Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (6): 1788-1805.doi: 10.19799/j.cnki.2095-4239.2022.0168
Previous Articles Next Articles
ZHOU Weidong( ), HUANG Qiu, XIE Xiaoxin, CHEN Kejun, LI Wei, QIU Jieshan(
), HUANG Qiu, XIE Xiaoxin, CHEN Kejun, LI Wei, QIU Jieshan( )
)
Received:2022-03-29
Revised:2022-05-11
Online:2022-06-05
Published:2022-06-13
Contact:
QIU Jieshan
E-mail:zhouwd@mail.buct.edu.cn;qiujs@mail.buct.edu.cn
CLC Number:
ZHOU Weidong, HUANG Qiu, XIE Xiaoxin, CHEN Kejun, LI Wei, QIU Jieshan. Research progress of polymer electrolyte for solid state lithium batteries[J]. Energy Storage Science and Technology, 2022, 11(6): 1788-1805.
Table 1
Ionic conductivities and electrochemical windows of PEO-SPEs"
| 类型 | 聚合物电解质 | 离子电导率/(S/cm) | 电化学窗口/V | 文献 |
|---|---|---|---|---|
| 有机-无机复合 | PEO/LiClO4 | 1.0×10-8 (35 ℃) 5×10-4 (80 ℃) | — | [ |
| PEO/LiClO4/10%TiO2 | 8×10-5 (35 ℃) 1×10-3 (80 ℃) | |||
| PEO/LiClO4/10%Al2O3 | 5×10-5 (35 ℃) 2×10-3 (80 ℃) | |||
| PEO/LiCF3SO3/5%SiO2 | 8.2×10-7 (30 ℃) 1.7×10-4 (70 ℃) | — | [ | |
| PEO/LLZTO | 2.1×10-4 (30 ℃) 5.6×10-4 (60 ℃) | 4.75 | [ | |
| PEO/LiTFSI/Al2O3 | 4.4×10-5 (30 ℃) 3.1×10-4 (60 ℃) | >4 | [ | |
| PEO/LiTFSI/LiZr2(PO4)3 | 1.2×10-4 (30 ℃) 2.1×10-3 (60 ℃) | >4.5 | ||
| PEO/LiTFSI/LLTO | 8.8×10-5 (25 ℃) | 4.5 | [ | |
| PEO/16%Ga-LLZO | 7.2×10-5 (30 ℃) 4.1×10-4 (60 ℃) | 4.6 | [ | |
| 交联或共聚 | PEO/LiFSI/30%C2epyrFSI① | 3.02×10-4 (50 ℃) | 5.1 | [ |
| PEO@AF② SPE | 6.57×10-4 (80 ℃) | 5.2 | [ | |
| 3PEO-7LATP-xBMP-TFSI③ | 2.42×10-4 (30 ℃) | 5 | [ | |
| PEO/PVP/LiClO4 | 2.31×10-6 (30 ℃) | — | [ | |
| 使用不同锂盐 | P(EO)20/LiBF4 | 6.32×10-7 (50 ℃) | — — | [ |
| P(EO)20/LiClO4 | 2.78×10-7 (50 ℃) | |||
| PEO/LiTFSI | 7.71×10-7 (30 ℃) | 3.8 | [ | |
| PEO-LiBOB | >10-6 (30 ℃) >10-4 (70 ℃) | — | [ |
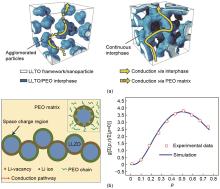
Fig. 2
(a) Schematic diagram of agglomeration of nanoparticles and 3D continuous framework in PEO/LiTFSI/LLTO composite electrolyte [33]; (b) Schematic illustration of the fast ionic conduction pathway along the space charge regions and comparison of the ionic conductivity data obtained from the Monte Carlo simulation with those acquired via the experimental measurement for the PEO:Ga-LLZO composite [34]"
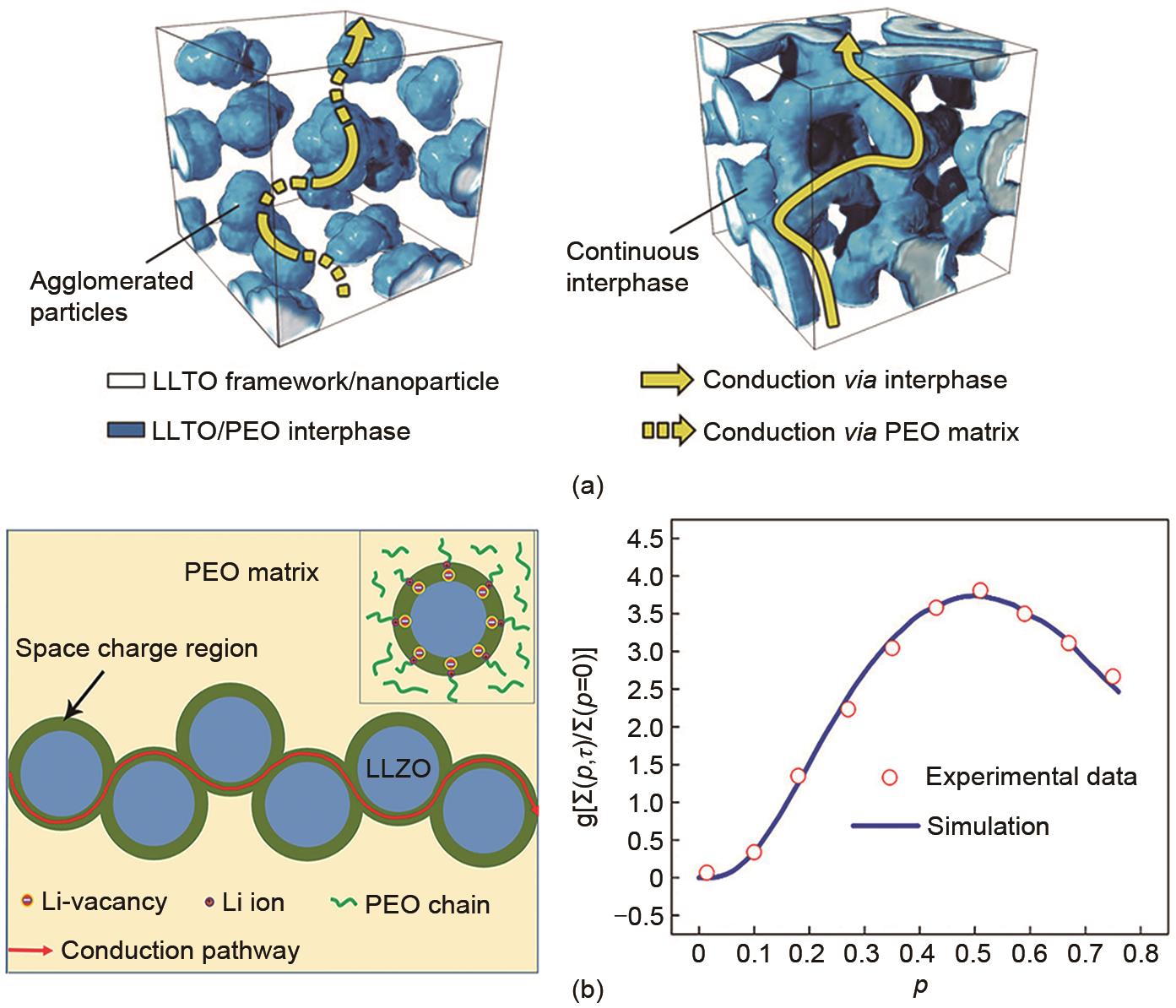
Table 2
Ionic conductivities and electrochemical windows of PS-SPE"
| 类型 | 聚合物电解质 | 离子电导率/(S/cm) | 电化学窗口/V | 文献 |
|---|---|---|---|---|
| 接枝 | VTMS-PMHS/LiPF6 | 1.12×10-3 (25 ℃) | >4.0 | [ |
| VC-PMHS/PVDF/LiTFSI | 1.55×10-4 (25 ℃) | 4.9 | [ | |
| ABPTP80/LiTFSI | 4.0×10-4 (60 ℃) | >4.5 | [ | |
| 交联 | PSi-S-CN/LiTFSI | 4.8×10-5 (60 ℃) | 3.95 | [ |
| CSPE-BFs/LiTFSI | 1.3×10-4 (60 ℃) | 5 | [ | |
| PSi-g-CN/LiClO4 | 1.15×10-5 (20 ℃) 1×10-4 (60 ℃) | 5 | [ | |
| 共聚(共混) | P(DMS-co-nEO)/LiClO4 | 2.6×10-4 (25 ℃) | 5 | [ |
