, LUO W B, et al. Alloy-based anode materials toward advanced sodium-ion batteries[J]. Advanced Materials, 2017, 29(48): 1700622. DOI:10.1002/adma.201700622. |
| 7 | WU X, LAN X X, HU R Z, et al. Tin-based anode materials for stable sodium storage: Progress and perspective[J]. Advanced Materials, 2022, 34(7): e2106895. DOI:10.1002/adma.202106895. |
| 8 | ZHOU L, CAO Z, WAHYUDI W, et al. Electrolyte engineering enables high stability and capacity alloying anodes for sodium and potassium ion batteries[J]. ACS Energy Letters, 2020, 5(3): 766-776. DOI:10.1021/acsenergylett.0c00148. |
| 9 | ZHANG B, ROUSSE G, FOIX D, et al. Microsized Sn as advanced anodes in glyme-based electrolyte for Na-ion batteries[J]. Advanced Materials, 2016, 28(44): 9824-9830. DOI:10.1002/adma.201603212. |
| 10 | TIAN Y, AN Y L, ZHANG B. Approaching microsized alloy anodes via solid electrolyte interphase design for advanced rechargeable batteries[J]. Advanced Energy Materials, 2023, 13(31): 2302119. DOI:10.1002/aenm.202302119. |
| 11 | HUANG J Q, GUO X Y, DU X Q, et al. Nanostructures of solid electrolyte interphases and their consequences for microsized Sn anodes in sodium ion batteries[J]. Energy & Environmental Science, 2019, 12(5): 1550-1557. DOI:10.1039/C8EE03632B. |
| 12 | WANG J W, LIU X H, MAO S X, et al. Microstructural evolution of tin nanoparticles during in situ sodium insertion and extraction[J]. Nano Letters, 2012, 12(11): 5897-5902. DOI:10.1021/nl303305c. |
| 13 | 周琳, 杨佯, 胡勇胜. 合金电极失效机制:体积膨胀?电解液分解? [J]. 储能科学与技术, 2021, 10(3): 813-20. |
| 14 | CHU C T, ZHOU L, CHENG Y, et al. Ultralow-concentration (0.1M) electrolyte for stable bulk alloy (Sn, Bi) anode in sodium-ion battery via regulating anions structure[J]. Chemical Engineering Journal, 2024, 482: 148915. DOI:10.1016/j.cej. 2024.148915. |
| 15 | ZHENG C, JI D L, YAO Q, et al. Electrostatic shielding boosts electrochemical performance of alloy-type anode materials of sodium-ion batteries[J]. Angewandte Chemie International Edition, 2023, 62(14): e202214258. DOI:10.1002/anie.202214258. |
| 16 | YAO Q, ZHENG C, JI D L, et al. Superior sodiophilicity and molecule crowding of crown ether boost the electrochemical performance of all-climate sodium-ion batteries[J]. Proceedings of the National Academy of Sciences of the United States of America, 2024, 121(27): e2312337121. DOI:10.1073/pnas. 2312337121. |
| 17 | NAM D H, KIM T H, HONG K S, et al. Template-free electrochemical synthesis of Sn nanofibers as high-performance anode materials for Na-ion batteries[J]. ACS Nano, 2014, 8(11): 11824-11835. DOI:10.1021/nn505536t. |
| 18 | LI T, GULZAR U, BAI X, et al. Insight on the failure mechanism of Sn electrodes for sodium-ion batteries: Evidence of pore formation during sodiation and crack formation during desodiation[J]. ACS Applied Energy Materials, 2019, 2(1): 860-866. DOI:10.1021/acsaem.8b01934. |
| 19 | FUKUNISHI M, HORIBA T, DAHBI M, et al. Optimizing micrometer-sized Sn powder composite electrodes for sodium-ion batteries[J]. Electrochemistry, 2019, 87(1): 70-77. DOI:10. 5796/electrochemistry.18-00069. |
