Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (9): 2900-2920.doi: 10.19799/j.cnki.2095-4239.2021.0595
• Special Issue for the 10th Anniversary • Previous Articles Next Articles
Zhizhan LI( ), Jinlei QIN, Jianing LIANG, Zhengrong LI, Rui WANG, Deli WANG
), Jinlei QIN, Jianing LIANG, Zhengrong LI, Rui WANG, Deli WANG
Received:2021-11-11
Revised:2021-12-27
Online:2022-09-05
Published:2022-08-30
Contact:
Deli WANG
E-mail:944264721@qq.com
CLC Number:
Zhizhan LI, Jinlei QIN, Jianing LIANG, Zhengrong LI, Rui WANG, Deli WANG. High-nickel ternary layered cathode materials for lithium-ion batteries: Research progress, challenges and improvement strategies[J]. Energy Storage Science and Technology, 2022, 11(9): 2900-2920.

Fig. 2
(a)-(c) Li[Ni x Co y Mn1-x-y ]O2 (x=0.6, 0.8, 0.9, 0.95, 1) cathodes: Initial charge-discharge profiles, cycling performance and variations of c-axis lattice parameters with cell voltage[17]; (d) Schematic diagram of structural degradation of NCM materials after cycling[18]; (e) Magnified cross-sectional SEM images of the first charged state of the NC90, NCM90, and NM90 cathodes[19]"
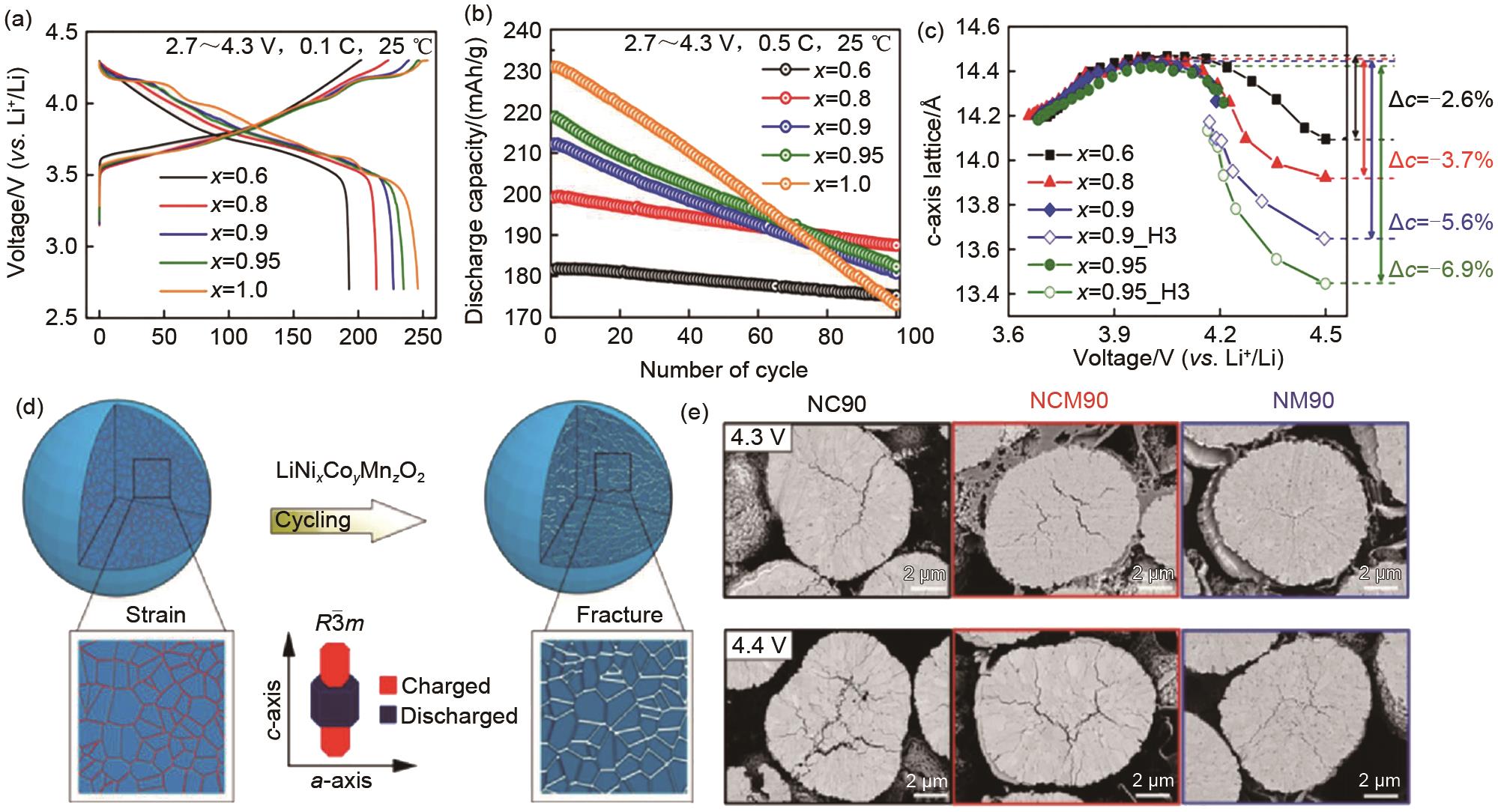
Fig. 9
(a) Schematic illustration of particle fracture in polycrystalline and single-crystal NCM materials[100]; SEM images of sintered SC-NCM; (b) Cross-sectional SEM images of single crystal and polycrystal NCM before and after 600 cycles; (c) Schematic illustration of crack evolution and the internal morphological difference for polycrystalline and single-crystal NCM cathodes during prolong cycling; (d) Cycling performance of polycrystalline and single-crystal NCM cathodes a coin-cell[103]"
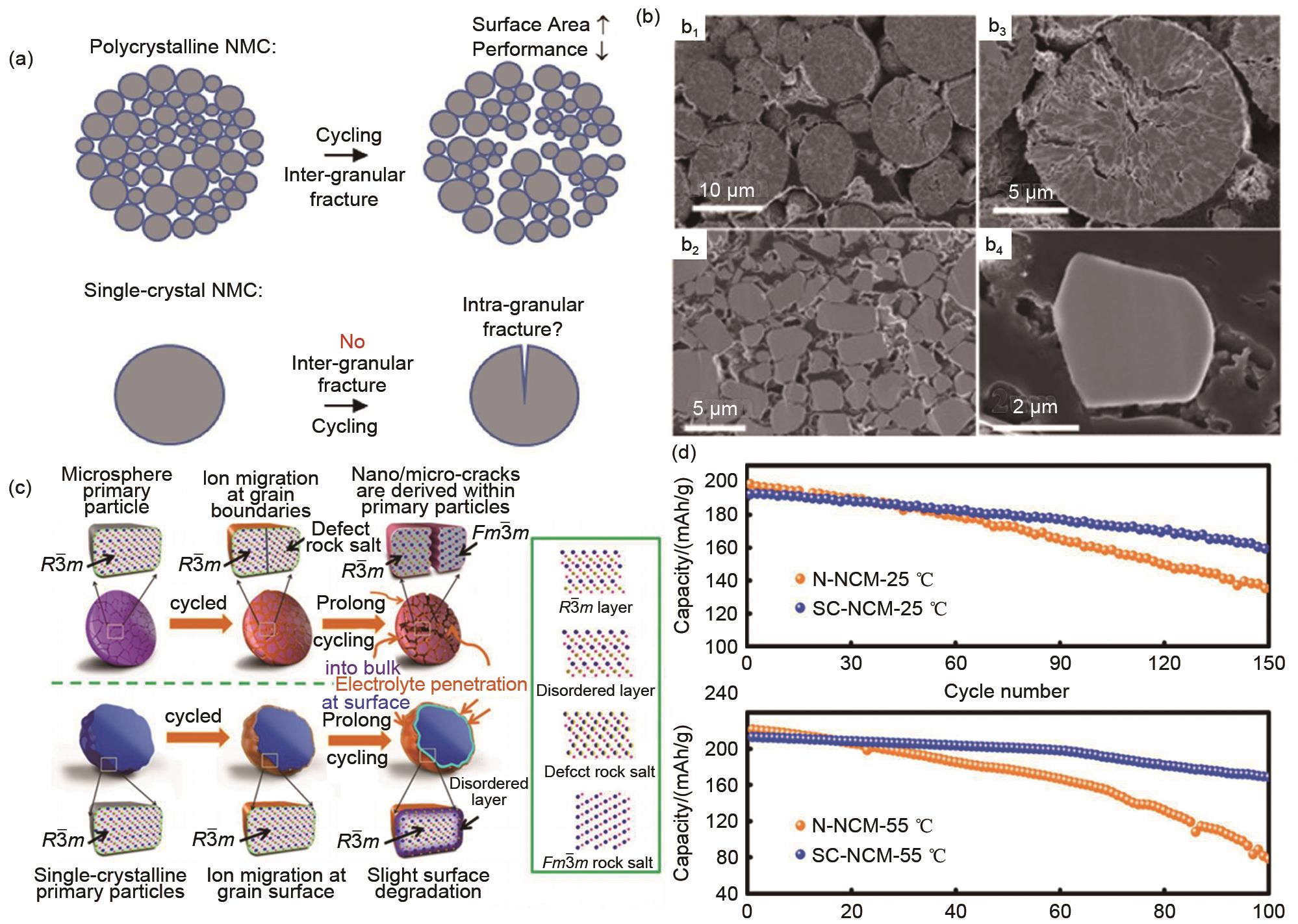

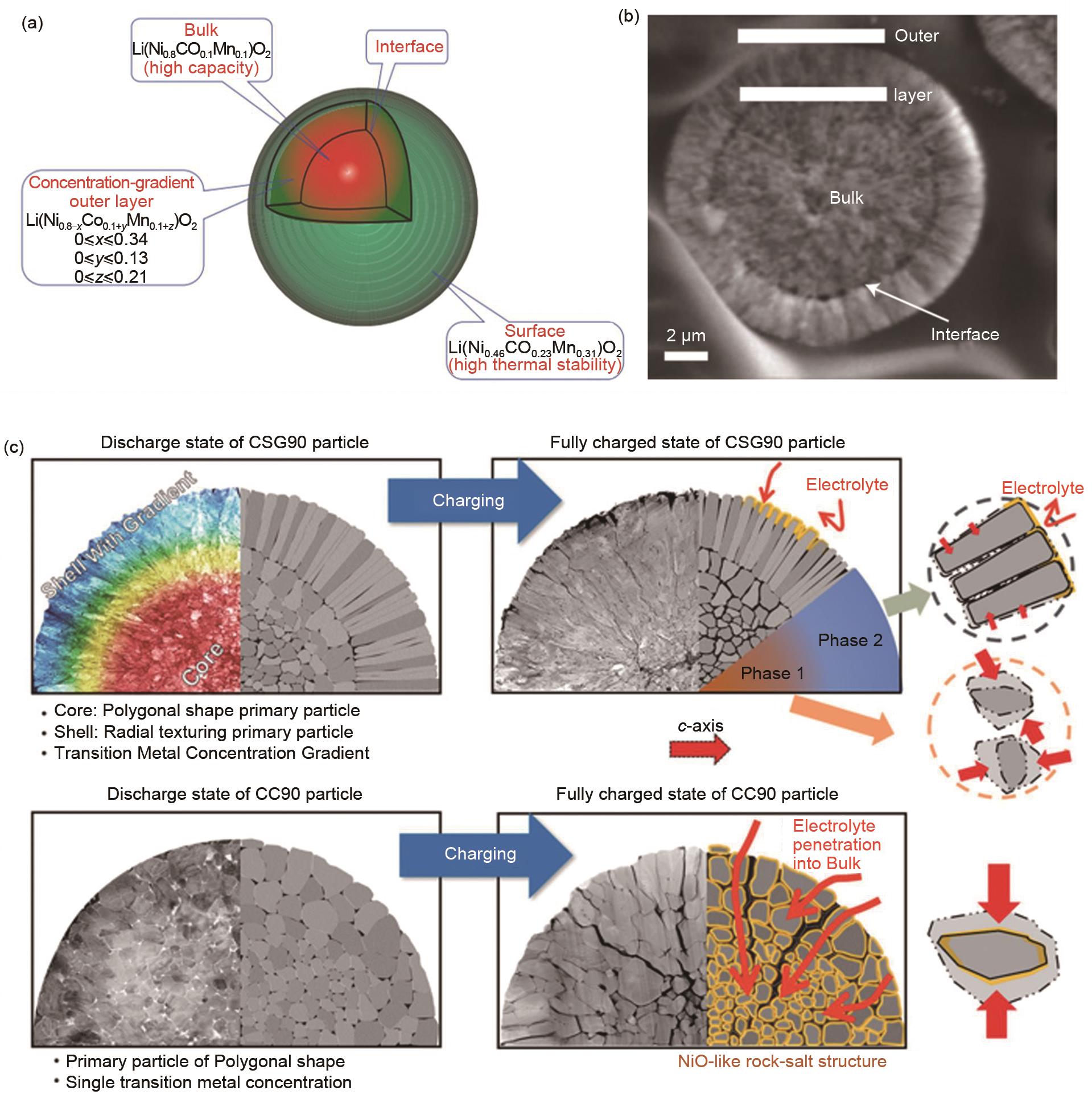
Fig. 10
(a)-(b) Schematic diagram and SEM of positive-electrode particle with Ni-rich core surrounded by concentration-gradient outer layer[109]; (c) Schematic description of discharge and charge state: CC90 and CSG90 cathodes showing the internal morphological difference and the sustained damage[111]"
| 1 | LI H Y, LIU A, ZHANG N, et al. An unavoidable challenge for Ni-rich positive electrode materials for lithium-ion batteries[J]. Chemistry of Materials, 2019, 31(18): 7574-7583. |
| 2 | GUAN P Y, ZHOU L, YU Z L, et al. Recent progress of surface coating on cathode materials for high-performance lithium-ion batteries[J]. Journal of Energy Chemistry, 2020, 43: 220-235. |
| 3 | LIANG J N, LU Y, LIU Y, et al. Oxides overlayer confined Ni3Sn2 alloy enable enhanced lithium storage performance[J]. Journal of Power Sources, 2019, 441: doi: 10.1016/j.jpowsour.2019.227185. |
| 4 | LANGDON J, MANTHIRAM A. A perspective on single-crystal layered oxide cathodes for lithium-ion batteries[J]. Energy Storage Materials, 2021, 37: 143-160. |
| 5 | CHOI J U, VORONINA N, SUN Y K, et al. Recent progress and perspective of advanced high-energy co-less Ni-rich cathodes for Li-ion batteries: Yesterday, today, and tomorrow[J]. Advanced Energy Materials, 2020, 10(42): doi: 10.1002/aenm.202002027. |
| 6 | LIANG C P, LONGO R C, KONG F T, et al. Obstacles toward unity efficiency of LiNi1-2 xCoxMnxO2 (x=0~1/3) (NCM) cathode materials: Insights from ab initio calculations[J]. Journal of Power Sources, 2017, 340: 217-228. |