| D m CS n /LiTFSI | 1.15×10-4 (25 ℃) | 4.5 | [ | |
| POEM-g-PMDS/LiCF3SO3 | 9×10-6 (25 ℃) 6×10-5 (60 ℃) | >4 | [ |
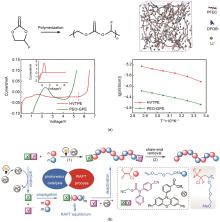
Fig. 7
(a) Polymerization process of PFEC and simulation of HVTPE system, where LiDFOB was uniformly distributed, Linear voltammetry curves of Li/PEO-GPE/SS and Li/HVTPE/SS cells, Comparison of temperature dependent ionic conductivity of PEO-GPE and HVTPE[70]; (b) Synthetic process of photo-organocatalyzed alternating copolymerization of CTFE/MEGVE and postmodification[71]"
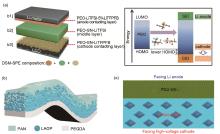
Fig. 11
(a) Schematic of differentiated salt-based multilayered solid polymer electrolyte (DSM-SPE) and Scheme representative of the interfacial stability of cathode enabled by the lower HOMO of DSM-SPE and CEI[78]; (b) Schematic diagram of the HMSE [79]; (c) Schematic diagram of the laminated dual-polymer/polymer-ceramic composite electrolyte LDPPCCE [80]"
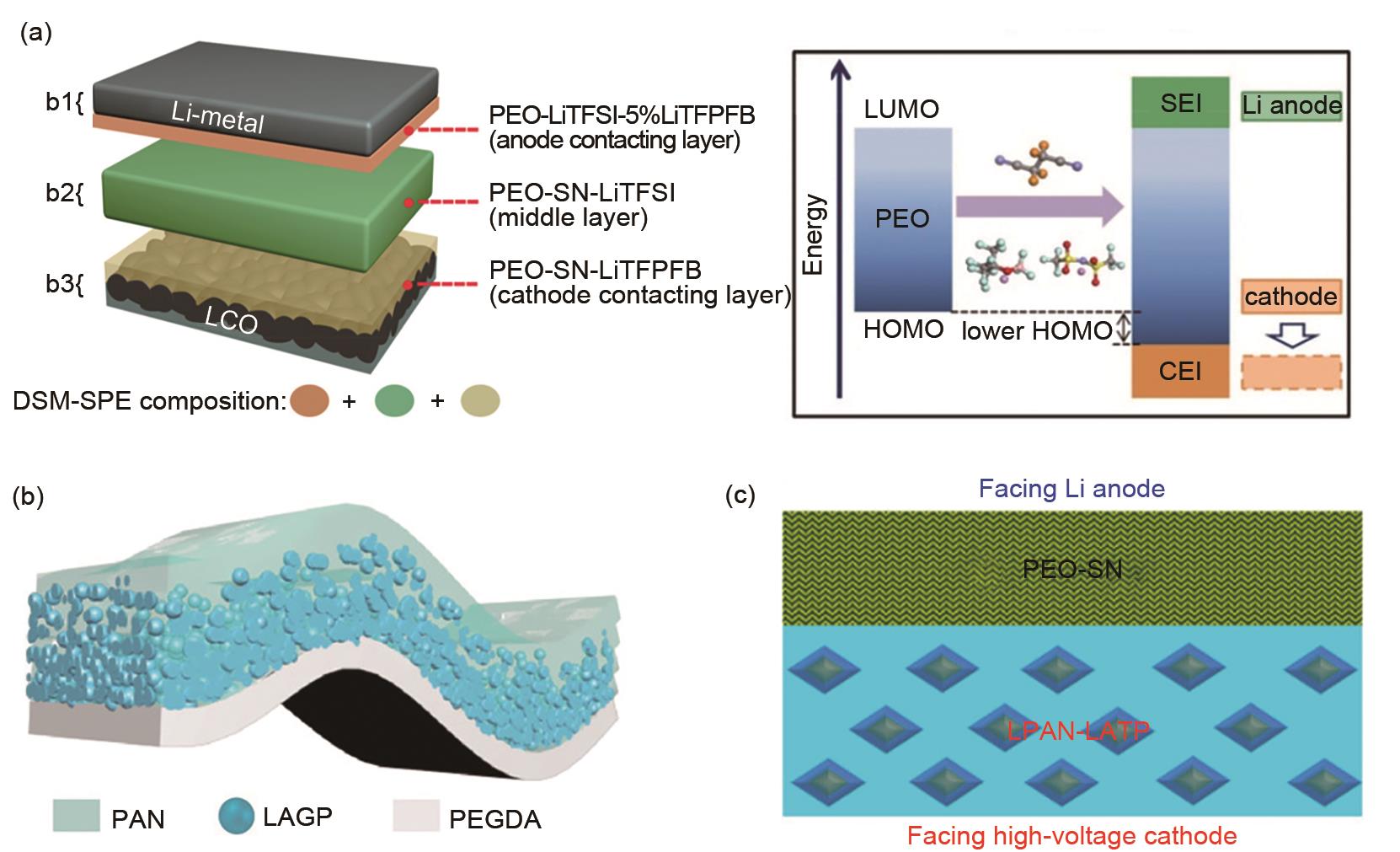

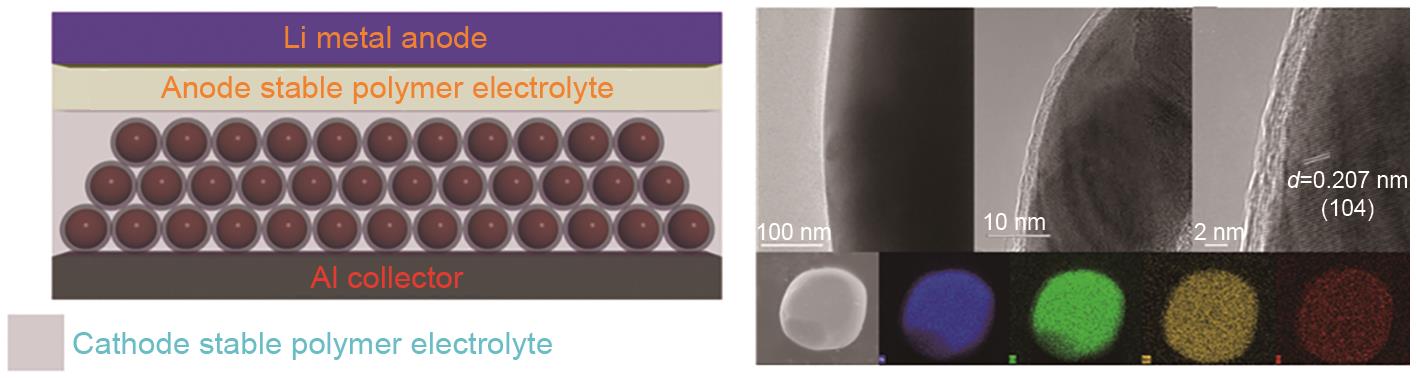
Fig. 12
The cross-section structure of a Li-polymer all-solid-state cell with cathode stable polymer as cathode bind, transmission electron microscope (TEM) and high-resolution TEM (HRTEM) images of TiO2-coated NCM particles in different magnifications, SEM and elemental mapping images of TiO2-coated NCM particles, Ni, Co, Mn, Ti[81]"
| 1 | LI H. Practical evaluation of Li-ion batteries[J]. Joule, 2019, 3(4): 911-914. |
| 2 | YE H, XIN S, YIN Y X, et al. Stable Li plating/stripping electrochemistry realized by a hybrid Li reservoir in spherical carbon granules with 3D conducting skeletons[J]. Journal of the American Chemical Society, 2017, 139(16): 5916-5922. |
| 3 | ZHOU B H, HE D, HU J, et al. A flexible, self-healing and highly stretchable polymer electrolyte via quadruple hydrogen bonding for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(25): 11725-11733. |
| 4 | ZHAO N, KHOKHAR W, BI Z J, et al. Solid garnet batteries[J]. Joule, 2019, 3(5): 1190-1199. |
| 5 | CHEN S J, XIE D J, LIU G Z, et al. Sulfide solid electrolytes for all-solid-state lithium batteries: Structure, conductivity, stability and application[J]. Energy Storage Materials, 2018, 14: 58-74. |
| 6 | XU J R, LI Y X, LU P S, et al. Water-stable sulfide solid electrolyte membranes directly applicable in all-solid-state batteries enabled by superhydrophobic Li+-conducting protection layer[J]. Advanced Energy Materials, 2022, 12(2): doi:10.10021aenm.202102348. |
| 7 | LI X N, LIANG J W, LUO J, et al. Air-stable Li3InCl6 electrolyte with high voltage compatibility for all-solid-state batteries[J]. Energy & Environmental Science, 2019, 12(9): 2665-2671. |
| 8 | CHEN G H, YE L, ZHANG K, et al. Hyperbranched polyether boosting ionic conductivity of polymer electrolytes for all-solid-state sodium ion batteries[J]. Chemical Engineering Journal, 2020, 394: doi:10.1016/j.cej.2020.124885 |