| 20 | PALANISELVAM T, GOKTAS M, ANOTHUMAKKOOL B, et al. Sodium storage and electrode dynamics of tin-carbon composite electrodes from bulk precursors for sodium-ion batteries[J]. Advanced Functional Materials, 2019, 29(18): 1900790. DOI:10.1002/adfm.201900790. |
| 21 | LIU Z, ZHANG S, QIU Z P, et al. Tin nanodots derived from Sn2+/graphene quantum dot complex as pillars into graphene blocks for ultrafast and ultrastable sodium-ion storage[J]. Small, 2020, 16(38): e2003557. DOI:10.1002/smll.202003557. |
| 22 | PALANISELVAM T, BABU B, MOON H, et al. Tin-containing graphite for sodium-ion batteries and hybrid capacitors[J]. Batteries & Supercaps, 2021, 4(1): 173-182. DOI:10.1002/batt.202000196. |
| 23 | TAN M D, HAN S H, LI Z B, et al. Compact Sn/C composite realizes long-life sodium-ion batteries[J]. Nano Research, 2023, 16(3): 3804-3813. DOI:10.1007/s12274-022-4255-0. |
| 24 | SAYED S Y, KALISVAART W P, LUBER E J, et al. Stabilizing tin anodes in sodium-ion batteries by alloying with silicon[J]. ACS Applied Energy Materials, 2020, 3(10): 9950-9962. DOI:10.1021/acsaem.0c01641. |
| 25 | ZHU Y S, YAO Q, SHAO R W, et al. Microsized gray tin as a high-rate and long-life anode material for advanced sodium-ion batteries[J]. Nano Letters, 2022, 22(19): 7976-7983. DOI:10.1021/acs.nanolett.2c03334. |
| 26 | KIM C, KIM I, KIM H, et al. A self-healing Sn anode with an ultra-long cycle life for sodium-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(45): 22809-22818. DOI:10.1039/C8TA09544B. |
| 27 | PARK J H, CHOI Y S, KIM C, et al. Self-assembly of pulverized nanoparticles: An approach to realize large-capacity, long-lasting, and ultra-fast-chargeable Na-ion batteries[J]. Nano Letters, 2021, 21(21): 9044-9051. DOI:10.1021/acs.nanolett.1c02518. |
| 28 | YAO Q, ZHU Y S, ZHENG C, et al. Intermolecular cross-linking reinforces polymer binders for durable alloy-type anode materials of sodium-ion batteries[J]. Advanced Energy Materials, 2023, 13(9): 2202939. DOI:10.1002/aenm.202202939. |
| 29 | ZHU Y S, QIAN Z, SONG J, et al. Voltage-modulated structure stress for enhanced electrochemcial performances: The case of μ-Sn in sodium-ion batteries[J]. Nano Letters, 2021, 21(8): 3588-3595. DOI:10.1021/acs.nanolett.1c00489. |
| 30 | BOLES S T, TAHMASEBI M H. Are foils the future of anodes?[J]. Joule, 2020, 4(7): 1342-1346. DOI:10.1016/j.joule.2020.05.009. |
| 31 | KIM C, KIM H, SADAN M K, et al. Development and evaluation of Sn foil anode for sodium-ion batteries[J]. Small, 2021, 17(50): e2102618. DOI:10.1002/smll.202102618. |
| 32 | DIRICAN M, LU Y, GE Y Q, et al. Carbon-confined SnO2-electrodeposited porous carbon nanofiber composite as high-capacity sodium-ion battery anode material[J]. ACS Applied Materials & Interfaces, 2015, 7(33): 18387-18396. DOI:10.1021/acsami.5b04338. |
| 33 | SU D W, AHN H J, WANG G X. SnO2@graphene nanocomposites as anode materials for Na-ion batteries with superior electrochemical performance[J]. Chemical Communications, 2013, 49(30): 3131-3133. DOI:10.1039/c3cc40448j. |
| 34 | SHIMIZU M, USUI H, SAKAGUCHI H. Electrochemical Na-insertion/extraction properties of SnO thick-film electrodes prepared by gas-deposition[J]. Journal of Power Sources, 2014, 248: 378-382. DOI:10.1016/j.jpowsour.2013.09.046. |