| 7 | LU Y, ZHANG Q, CHEN J. Recent progress on lithium-ion batteries with high electrochemical performance[J]. Science China Chemistry, 2019, 62(5): 533-548. |
| 8 | 张欣, 孔令丽, 高腾跃, 等. 高镍三元锂离子电池循环衰减分析及改善[J]. 储能科学与技术, 2020, 9(3): 813-817. |
| ZHANG X, KONG L L, GAO T Y, et al. Analysis and improvement of cycle performance for Ni-rich lithium ion battery[J]. Energy Storage Science and Technology, 2020, 9(3): 813-817. | |
| 9 | LIU Z L, YU A S, LEE J Y. Synthesis and characterization of LiNi1- x- yCoxMnyO2 as the cathode materials of secondary lithium batteries[J]. Journal of Power Sources, 1999, 81/82: 416-419. |
| 10 | OHZUKU T, MAKIMURA Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries[J]. Chemistry Letters, 2001(7): 642-643. |
| 11 | KOYAMA Y, TANAKA I, ADACHI H, et al. Crystal and electronic structures of superstructural Li1- x[Co1/3Ni1/3Mn1/3]O2 (0≤x≤1)[J]. Journal of Power Sources, 2003, 119/120/121: 644-648. |
| 12 | OH S W, PARK S H, PARK C W, et al. Structural and electrochemical properties of layered Li[Ni0.5Mn0.5]1- xCoxO2 positive materials synthesized by ultrasonic spray pyrolysis method[J]. Solid State Ionics, 2004, 171(3/4): 167-172. |
| 13 | LIAO P Y, DUH J G, SHEEN S R. Microstructure and electrochemical performance of LiNi0.6Co0.4- xMnxO2 cathode materials[J]. Journal of Power Sources, 2005, 143(1/2): 212-218. |
| 14 | LIAO P Y, DUH J G, SHEEN S R. Effect of Mn content on the microstructure and electrochemical performance of LiNi0.75- xCo0.25MnxO2 cathode materials[J]. Journal of the Electrochemical Society, 2005, 152(9): doi: 10.1149/1.1952687. |
| 15 | KIM M H, SHIN H S, SHIN D, et al. Synthesis and electrochemical properties of Li[Ni0.8Co0.1Mn0.1]O2 and Li[Ni0.8Co0.2]O2 via co-precipitation[J]. Journal of Power Sources, 2006, 159(2): 1328-1333. |
| 16 | SUN H H, MANTHIRAM A. Impact of microcrack generation and surface degradation on a nickel-rich layered Li[Ni0.9Co0.05Mn0.05]O2 cathode for lithium-ion batteries[J]. Chemistry of Materials, 2017, 29(19): 8486-8493. |
| 17 | RYU H H, PARK K J, YOON C S, et al. Capacity fading of Ni-rich Li[NixCoyMn1- x- y]O2 (0.6≤x≤0.95) cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation?[J]. Chemistry of Materials, 2018, 30(3): 1155-1163. |
| 18 | KONDRAKOV A O, SCHMIDT A, XU J, et al. Anisotropic lattice strain and mechanical degradation of high-and low-nickel NCM cathode materials for Li-ion batteries[J]. The Journal of Physical Chemistry C, 2017, 121(6): 3286-3294. |
| 19 | AISHOVA A, PARK G T, YOON C S, et al. Cobalt-free high-capacity Ni-rich layered Li[Ni0.9Mn0.1]O2 cathode[J]. Advanced Energy Materials, 2020, 10(4): doi: 10.1002/aenm.201903179. |
| 20 | NAM G W, PARK N Y, PARK K J, et al. Capacity fading of Ni-rich NCA cathodes: Effect of microcracking extent[J]. ACS Energy Letters, 2019, 4(12): 2995-3001. |
| 21 | LI W D, LEE S, MANTHIRAM A. High-nickel NMA: A cobalt-free alternative to NMC and NCA cathodes for lithium-ion batteries[J]. Advanced Materials, 2020, 32(33): doi: 10.1002/adma.202002718. |
| 22 | QIAN G N, ZHANG J, CHU S Q, et al. Understanding the mesoscale degradation in nickel-rich cathode materials through machine-learning-revealed strain-redox decoupling[J]. ACS Energy Letters, 2021, 6(2): 687-693. |