| 9 | FENTON D E, PARKER J M, WRIGHT P V. Complexes of alkali metal ions with poly(ethylene oxide)[J]. Polymer, 1973, 14(11): 589. |
| 10 | BERTHIER C, GORECKI W, MINIER M, et al. Microscopic investigation of ionic conductivity in alkali metal salts-poly(ethylene oxide) adducts[J]. Solid State Ionics, 1983, 11(1): 91-95. |
| 11 | NAGAOKA K, NARUSE H, SHINOHARA I, et al. High ionic conductivity in poly (dimethyl siloxane-co-ethylene oxide) dissolving lithium perchlorate[J]. Journal of Polymer Science: Polymer Letters Edition, 1984, 22(12): 659-663. |
| 12 | WATANABE M, TOGO M, SANUI K, et al. Ionic conductivity of polymer complexes formed by poly (β-propiolactone) and lithium perchlorate[J]. Macromolecules, 1984, 17(12): 2908-2912. |
| 13 | WATANABE M, TOGO M, SANUI K, et al. Ionic conductivity of polymer complexes formed by poly (β-propiolactone) and lithium perchlorate[J]. Macromolecules, 1984, 17(12): 2908-2912. |
| 14 | BANNISTER D J, DAVIES G R, WARD I M, et al. Ionic conductivities of poly (methoxy polyethylene glycol monomethacrylate) complexes with LiSO3CH3[J]. Polymer, 1984, 25(11): 1600-1602. |
| 15 | ALAMGIR M, MOULTON R D, ABRAHAM K M. Li+-conductive polymer electrolytes derived from poly (1,3-dioxolane) and polytetrahydrofuran[J]. Electrochimica Acta, 1991, 36(5/6): 773-782. |
| 16 | BLONSKY P M, SHRIVER D F, AUSTIN P, et al. Polyphosphazene solid electrolytes[J]. Journal of the American Chemical Society, 1984, 106(22): 6854-6855. |
| 17 | WEI X Y, SHRIVER D F. Highly conductive polymer electrolytes containing rigid polymers[J]. Chemistry of Materials, 1998, 10(9): 2307-2308. |
| 18 | SMITH M J, SILVA M M, CERQUEIRA S, et al. Preparation and characterization of a lithium ion conducting electrolyte based on poly (trimethylene carbonate)[J]. Solid State Ionics, 2001, 140(3/4): 345-351. |
| 19 | YU X Y, XIAO M, WANG S J, et al. Fabrication and characterization of PEO/PPC polymer electrolyte for lithium-ion battery[J]. Journal of Applied Polymer Science, 2010, 115(5): 2718-2722. |
| 20 | HU P, DUAN Y L, HU D P, et al. Rigid-flexible coupling high ionic conductivity polymer electrolyte for an enhanced performance of LiMn2O4/graphite battery at elevated temperature[J]. ACS Applied Materials & Interfaces, 2015, 7(8): 4720-4727. |
| 21 | ZHOU W D, WANG Z X, PU Y, et al. Double-layer polymer electrolyte for high-voltage all-solid-state rechargeable batteries[J]. Advanced Materials, 2019, 31(4): doi:10.10021adma.201805574.. |
| 22 | 刘如亮, 高兴远, 尹伟, 等. PVDF-HFP基凝胶固态聚合物电解质的合成与锂离子电池性能[J]. 储能科学与技术, 2021, 10(6): 2077-2081. |
| LIU R L, GAO X Y, YIN W, et al. Synthesis of PVDF-HFP based gel polymer electrolyte and study of lithium ion battery performance[J]. Energy Storage Science and Technology, 2021, 10(6): 2077-2081. | |