| 35 | PATRA J, CHEN H C, YANG C H, et al. High dispersion of 1-nm SnO2 particles between graphene nanosheets constructed using supercritical CO2 fluid for sodium-ion battery anodes[J]. Nano Energy, 2016, 28: 124-134. DOI:10.1016/j.nanoen.2016.08.044. |
| 36 | OEHL N, MICHALOWSKI P, KNIPPER M, et al. Size-dependent strain of Sn/SnOx core/shell nanoparticles[J]. The Journal of Physical Chemistry C, 2014, 118(51): 30238-30243. DOI:10.1021/jp5096147. |
| 37 | MA D T, LI Y L, MI H W, et al. Robust SnO2- x nanoparticle-impregnated carbon nanofibers with outstanding electrochemical performance for advanced sodium-ion batteries[J]. Angewandte Chemie (International Ed), 2018, 57(29): 8901-8905. DOI:10.1002/anie.201802672. |
| 38 | LIANG J J, YUAN C C, LI H H, et al. Growth of SnO2 nanoflowers on N-doped carbon nanofibers as anode for Li- and Na-ion batteries[J]. Nano-Micro Letters, 2018, 10(2): 21. DOI:10.1007/s40820-017-0172-2. |
| 39 | ZHAO Y, WEI C, SUN S N, et al. Reserving interior void space for volume change accommodation: An example of cable-like MWNTs@SnO2@C composite for superior lithium and sodium storage[J]. Advanced Science, 2015, 2(6): 1500097. DOI:10.1002/advs.201500097. |
| 40 | WANG Y, SU D W, WANG C Y, et al. SnO2@MWCNT nanocomposite as a high capacity anode material for sodium-ion batteries[J]. Electrochemistry Communications, 2013, 29: 8-11. DOI:10.1016/j.elecom.2013.01.001. |
| 41 | HUANG S F, WANG M, JIA P, et al. N-graphene motivated SnO2@SnS2 heterostructure quantum dots for high performance lithium/sodium storage[J]. Energy Storage Materials, 2019, 20: 225-233. DOI:10.1016/j.ensm.2018.11.024. |
| 42 | CHEN W H, SONG K M, MI L W, et al. Synergistic effect induced ultrafine SnO2/graphene nanocomposite as an advanced lithium/sodium-ion batteries anode[J]. Journal of Materials Chemistry A, 2017, 5(20): 10027-10038. DOI:10.1039/C7TA01634D. |
| 43 | XIE X Q, CHEN S Q, SUN B, et al. 3D networked tin oxide/graphene aerogel with a hierarchically porous architecture for high-rate performance sodium-ion batteries[J]. ChemSusChem, 2015, 8(17): 2948-2955. DOI:10.1002/cssc.201500149. |
| 44 | GABRIEL E, MA C R, GRAFF K, et al. Heterostructure engineering in electrode materials for sodium-ion batteries: Recent progress and perspectives[J]. eScience, 2023, 3(5): 100139. DOI:10.1016/j.esci.2023.100139. |
| 45 | LI Z T, FENG J Z, HU H, et al. An amorphous tin-based nanohybrid for ultra-stable sodium storage[J]. Journal of Materials Chemistry A, 2018, 6(39): 18920-18927. DOI:10.1039/C8TA05390A. |
| 46 | CHOI I Y, JO C, LIM W G, et al. Amorphous tin oxide nanohelix structure based electrode for highly reversible Na-ion batteries[J]. ACS Nano, 2019, 13(6): 6513-6521. DOI:10.1021/acsnano.8b09773. |
| 47 | WANG J J, LUO C, MAO J F, et al. Solid-state fabrication of SnS2/C nanospheres for high-performance sodium ion battery anode[J]. ACS Applied Materials & Interfaces, 2015, 7(21): 11476-11481. DOI:10.1021/acsami.5b02413. |
| 48 | SUN W P, RUI X H, YANG D, et al. Two-dimensional tin disulfide nanosheets for enhanced sodium storage[J]. ACS Nano, 2015, 9(11): 11371-11381. DOI:10.1021/acsnano.5b05229. |