| 23 | 杜光超, 郑莉莉, 张志超, 等. 锂离子电池热安全性研究进展[J]. 储能科学与技术, 2019, 8(3): 500-505. |
| DU G C, ZHENG L L, ZHANG Z C, et al. Overview of research on thermal safety of lithium-ion batteries[J]. Energy Storage Science and Technology, 2019, 8(3): 500-505. | |
| 24 | SUN Y K. High-capacity layered cathodes for next-generation electric vehicles[J]. ACS Energy Letters, 2019, 4(5): 1042-1044. |
| 25 | KO D S, PARK J H, YU B Y, et al. Degradation of high-nickel-layered oxide cathodes from surface to bulk: A comprehensive structural, chemical, and electrical analysis[J]. Advanced Energy Materials, 2020, 10(36): doi: 10.1002/aenm.202001035. |
| 26 | YAN P F, ZHENG J M, LV D P, et al. Atomic-resolution visualization of distinctive chemical mixing behavior of Ni, Co, and Mn with Li in layered lithium transition-metal oxide cathode materials[J]. Chemistry of Materials, 2015, 27(15): 5393-5401. |
| 27 | ZHAO E Y, FANG L C, CHEN M M, et al. New insight into Li/Ni disorder in layered cathode materials for lithium ion batteries: A joint study of neutron diffraction, electrochemical kinetic analysis and first-principles calculations[J]. Journal of Materials Chemistry A, 2017, 5(4): 1679-1686. |
| 28 | XIA Y, ZHENG J M, WANG C M, et al. Designing principle for Ni-rich cathode materials with high energy density for practical applications[J]. Nano Energy, 2018, 49: 434-452. |
| 29 | LIANG J N, LU Y, WANG J, et al. Well-ordered layered LiNi0.8Co0.1Mn0.1O2 submicron sphere with fast electrochemical kinetics for cathodic lithium storage[J]. Journal of Energy Chemistry, 2020, 47: 188-195. |
| 30 | ZHANG J C, ZHOU D, YANG W Y, et al. Probing the nature of Li+/Ni2+ disorder on the structure and electrochemical performance in Ni-based layered oxide cathodes[J]. Journal of the Electrochemical Society, 2019, 166(16): doi: 10.1149/2.0641916jes. |
| 31 | TANG Z F, WANG S, LIAO J Y, et al. Facilitating lithium-ion diffusion in layered cathode materials by introducing Li+/Ni2+ antisite defects for high-rate Li-ion batteries[J]. Research, 2019: 162-171. |
| 32 | BAK S M, HU E Y, ZHOU Y N, et al. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy[J]. ACS Applied Materials & Interfaces, 2014, 6(24): 22594-22601. |
| 33 | YU T H, LI J L, XU G F, et al. Improved cycle performance of Li[Li0.2Mn0.54Co0.13Ni0.13]O2 by Ga doping for lithium ion battery cathode material[J]. Solid State Ionics, 2017, 301: 64-71. |
| 34 | LIANG C P, LONGO R C, KONG F T, et al. Ab initio study on surface segregation and anisotropy of Ni-rich LiNi1-2 yCoyMnyO2 (NCM) (y≤0.1) cathodes[J]. ACS Applied Materials & Interfaces, 2018, 10(7): 6673-6680. |
| 35 | YIN S Y, DENG W T, CHEN J, et al. Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries[J]. Nano Energy, 2021, 83: 10.1016/j.nanoen.2021. 105854. |
| 36 | PARK K J, HWANG J Y, RYU H H, et al. Degradation mechanism of Ni-enriched NCA cathode for lithium batteries: Are microcracks really critical?[J]. ACS Energy Letters, 2019, 4(6): 1394-1400. |
| 37 | WATANABE S, KINOSHITA M, HOSOKAWA T, et al. Capacity fade of LiAlyNi1- x- yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (surface analysis of LiAlyNi1- x- yCoxO2 cathode after cycle tests in restricted depth of discharge ranges)[J]. Journal of Power Sources, 2014, 258: 210-217. |