| 23 | XUE Z G, HE D, XIE X L. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2015, 3(38): 19218-19253. |
| 24 | MYUNG S T, MAGLIA F, PARK K J, et al. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives[J]. ACS Energy Letters, 2017, 2(1): 196-223. |
| 25 | FENG J N, WANG L, CHEN Y J, et al. PEO based polymer-ceramic hybrid solid electrolytes: A review[J]. Nano Convergence, 2021, 8(1): 2. |
| 26 | QUARTARONE E, MUSTARELLI P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives[J]. Chemical Society Reviews, 2011, 40(5): 2525-2540. |
| 27 | MEYER W H. Polymer electrolytes for lithium-ion batteries[J]. Advanced Materials, 1998, 10(6): 439-448. |
| 28 | CROCE F, APPETECCHI G B, PERSI L, et al. Nanocomposite polymer electrolytes for lithium batteries[J]. Nature, 1998, 394(6692): 456-458. |
| 29 | KHURANA R, SCHAEFER J L, ARCHER L A, et al. Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: A new approach for practical lithium-metal polymer batteries[J]. Journal of the American Chemical Society, 2014, 136(20): 7395-7402. |
| 30 | APPETECCHI G B, CROCE F, HASSOUN J, et al. Hot-pressed, dry, composite, PEO-based electrolyte membranes: I. ionic conductivity characterization[J]. Journal of Power Sources, 2003, 114(1): 105-112. |
| 31 | ZHANG J X, ZHAO N, ZHANG M, et al. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide[J]. Nano Energy, 2016, 28: 447-454. |
| 32 | WU N, CHIEN P H, LI Y T, et al. Fast Li+conduction mechanism and interfacial chemistry of a NASICON/polymer composite electrolyte[J]. Journal of the American Chemical Society, 2020, 142(5): 2497-2505. |
| 33 | BAE J, LI Y T, ZHANG J, et al. A 3D nanostructured hydrogel-framework-derived high-performance composite polymer lithium-ion electrolyte[J]. Angewandte Chemie International Edition, 2018, 57(8): 2096-2100. |
| 34 | LI Z, HUANG H M, ZHU J K, et al. Ionic conduction in composite polymer electrolytes: Case of PEO: Ga-LLZO composites[J]. ACS Applied Materials & Interfaces, 2019, 11(1): 784-791. |
| 35 | FANG Z Q, ZHAO M, PENG Y, et al. Organic ionic plastic crystal enhanced interface compatibility of PEO-based solid polymer electrolytes for lithium-metal batteries[J]. Solid State Ionics, 2021, 373: doi:10.1016/j.ssi.2021.115806. |
| 36 | ZENG F Y, SUN Y Y, HUI B, et al. Three-dimensional porous alginate fiber membrane reinforced PEO-based solid polymer electrolyte for safe and high-performance lithium ion batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(39): 43805-43812. |
| 37 | ZHANG D C, XU X J, HUANG X Y, et al. A flexible composite solid electrolyte with a highly stable interphase for dendrite-free and durable all-solid-state lithium metal batteries[J]. Journal of Materials Chemistry A, 2020, 8(35): 18043-18054. |
