Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (12): 3836-3844.doi: 10.19799/j.cnki.2095-4239.2022.0414
• Energy Storage Materials and Devices • Previous Articles Next Articles
Bochao YANG( ), Jie LÜ, Ziwei ZHEN, Jianjun WANG, Yuxia SHEN, Yu ZHANG, Yi WANG(
), Jie LÜ, Ziwei ZHEN, Jianjun WANG, Yuxia SHEN, Yu ZHANG, Yi WANG( )
)
Received:2022-07-25
Revised:2022-08-08
Online:2022-12-05
Published:2022-12-29
Contact:
Yi WANG
E-mail:baochaoyang2022@163.com;wangyi@lut.edu.cn
CLC Number:
Bochao YANG, Jie LÜ, Ziwei ZHEN, Jianjun WANG, Yuxia SHEN, Yu ZHANG, Yi WANG. Crystallization kinetics of stearic acid and stearic acid/MXene composite phase change materials[J]. Energy Storage Science and Technology, 2022, 11(12): 3836-3844.
Table 1
Kinetic parameters of SA and SA/MXene during isothermal crystallization process"
| 样品 | 结晶过程 | Tc/℃ | t1/2/min | G/min-1 | n | K | R2 |
|---|---|---|---|---|---|---|---|
| SA | 熔融结晶 | 57 | 0.36 | 2.78 | 1.94 | 5.91 | 0.99 |
| 65 | 0.55 | 1.82 | 1.57 | 2.04 | 0.99 | ||
| 冷结晶 | 57 | 0.50 | 2.00 | 2.72 | 3.76 | 0.96 | |
| 65 | 0.46 | 2.17 | 2.51 | 4.39 | 0.99 | ||
| SA/MXene | 熔融结晶 | 73 | 0.31 | 3.23 | 2.43 | 13.07 | 0.99 |
| 81 | 0.26 | 3.85 | 1.82 | 8.37 | 0.99 | ||
| 冷结晶 | 73 | 0.35 | 2.86 | 2.14 | 6.49 | 0.99 | |
| 81 | 0.48 | 2.08 | 3.13 | 4.76 | 0.95 |

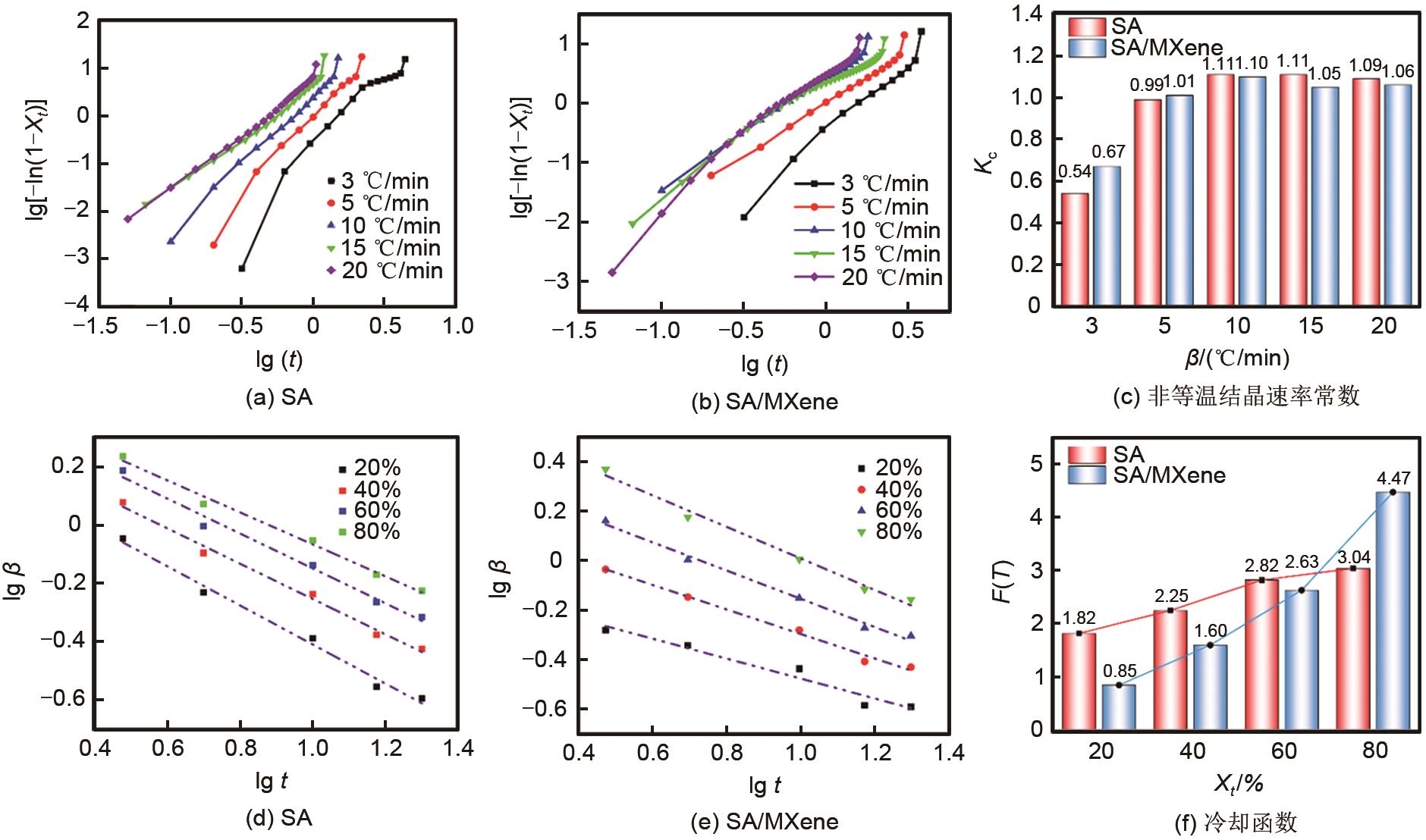
Fig. 7
The relationship between lg[-ln(1- Xt )] and lg(t) [(a),(b)], the plots of non-isothermal crystallization rate versus cooling rate (c), the relationship between cooling rate and the crystallization time [(d),(e)] and the relationship between cooling function and relative crystallinity (f) of SA and SA/MXene"
| 1 | MEHRALI M, ELSHOF J E, SHAHI M, et al. Simultaneous solar-thermal energy harvesting and storage via shape stabilized salt hydrate phase change material[J]. Chemical Engineering Journal, 2021, 405: doi: 10.1016/j.cej.2020.126624. |
| 2 | 包信和. 纳米限域及能源分子的催化转化[J]. 科学通报, 2018, 63(14): 1266-1274, 1265. |
| BAO X H. Nano confinement and catalytic conversion of energy molecules[J]. Chinese Science Bulletin, 2018, 63(14): 1266-1274, 1265. | |
| 3 | 邢晓红, 欧阳金波, 周利民, 等. 限域空间内的结晶研究进展[J]. 化学工业与工程, 2022, 39(5): 39-48. |
| XING X H, OUYANG J B, ZHOU L M, et al. Research progress of crystallization in confined space[J]. Chemical Industry and Engineering, 2022, 39(5): 39-48. | |