| 38 | CHO D H, JO C H, CHO W, et al. Effect of residual lithium compounds on layer Ni-rich Li[Ni0.7Mn0.3]O2[J]. Journal of the Electrochemical Society, 2014, 161(6): doi: 10.1149/2.042406jes. |
| 39 | WANDT J, FREIBERG A T S, OGRODNIK A, et al. Singlet oxygen evolution from layered transition metal oxide cathode materials and its implications for lithium-ion batteries[J]. Materials Today, 2018, 21(8): 825-833. |
| 40 | 陈晓轩, 李晟, 胡泳钢, 等. 锂离子电池三元层状氧化物正极材料失效模式分析[J]. 储能科学与技术, 2019, 8(6): 1003-1016. |
| CHEN X X, LI S, HU Y G, et al. Failure mechanism of Li1+ x(NCM)1- xO2 layered oxide cathode material during capacity degradation[J]. Energy Storage Science and Technology, 2019, 8(6): 1003-1016. | |
| 41 | ZHUANG G V, CHEN G Y, SHIM J, et al. Li2CO3 in LiNi0.8Co0.15Al0.05O2 cathodes and its effects on capacity and power[J]. Journal of Power Sources, 2004, 134(2): 293-297. |
| 42 | ZHENG H Y, QU Q T, ZHU G B, et al. Quantitative characterization of the surface evolution for LiNi 0.5 Co0.2Mn0.3O2/graphite cell during long-term cycling[J]. ACS Applied Materials & Interfaces, 2017, 9(14): 12445-12452. |
| 43 | LIU W, OH P, LIU X E, et al. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries[J]. Angewandte Chemie International Edition, 2015, 54(15): 4440-4457. |
| 44 | BELHAROUAK I, LU W Q, VISSERS D, et al. Safety characteristics of Li(Ni0.8Co0.15Al0.05)O2 and Li(Ni1/3Co1/3Mn1/3)O2[J]. Electrochemistry Communications, 2006, 8(2): 329-335. |
| 45 | WU L J, NAM K W, WANG X J, et al. Structural origin of overcharge-induced thermal instability of Ni-containing layered-cathodes for high-energy-density lithium batteries[J]. Chemistry of Materials, 2011, 23(17): 3953-3960. |
| 46 | LIANG C, ZHANG W H, WEI Z S, et al. Transition-metal redox evolution and its effect on thermal stability of LiNixCoyMnzO2 based on synchrotron soft X-ray absorption spectroscopy[J]. Journal of Energy Chemistry, 2021, 59: 446-454. |
| 47 | CHAE B J, PARK J H, SONG H J, et al. Thiophene-initiated polymeric artificial cathode-electrolyte interface for Ni-rich cathode material[J]. Electrochimica Acta, 2018, 290: 465-473. |
| 48 | FENG Z, RAJAGOPALAN R, SUN D, et al. In-situ formation of hybrid Li3PO4-AlPO4-Al(PO3)3 coating layer on LiNi0.8Co0.1Mn0.1O2 cathode with enhanced electrochemical properties for lithium-ion battery[J]. Chemical Engineering Journal, 2020, 382: doi: 10.1016/j.cej.2019.122959. |
| 49 | 王栋, 郑莉莉, 杜光超, 等. 锂离子电池正极材料掺杂和表面包覆研究综述[J]. 储能科学与技术, 2019, 8(S1): 43-48. |
| WANG D, ZHENG L L, DU G C, et al. Review of doping and surface coating of cathode materials for lithium ion batteries[J]. Energy Storage Science and Technology, 2019, 8(S1): 43-48. | |
| 50 | CHENG X P, ZHENG J M, LU J X, et al. Realizing superior cycling stability of Ni-Rich layered cathode by combination of grain boundary engineering and surface coating[J]. Nano Energy, 2019, 62: 30-37. |
| 51 | GUO S H, YUAN B, ZHAO H M, et al. Dual-component LixTiO2@silica functional coating in one layer for performance enhanced LiNi0.6Co0.2Mn0.2O2 cathode[J]. Nano Energy, 2019, 58: 673-679. |
| 52 | JIN M H, LI B, HU L L, et al. Functional copolymer binder for nickel-rich cathode with exceptional cycling stability at high temperature through coordination interaction[J]. Journal of Energy Chemistry, 2021, 60: 156-161. |