| 38 | KESAVAN K, MATHEW C M, RAJENDRAN S, et al. Solid Polymer Blend Electrolyte Based on Poly (ethylene oxide) and Poly (vinyl pyrrolidone) for Lithium Secondary Batteries[J]. Brazilian Journal of Physics, 2015, 45(1): 19-27. |
| 39 | CHOUDHARY S, SENGWA R J. Effect of different anions of lithium salt and MMT nanofiller on ion conduction in melt-compounded PEO-LiX-MMT electrolytes[J]. Ionics, 2012, 18(4): 379-384. |
| 40 | ZHANG N, HE J W, HAN W M, et al. Composite solid electrolyte PEO/SN/LiAlO2 for a solid-state lithium battery[J]. Journal of Materials Science, 2019, 54(13): 9603-9612. |
| 41 | APPETECCHI G B, ZANE D, SCROSATI B. PEO-based electrolyte membranes based on LiBC4O8 salt[J]. Journal of the Electrochemical Society, 2004, 151(9): A1369. |
| 42 | AIHARA Y, KURATOMI J, BANDO T, et al. Investigation on solvent-free solid polymer electrolytes for advanced lithium batteries and their performance[J]. Journal of Power Sources, 2003, 114(1): 96-104. |
| 43 | NAVA D P, GUZMÁN G, VAZQUEZ-ARENAS J, et al. An experimental and theoretical correlation to account for the effect of LiPF6 concentration on the ionic conductivity of poly (poly (ethylene glycol) methacrylate)[J]. Solid State Ionics, 2016, 290: 98-107. |
| 44 | CARDOSO J, SORIA-ARTECHE O, VÁZQUEZ G, et al. Synthesis and characterization of zwitterionic polymers with a flexible lateral chain[J]. The Journal of Physical Chemistry C, 2010, 114(33): 14261-14268. |
| 45 | WANG S, ZHANG L, ZENG Q H, et al. Cellulose microcrystals with brush-like architectures as flexible all-solid-state polymer electrolyte for lithium-ion battery[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(8): 3200-3207. |
| 46 | KALE S B, NIRMALE T C, KHUPSE N D, et al. Cellulose-derived flame-retardant solid polymer electrolyte for lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(4): 1559-1567. |
| 47 | MUNCH ELMÉR A, JANNASCH P. Solid electrolyte membranes from semi-interpenetrating polymer networks of PEG-grafted polymethacrylates and poly(methyl methacrylate)[J]. Solid State Ionics, 2006, 177(5/6): 573-579. |
| 48 | LIU Q, CAI B Y, LI S, et al. Long-cycling and safe lithium metal batteries enabled by the synergetic strategy of ex situ anodic pretreatment and an in-built gel polymer electrolyte[J]. Journal of Materials Chemistry A, 2020, 8(15): 7197-7204. |
| 49 | MA Q, YUE J P, FAN M, et al. Formulating the electrolyte towards high-energy and safe rechargeable lithium-metal batteries[J]. Angewandte Chemie International Edition, 2021, 60(30): 16554-16560. |
| 50 | LI W, GAO J, TIAN H Y, et al. SnF2-catalyzed formation of polymerized dioxolane as solid electrolyte and its thermal decomposition behavior[J]. Angewandte Chemie International Edition, 2022, 61(6): doi:10.1002/ange.202114805. |