| 4 | UVANESH K, SAGIRI S S, SENTHILGURU K, et al. Effect of span 60 on the microstructure, crystallization kinetics, and mechanical properties of stearic acid oleogels: An in-depth analysis[J]. Journal of Food Science, 2016, 81(2): E380-E387. |
| 5 | PIELICHOWSKA K, PIELICHOWSKI K. Kinetics of isothermal and nonisothermal crystallization of poly(ethylene oxide) (PEO) in PEO/fatty acid blends[J]. Journal of Macromolecular Science, Part B, 2011, 50(9): 1714-1738. |
| 6 | 周卫兵, 张磊, 朱教群, 等. 硬脂酸/膨胀石墨复合相变储热的动力学研究[J]. 武汉理工大学学报, 2012, 34(7): 9-13. |
| ZHOU W B, ZHANG L, ZHU J Q, et al. Kinetics study of phase change on stearic acid/expanded graphite composite as heat storage material[J]. Journal of Wuhan University of Technolgy, 2012, 34(7): 9-13. | |
| 7 | HA J M, HAMILTON B D, HILLMYER M A, et al. Phase behavior and polymorphism of organic crystals confined within nanoscale chambers[J]. Crystal Growth & Design, 2009, 9(11): 4766-4777. |
| 8 | WANG L P, SUI J, ZHAI M, et al. Physical control of phase behavior of hexadecane in nanopores[J]. The Journal of Physical Chemistry C, 2015, 119(32): 18697-18706. |
| 9 | LI B X, LIU T X, HU L Y, et al. Facile preparation and adjustable thermal property of stearic acid-graphene oxide composite as shape-stabilized phase change material[J]. Chemical Engineering Journal, 2013, 215/216: 819-826. |
| 10 | LIU Z F, CHEN Z H, YU F. Preparation and characterization of microencapsulated phase change materials containing inorganic hydrated salt with silica shell for thermal energy storage[J]. Solar Energy Materials and Solar Cells, 2019, 200: doi: 10.1016/j.solmat.2019.110004 |
| 11 | KADOONO T, OGURA M. Heat storage properties of organic phase-change materials confined in the nanospace of mesoporous SBA-15 and CMK-3[J]. Physical Chemistry Chemical Physics: PCCP, 2014, 16(12): 5495-5498. |
| 12 | ZHANG Y Z, ZHENG S L, ZHU S Q, et al. Evaluation of paraffin infiltrated in various porous silica matrices as shape-stabilized phase change materials for thermal energy storage[J]. Energy Conversion and Management, 2018, 171: 361-370. |
| 13 | WANG J J, ZHANG T, SHEN Y X, et al. Polyethylene glycol/nanofibrous Kevlar aerogel composite: Fabrication, confinement effect, thermal energy storage and insulation performance[J]. Materials Today Communications, 2022, 32: doi: 10.1016/j.mtcomm.2022.104011. |
| 14 | YU K Y, LIU Y S, YANG Y Z. Review on form-stable inorganic hydrated salt phase change materials: Preparation, characterization and effect on the thermophysical properties[J]. Applied Energy, 2021, 292: doi: 10.1016/j.apenergy.2021.116845. |