| 53 | YUAN K, LI N, NING R Q, et al. Stabilizing surface chemical and structural Ni-rich cathode via a non-destructive surface reinforcement strategy[J]. Nano Energy, 2020, 78: doi: 10.1016/j.nanoen.2020.105239. |
| 54 | LV H J, LI C L, ZHAO Z K, et al. A review: Modification strategies of nickel-rich layer structure cathode (Ni≥0.8) materials for lithium ion power batteries[J]. Journal of Energy Chemistry, 2021, 60: 435-450. |
| 55 | TIAN L Y, LIANG K, WEN X F, et al. Enhanced cycling stability and rate capability of LiNi0.80Co0.15Al0.05O2 cathode material by a facile coating method[J]. Journal of Electroanalytical Chemistry, 2018, 812: 22-27. |
| 56 | LEE Y S, SHIN W K, KANNAN A G, et al. Improvement of the cycling performance and thermal stability of lithium-ion cells by double-layer coating of cathode materials with Al2O3 nanoparticles and conductive polymer[J]. ACS Applied Materials & Interfaces, 2015, 7(25): 13944-13951. |
| 57 | MAKHONINA E V, MEDVEDEVA A E, DUBASOVA V S, et al. A new coating for improving the electrochemical performance of cathode materials[J]. International Journal of Hydrogen Energy, 2016, 41(23): 9901-9907. |
| 58 | MA F, WU Y H, WEI G Y, et al. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode via wet-chemical coating of MgO[J]. Journal of Solid State Electrochemistry, 2019, 23(7): 2213-2224. |
| 59 | XU C L, XIANG W, WU Z G, et al. Highly stabilized Ni-rich cathode material with Mo induced epitaxially grown nanostructured hybrid surface for high-performance lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(18): 16629-16638. |
| 60 | ZHU Z, WANG H, LI Y, et al. A surface Se-substituted LiCo[O2- δSeδ]cathode with ultrastable high-voltage cycling in pouch full-cells[J]. Advanced Materials, 2020, 32(50): doi: 10.1002/adma.202005182. |
| 61 | LAI Y Q, XU M, ZHANG Z A, et al. Optimized structure stability and electrochemical performance of LiNi0.8Co0.15Al0.05O2 by sputtering nanoscale ZnO film[J]. Journal of Power Sources, 2016, 309: 20-26. |
| 62 | YAO L, LIANG F Q, JIN J, et al. Improved electrochemical property of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode via in situ ZrO2 coating for high energy density lithium ion batteries[J]. Chemical Engineering Journal, 2020, 389: doi: 10.1016/j.cej.2020.124403. |
| 63 | DU Z L, PENG W J, WANG Z X, et al. Improving the electrochemical performance of Li-rich Li1.2Ni0.13Co0.13Mn0.54O2 cathode material by LiF coating[J]. Ionics, 2018, 24(12): 3717-3724. |
| 64 | PARK B C, KIM H B, BANG H J, et al. Improvement of electrochemical performance of Li[Ni0.8Co0.15Al0.05]O2 cathode materials by AlF3 coating at various temperatures[J]. Industrial & Engineering Chemistry Research, 2008, 47(11): 3876-3882. |
| 65 | ZHANG C L, SHEN L F, LI H S, et al. Enhanced electrochemical properties of MgF2 and C co-coated Li3V2(PO4)3 composite for Li-ion batteries[J]. Journal of Electroanalytical Chemistry, 2016, 762: 1-6. |
| 66 | REN D, YANG Y, SHEN L X, et al. Ni-rich LiNi0.88Mn0.06Co0.06O2 cathode interwoven by carbon fiber with improved rate capability and stability[J]. Journal of Power Sources, 2020, 447: doi: 10.1016/j.jpowsour.2019.227344. |