| 51 | KUO S W, CHANG F C. POSS related polymer nanocomposites[J]. Progress in Polymer Science, 2011, 36(12): 1649-1696. |
| 52 | WANG Q L, ZHANG H R, CUI Z L, et al. Siloxane-based polymer electrolytes for solid-state lithium batteries[J]. Energy Storage Materials, 2019, 23: 466-490. |
| 53 | LONG L Z, WANG S J, XIAO M, et al. Polymer electrolytes for lithium polymer batteries[J]. Journal of Materials Chemistry A, 2016, 4(26): 10038-10069. |
| 54 | WALKOWIAK M, SCHROEDER G, GIERCZYK B, et al. New lithium ion conducting polymer electrolytes based on polysiloxane grafted with Si-tripodand centers[J]. Electrochemistry Communications, 2007, 9(7): 1558-1562. |
| 55 | LIN Y, LI J, LAI Y Q, et al. A wider temperature range polymer electrolyte for all-solid-state lithium ion batteries[J]. RSC Advances, 2013, 3(27): 10722. |
| 56 | SHIM J, KIM L, KIM H J, et al. All-solid-state lithium metal battery with solid polymer electrolytes based on polysiloxane crosslinked by modified natural Gallic acid[J]. Polymer, 2017, 122: 222-231. |
| 57 | FU C Y, IACOB M, SHEIMA Y, et al. A highly elastic polysiloxane-based polymer electrolyte for all-solid-state lithium metal batteries[J]. Journal of Materials Chemistry A, 2021, 9(19): 11794-11801. |
| 58 | HONG D G, BAIK J H, KIM S, et al. Solid polymer electrolytes based on polysiloxane with anion-trapping boron moieties for all-solid-state lithium metal batteries[J]. Polymer, 2022, 240: doi:10.1016/j.polymer.2022.124517. |
| 59 | LEE Y S, SONG G S, KANG Y K, et al. The polymer electrolyte based on polysiloxane containing both alkyl cyanide and oligo ethylene oxide pendants[J]. Electrochimica Acta, 2004, 50(2/3): 311-316. |
| 60 | FONSECA C P, NEVES S. Characterization of polymer electrolytes based on poly(dimethyl siloxane-co-ethylene oxide)[J]. Journal of Power Sources, 2002, 104(1): 85-89. |
| 61 | WANG F M, HU C C, LO S C, et al. The investigation of electrochemical properties and ionic motion of functionalized copolymer electrolytes based on polysiloxane[J]. Solid State Ionics, 2009, 180(4/5): 405-411. |
| 62 | TRAPA P E, WON Y Y, MUI S C, et al. Rubbery graft copolymer electrolytes for solid-state, thin-film lithium batteries[J]. Journal of the Electrochemical Society, 2005, 152(1): A1. |
| 63 | CHEN L, FAN L Z. Dendrite-free Li metal deposition in all-solid-state lithium sulfur batteries with polymer-in-salt polysiloxane electrolyte[J]. Energy Storage Materials, 2018, 15: 37-45. |
| 64 | FISCHER F, HAHN T, BÄSSLER H, et al. Measuring reduced C60 diffusion in crosslinked polymer films by optical spectroscopy[J]. Advanced Functional Materials, 2014, 24(39): 6172-6177. |
| 65 | 王星星, 宋子钰, 吴浩, 等. 固态聚合物电解质导电锂盐的研究进展[J]. 储能科学与技术, 2022, 11(4): 1226-1235. |