| 15 | MO Z J, MO P J, YI M M, et al. Ti3C2Tx@Polyvinyl alcohol foam-supported phase change materials with simultaneous enhanced thermal conductivity and solar-thermal conversion performance[J]. Solar Energy Materials and Solar Cells, 2021, 219: doi: 10.1016/j.solmat.2020.110813 |
| 16 | 牛慧, 秦亚伟, 董金勇. 用DSC方法研究β-定向结晶聚丙烯树脂的结晶动力学[J]. 石油化工, 2014, 43(11): 1240-1245. |
| NIU H, QIN Y W, DONG J Y. Study of crystallization kinetics of β-crystalline-specified polypropylene resins by DSC[J]. Petrochemical Technolgy, 2014, 43(11): 1240-1245. | |
| 17 | WINSECK M M, CHENG H Y, SANTALA M K. Characterization of low temperature crystal growth parameters of the growth-dominated phase change materials GeSb6Te[J]. Journal of Non-Crystalline Solids, 2020, 547: doi: 10.1016/j.jnoncrysol. 2020.120317. |
| 18 | 张予东, 崔新盼, 邹易谙, 等. 聚乳酸/可分散性纳米二氧化硅复合材料等温结晶行为研究[J]. 化学研究, 2018, 29(6): 614-620. |
| ZHANG Y D, CUI X P, ZOU Y A, et al. Isothermal crystallization behavior of PLA/dispersible nano-SiO2 composites[J]. Chemical Research, 2018, 29(6): 614-620. | |
| 19 | AVRAMI M. Kinetics of phase change. I: general theory[J]. The Journal of Chemical Physics, 1939, 7(12): 1103-1112. |
| 20 | JEZIORNY A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by D.S.C[J]. Polymer, 1978, 19(10): 1142-1144. |
| 21 | OZAWA T. Kinetics of non-isothermal crystallization[J]. Polymer, 1971, 12(3): 150-158. |
| 22 | LIU T X, MO Z S, WANG S E, et al. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone)[J]. Polymer Engineering & Science, 1997, 37(3): 568-575. |
| 23 | KISSINGER H E. Reaction kinetics in differential thermal analysis[J]. Analytical Chemistry, 1957, 29(11): 1702-1706. |
| 24 | LAYACHI A, MAKHLOUF A, FRIHI D, et al. Non-isothermal crystallization kinetics and nucleation behavior of isotactic polypropylene composites with micro-talc[J]. Journal of Thermal Analysis and Calorimetry, 2019, 138(2): 1081-1095. |
| 25 | 何曼君, 张红东, 陈维孝. 高分子物理[M]. 3版. 上海: 复旦大学出版社, 2007. |
| HE M J, ZHANG H D, CHEN W X. Polymer physics[M]. 3rd. Shanghai: Fudan University Press, 2007. | |
| 26 | ZHISHEN M. A method for the non-isothermal crystallization kinetics of polymers[J]. Acta Polymerica Sinica, 2008, 1(7): 656-661. |
| 27 | MAFFEZZOLI A, KENNY J, TORRE L. On the physical dimensions of the Avrami constant[J]. Thermochimica Acta, 1995, 269/270: 185-190. |