| 67 | WALKER B A, PLAZA-RIVERA C O, SUN S S, et al. Dry-pressed lithium nickel cobalt manganese oxide (NCM) cathodes enabled by holey graphene host[J]. Electrochimica Acta, 2020, 362: doi: 10.1016/j.electacta.2020.137129. |
| 68 | PARK K Y, LIM J M, LUU N S, et al. Concurrently approaching volumetric and specific capacity limits of lithium battery cathodes via conformal Pickering emulsion graphene coatings[J]. Advanced Energy Materials, 2020, 10(25): doi: 10.1002/aenm.202001216. |
| 69 | CHEN S, HE T, SU Y F, et al. Ni-rich LiNi0.8Co0.1Mn0.1O2 oxide coated by dual-conductive layers as high performance cathode material for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(35): 29732-29743. |
| 70 | XU G L, LIU Q, LAU K K S, et al. Building ultraconformal protective layers on both secondary and primary particles of layered lithium transition metal oxide cathodes[J]. Nature Energy, 2019, 4(6): 484-494. |
| 71 | ZHU H W, YU H F, JIANG H B, et al. High-efficiency Mo doping stabilized LiNi0.9Co0.1O2 cathode materials for rapid charging and long-life Li-ion batteries[J]. Chemical Engineering Science, 2020, 217: 10.1016/j.ces.2020.115518. |
| 72 | XIE Q, LI W D, MANTHIRAM A. A Mg-doped high-nickel layered oxide cathode enabling safer, high-energy-density Li-ion batteries[J]. Chemistry of Materials, 2019, 31(3): 938-946. |
| 73 | WU F, LIU N, CHEN L, et al. Improving the reversibility of the H2-H3 phase transitions for layered Ni-rich oxide cathode towards retarded structural transition and enhanced cycle stability[J]. Nano Energy, 2019, 59: 50-57. |
| 74 | CHU B B, LIU S Y, YOU L Z, et al. Enhancing the cycling stability of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode at a high cutoff voltage with Ta doping[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(8): 3082-3090. |
| 75 | YU H, LI Y, HU Y, et al. 110th Anniversary: Concurrently coating and doping high-valence vanadium in nickel-rich lithiated oxides for high-rate and stable lithium-ion batteries[J]. Industrial & Engineering Chemistry Research, 2019, 58(10): 4108-4115. |
| 76 | KIM U H, JUN D W, PARK K J, et al. Pushing the limit of layered transition metal oxide cathodes for high-energy density rechargeable Li ion batteries[J]. Energy & Environmental Science, 2018, 11(5): 1271-1279. |
| 77 | KIM U H, PARK N Y, PARK G T, et al. High-energy W-doped Li[Ni0.95Co0.04Al0.01]O2 cathodes for next-generation electric vehicles[J]. Energy Storage Materials, 2020, 33: 399-407. |
| 78 | RYU H H, PARK K J, YOON D R, et al. Li[Ni0.9Co0.09W0.01]O2: A new type of layered oxide cathode with high cycling stability[J]. Advanced Energy Materials, 2019, 9(44): doi: 10.1002/aenm. 201902698. |
| 79 | WEIGEL T, SCHIPPER F, ERICKSON E M, et al. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2 cathode materials doped by various cations[J]. ACS Energy Letters, 2019, 4(2): 508-516. |
| 80 | LUN Z Y, OUYANG B, KWON D H, et al. Cation-disordered rocksalt-type high-entropy cathodes for Li-ion batteries[J]. Nature Materials, 2021, 20(2): 214-221. |
| 81 | PARK G T, RYU H H, PARK N Y, et al. Tungsten doping for stabilization of Li[Ni0.90Co0.05Mn0.05]O2 cathode for Li-ion battery at high voltage[J]. Journal of Power Sources, 2019, 442: doi: 10.1016/j.jpowsour.2019.227242. |