| WANG X X, SONG Z Y, WU H, et al. Advances in conducting lithium salts for solid polymer electrolytes[J]. Energy Storage Science and Technology, 2022, 11(4): 1226-1235. | |
| 66 | FONSECA C P, ROSA D S, GABOARDI F, et al. Development of a biodegradable polymer electrolyte for rechargeable batteries[J]. Journal of Power Sources, 2006, 155(2): 381-384. |
| 67 | LIN C K, WU I D. Investigating the effect of interaction behavior on the ionic conductivity of Polyester/LiClO4 blend systems[J]. Polymer, 2011, 52(18): 4106-4113. |
| 68 | ZHANG J J, ZHAO J H, YUE L P, et al. Safety-reinforced poly(propylene carbonate)-based all-solid-state polymer electrolyte for ambient-temperature solid polymer lithium batteries[J]. Advanced Energy Materials, 2015, 5(24): doi:10.1002/aenm.201501082. |
| 69 | CHAI J C, LIU Z H, MA J, et al. In situ generation of poly (vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries[J]. Advanced Science, 2017, 4(2): doi:10.1002/adv.201600377. |
| 70 | LIU J, SHEN X W, ZHOU J Q, et al. Nonflammable and high-voltage-tolerated polymer electrolyte achieving high stability and safety in 4.9 V-class lithium metal battery[J]. ACS Applied Materials & Interfaces, 2019, 11(48): 45048-45056. |
| 71 | MA M Y, SHAO F, WEN P, et al. Designing weakly solvating solid main-chain fluoropolymer electrolytes: Synergistically enhancing stability toward Li anodes and high-voltage cathodes[J]. ACS Energy Letters, 2021, 6(12): 4255-4264. |
| 72 | CUI Y Y, CHAI J C, DU H P, et al. Facile and reliable in situ polymerization of poly(ethylcyanoacrylate)-based polymer electrolytes toward flexible lithium batteries[J]. ACS Applied Materials Interfaces, 2017, 9(10): 8737-8741. |
| 73 | SUN H, XIE X X, HUANG Q, et al. Fluorinated poly-oxalate electrolytes stabilizing both anode and cathode interfaces for all-solid-state Li/NMC811 batteries[J]. Angewandte Chemie International Edition, 2021, 60(33): 18335-18343. |
| 74 | LU J Z, ZHOU J H, CHEN R S, et al. 4.2 V poly (ethylene oxide)-based all-solid-state lithium batteries with superior cycle and safety performance[J]. Energy Storage Materials, 2020, 32: 191-198. |
| 75 | QIU J L, LIU X Y, CHEN R S, et al. Enabling stable cycling of 4.2 V high-voltage all-solid-state batteries with PEO-based solid electrolyte[J]. Advanced Functional Materials, 2020, 30(22): doi:10.1002/adfm.201909392. |
| 76 | QIU J L, YANG L F, SUN G C, et al. A stabilized PEO-based solid electrolyte via a facile interfacial engineering method for a high voltage solid-state lithium metal battery[J]. Chemical Communications, 2020, 56: 5633-5636. |
| 77 | ZHOU W D, WANG Z X, PU Y, et al. Double-layer polymer electrolyte for high-voltage all-solid-state rechargeable batteries[J]. Advanced Materials, 2019, 31(4): doi:10.1002/adma.201805574. |