| 49 | ZHANG Y D, ZHU P Y, HUANG L L, et al. Few-layered SnS2 on few-layered reduced graphene oxide as Na-ion battery anode with ultralong cycle life and superior rate capability[J]. Advanced Functional Materials, 2015, 25(3): 481-489. DOI:10.1002/adfm.201402833. |
| 50 | WANG Y P, ZHANG Y F, SHI J R, et al. Tin sulfide nanoparticles embedded in sulfur and nitrogen dual-doped mesoporous carbon fibers as high-performance anodes with battery-capacitive sodium storage[J]. Energy Storage Materials, 2019, 18: 366-374. DOI:10.1016/j.ensm.2018.08.014. |
| 51 | KIM Y, KIM Y, PARK Y, et al. SnSe alloy as a promising anode material for Na-ion batteries[J]. Chemical Communications, 2015, 51(1): 50-53. DOI:10.1039/c4cc06106c. |
| 52 | ZHANG Z A, ZHAO X X, LI J. SnSe/carbon nanocomposite synthesized by high energy ball milling as an anode material for sodium-ion and lithium-ion batteries[J]. Electrochimica Acta, 2015, 176: 1296-1301. DOI:10.1016/j.electacta.2015.07.140. |
| 53 | YANG K W, ZHANG X X, SONG K M, et al. Se–C bond and reversible SEI in facile synthesized SnSe2⊂3D carbon induced stable anode for sodium-ion batteries[J]. Electrochimica Acta, 2020, 337: 135783. DOI:10.1016/j.electacta.2020.135783. |
| 54 | XIAO S H, LI Z Z, LIU J T, et al. Se C bonding promoting fast and durable Na+ storage in yolk-shell SnSe2@Se C[J]. Small, 2020, 16(41): 2002486. DOI:10.1002/smll.202002486. |
| 55 | WANG W H, ZHANG J L, YU D Y W, et al. Improving the cycling stability of Sn4P3 anode for sodium-ion battery[J]. Journal of Power Sources, 2017, 364: 420-425. DOI:10.1016/j.jpowsour. 2017.08.060. |
| 56 | KIM Y, KIM Y, CHOI A, et al. Tin phosphide as a promising anode material for Na-ion batteries[J]. Advanced Materials, 2014, 26(24): 4139-4144. DOI:10.1002/adma.201305638. |
| 57 | MA L B, YAN P J, WU S K, et al. Engineering tin phosphides@carbon yolk-shell nanocube structures as a highly stable anode material for sodium-ion batteries[J]. Journal of Materials Chemistry A, 2017, 5(32): 16994-17000. DOI:10.1039/c7ta04900e. |
| 58 | GÓMEZ-CÁMER J L, ACEBEDO B, ORTIZ-VITORIANO N, et al. Unravelling the impact of electrolyte nature on Sn4P3/C negative electrodes for Na-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(31): 18434-18441. DOI:10.1039/C9TA04288A. |
| 59 | PALANISELVAM T, MUKUNDAN C, HASA I, et al. Assessment on the use of high capacity "Sn4P3" /NHC composite electrodes for sodium-ion batteries with ether and carbonate electrolytes[J]. Advanced Functional Materials, 2020, 30(42): 2004798. DOI:10.1002/adfm.202004798. |
| 60 | WANG Y, SHI H T, NIU J R, et al. Self-healing Sn4P3@hard carbon co-storage anode for sodium-ion batteries[J]. Journal of Alloys and Compounds, 2021, 851: 156746. DOI:10.1016/j.jallcom.2020.156746. |
| [1] | Weixiang CHENG, Xingwen HUANG, Yuezhu LI, Junqi HU, Songyi LIAO, Yonggang MIN. Advances in layered metal disulfide as anode material for Na-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(10): 3062-3075. |
| [2] | Quan ZHOU, Xingguo QI, Yaxiang LU, Xiaohui RONG, Fei TANG, Weihe KONG, Kun TANG, Liquan CHEN, Yongsheng HU. The necessity of establishing Na-ion battery standards [J]. Energy Storage Science and Technology, 2020, 9(5): 1225-1233. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