| 82 | RYU H H, PARK G T, YOON C S, et al. Suppressing detrimental phase transitions via tungsten doping of LiNiO2 cathode for next-generation lithium-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(31): 18580-18588. |
| 83 | YU H F, ZHU H W, YANG Z F, et al. Bulk Mg-doping and surface polypyrrole-coating enable high-rate and long-life for Ni-rich layered cathodes[J]. Chemical Engineering Journal, 2021, 412: doi: 10.1016/j.cej.2021.128625. |
| 84 | LIU D M, FAN X J, LI Z H, et al. A cation/anion co-doped Li1.12Na0.08Ni0.2Mn0.6O1.95F0.05 cathode for lithium ion batteries[J]. Nano Energy, 2019, 58: 786-796. |
| 85 | VANAPHUTI P, CHEN J J, CAO J Y, et al. Enhanced electrochemical performance of the lithium-manganese-rich cathode for Li-ion batteries with Na and F codoping[J]. ACS Applied Materials & Interfaces, 2019, 11(41): 37842-37849. |
| 86 | FENG Z, RAJAGOPALAN R, ZHANG S, et al. A three in one strategy to achieve zirconium doping, boron doping, and interfacial coating for stable LiNi0.8Co0.1Mn0.1O2 cathode[J]. Advanced Science, 2021, 8(2): 10.1002/advs.202001809. |
| 87 | PARK K J, JUNG H G, KUO L Y, et al. Improved cycling stability of Li[Ni0.90Co0.05Mn0.05]O2 through microstructure modification by boron doping for Li-ion batteries[J]. Advanced Energy Materials, 2018, 8(25): doi: 10.1002/aenm.201801202. |
| 88 | KIM U H, PARK G T, SON B K, et al. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge[J]. Nature Energy, 2020, 5(11): 860-869. |
| 89 | JI H W, WU J P, CAI Z J, et al. Ultrahigh power and energy density in partially ordered lithium-ion cathode materials[J]. Nature Energy, 2020, 5(3): 213-221. |
| 90 | FANG L L, WANG M, ZHOU Q H, et al. Suppressing cation mixing and improving stability by F doping in cathode material LiNiO2 for Li-ion batteries: First-principles study[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 600: doi: 10.1016/j.colsurfa.2020.124940. |
| 91 | LI X J, TANG Y C, ZHU J X, et al. Boosting the cycling stability of aqueous flexible Zn batteries via F doping in nickel-cobalt carbonate hydroxide cathode[J]. Small, 2020, 16(31): doi: 10.1002/smll.202001935. |
| 92 | LI C L, KAN W H, XIE H L, et al. Inducing favorable cation antisite by doping halogen in Ni-rich layered cathode with ultrahigh stability[J]. Advanced Science, 2019, 6(4): doi: 10.1002/advs.201801406. |
| 93 | TENG R, YU H T, GUO C F, et al. Effect of F dopant on the structural stability, redox mechanism, and electrochemical performance of Li2MoO3 cathode materials[J]. Advanced Sustainable Systems, 2020, 4(12): doi: 10.1002/adsu.202000104. |
| 94 | KIM U H, PARK G T, CONLIN P, et al. Cation ordered Ni-rich layered cathode for ultra-long battery life[J]. Energy & Environmental Science, 2021, 14(3): 1573-1583. |
| 95 | KONG F T, LIANG C P, LONGO R C, et al. Conflicting roles of anion doping on the electrochemical performance of Li-ion battery cathode materials[J]. Chemistry of Materials, 2016, 28(19): 6942-6952. |
| 96 | BINDER J O, CULVER S P, PINEDO R, et al. Investigation of fluorine and nitrogen as anionic dopants in nickel-rich cathode materials for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(51): 44452-44462. |