| 78 | WANG C, WANG T, WANG L, et al. Differentiated lithium salt design for multilayered PEO electrolyte enables a high‐voltage solid‐state lithium metal battery[J]. Advanced Science, 2019, 6: doi:10.1002/adv.201901036. |
| 79 | DUAN H, FAN M, CHEN W P, et al. Extended electrochemical window of solid electrolytes via heterogeneous multilayered structure for high-voltage lithium metal batteries[J]. Advanced Materials, 2019, 31(12): doi:10.10021/adma.201807789. |
| 80 | YU X W, LI J Y, MANTHIRAM A. Rational design of a laminated dual-polymer/polymer-ceramic composite electrolyte for high-voltage all-solid-state lithium batteries[J]. ACS Materials Letters, 2020, 2(4): 317-324. |
| 81 | PAN X Y, SUN H, WANG Z X, et al. High voltage stable polyoxalate catholyte with cathode coating for all-solid-state Li-metal/NMC622 batteries[J]. Advanced Energy Materials, 2020, 10(42): doi:10.1002/aenm.202002416. |
| [1] | Yun TANG, Fang YUE, Kaimo GUO, Lanchun LI, Wangsong KE, Wei CHEN. Analysis of the development trend and the innovation ability of an all-solid-state lithium battery technology [J]. Energy Storage Science and Technology, 2022, 11(1): 359-369. |
| [2] | Saisai ZHANG, Hailei ZHAO. Electrode/electrolyte interfaces in Li7La3Zr2O12 garnet-based solid-state lithium metal battery: Challenges and progress [J]. Energy Storage Science and Technology, 2021, 10(3): 863-871. |
| [3] | Yanming CUI, Zhihua ZHANG, Yuanqiao HUANG, Jiu LIN, Xiayin YAO, Xiaoxiong XU. Prototype all-solid-state battery electrodes preparation and assembly technology [J]. Energy Storage Science and Technology, 2021, 10(3): 836-847. |
| [4] | HUANG Xiao, WU Linbin, HUANG Zhen, LIN Jiu, XU Xiaoxiong. Characterization and testing of key electrical and electrochemical properties of lithium-ion solid electrolytes [J]. Energy Storage Science and Technology, 2020, 9(2): 479-500. |
| [5] | WU Jinghua, YAO Xiayin. Recent progress in interfaces of all-solid-state lithium batteries based on sulfide electrolytes [J]. Energy Storage Science and Technology, 2020, 9(2): 501-514. |
| [6] | ZHANG Jianjun, DONG Tiantian, YANG Jinfeng, ZHANG Min, CUI Guanglei. Research progress, challenge and perspective of all-solid-state polymer lithium batteries [J]. Energy Storage Science and Technology, 2018, 7(5): 861-868. |
| [7] | XIA Qiuying, SUN Shuo, XU Jing, ZAN Feng, YUE Jili, XIA Hui. All-solid-state thin film lithium batteries [J]. Energy Storage Science and Technology, 2018, 7(4): 565-574. |
| [8] | DU Aobing, CHAI Jingchao, ZHANG Jianjun, LIU Zhihong, CUI Guanglei. All-solid-state lithium-ion batteries based on polymer electrolytes: State of the art, challenges and future trends [J]. Energy Storage Science and Technology, 2016, 5(5): 627-648. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