| 28 | MORENO E, CORDOBILLA R, CALVET T, et al. Polymorphism of even saturated carboxylic acids from n-decanoic to n-eicosanoic acid [J]. New Journal of Chemistry, 2007, 31(6):947-957. |
| 29 | KANEKO F, KOBAYASHI M, KITAGAWA Y, et al. Structure of stearic acid form[J]. Acta Crystallgr. C, 1990, 46: 1490-1492. |
| 30 | YONG D. Effect of Ag nanowires on crystallization behavior of polyethylene glycol/expanded vermiculite composite phase change material[J]. Journal of Energy Storage, 2021, 34: doi: 10.1016/j.est.2020.102223. |
| 31 | ZHANG S D, WANG S S, ZHANG J, et al. Increasing phase change latent heat of stearic acid via nanocapsule interface confinement[J]. The Journal of Physical Chemistry C, 2013, 117(44): 23412-23417. |
| [1] | Tiezhu GUO, Di ZHOU, Chuanfang ZHANG. Strategies for improving MXene colloidal stability and impact on their supercapacitor performance [J]. Energy Storage Science and Technology, 2022, 11(4): 1165-1174. |
| [2] | Zan DUAN, Lingfang LI, Penghui LIU, Dongfang XIAO. Review on advanced preparation methods and energy storage mechanism of MXenes as energy storage materials [J]. Energy Storage Science and Technology, 2022, 11(3): 982-990. |
| [3] | Yuexia LI, Quanbing LIU. Application of MXene-based nanomaterials in electrocatalysis for oxygen reduction reaction [J]. Energy Storage Science and Technology, 2021, 10(6): 1918-1930. |
| [4] | MENG Qi, LIU Xiaohui, SUN Mingze, WANG Qiyang, BI Hong. Elecrochemical performance of MXene/silver nanowire supercapacitor electrode material [J]. Energy Storage Science and Technology, 2019, 8(6): 1126-1131. |
| [5] | YANG Yuehao, CHENG Xiaomin, LI Dan, LI Yuanyuan. Properties of stearic acid/modified carbon nanotube composite phase change materials [J]. Energy Storage Science and Technology, 2019, 8(4): 759-763. |
| [6] | SUN Helei, LI Yunfei, YI Ronghua, WANG Ruochong, ZHOU Aijun, SUN Yimin. Preparation and characterization of electrochemical properties of nitrogen and boron co-doped MXene composite materials [J]. Energy Storage Science and Technology, 2019, 8(1): 130-137. |
| [7] | LI Dan, CHENG Xiaomin, LI Yuanyuan. Thermal properties of a modified MOF-stearic acid composite phase change materials [J]. Energy Storage Science and Technology, 2018, 7(4): 654-660. |
| [8] | YAO Naiyuan, XIAN Cunni. Research progress of two-dimensional transition metal carbides and carbonitrides materials for fuel-cell catalysts [J]. Energy Storage Science and Technology, 2018, 7(4): 631-638. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