| 97 | KONG X B, ZHANG Y G, PENG S Y, et al. Superiority of single-crystal to polycrystalline LiNixCoyMn1- x- yO2 cathode materials in storage behaviors for lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(39): 14938-14948. |
| 98 | GE M Y, WI S, LIU X, et al. Kinetic limitations in single-crystal high-nickel cathodes[J]. Angewandte Chemie International Edition, 2021, 60(32): 17350-17355. |
| 99 | LIU Y L, HARLOW J, DAHN J. Microstructural observations of "single crystal" positive electrode materials before and after long term cycling by cross-section scanning electron microscopy[J]. Journal of the Electrochemical Society, 2020, 167(2): doi: 10.1149/1945-7111/ab6288. |
| 100 | QIAN G N, ZHANG Y T, LI L S, et al. Single-crystal nickel-rich layered-oxide battery cathode materials: Synthesis, electrochemistry, and intra-granular fracture[J]. Energy Storage Materials, 2020, 27: 140-149. |
| 101 | HAN Y K, XU J M, WANG W, et al. Implanting an electrolyte additive on a single crystal Ni-rich cathode surface for improved cycleability and safety[J]. Journal of Materials Chemistry A, 2020, 8(46): 24579-24589. |
| 102 | WEBER R, FELL C R, DAHN J R, et al. Operando X-ray diffraction study of polycrystalline and single-crystal LixNi0.5Mn0.3Co0.2O2[J]. Journal of the Electrochemical Society, 2017, 164(13): doi: 10.1149/2.0441713jes. |
| 103 | FAN X M, HU G R, ZHANG B, et al. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries[J]. Nano Energy, 2020, 70: doi: 10.1016/j.nanoen.2020.104450. |
| 104 | ZHENG L T, BENNETT J C, OBROVAC M N. All-dry synthesis of single crystal NMC cathode materials for Li-ion batteries[J]. Journal of the Electrochemical Society, 2020, 167(13): doi: 10.1149/1945-7111/abbcb1. |
| 105 | XU X, HUO H, JIAN J Y, et al. Radially oriented single-crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries[J]. Advanced Energy Materials, 2019, 9(15): doi: 10.1002/aenm. 201803963. |
| 106 | CHEN X, TANG Y, FAN C L, et al. A highly stabilized single crystalline nickel-rich LiNi0.8Co0.1Mn0.1O2 cathode through a novel surface spinel-phase modification[J]. Electrochimica Acta, 2020, 341: doi: 10.1016/j.electacta.2020.136075. |
| 107 | 熊凡, 张卫新, 杨则恒, 等. 高比能量锂离子电池正极材料的研究进展[J]. 储能科学与技术, 2018, 7(4): 607-617. |
| XIONG F, ZHANG W X, YANG Z H, et al. Research progress on cathode materials for high energy density lithium ion batteries[J]. Energy Storage Science and Technology, 2018, 7(4): 607-617. | |
| 108 | ZHANG Y D, LI H, LIU J X, et al. LiNi0.90Co0.07Mg0.03O2 cathode materials with Mg-concentration gradient for rechargeable lithium-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(36): 20958-20964. |
| 109 | SUN Y K, MYUNG S T, PARK B C, et al. High-energy cathode material for long-life and safe lithium batteries[J]. Nature Materials, 2009, 8(4): 320-324. |
| 110 | LIM B B, MYUNG S T, YOON C S, et al. Comparative study of Ni-rich layered cathodes for rechargeable lithium batteries: Li[Ni0.85Co0.11Al0.04]O2 and Li[Ni0.84Co0.06Mn0.09Al0.01]O2 with two-step full concentration gradients[J]. ACS Energy Letters, 2016, doi: 10.1021/acsenergylett.6b00150. |
| 111 | KIM U H, RYU H H, KIM J H, et al. Microstructure-controlled Ni-rich cathode material by microscale compositional partition for next-generation electric vehicles[J]. Advanced Energy Materials, 2019, 9(15): doi: 10.1002/aenm.201803902. |
| [1] | Linwang DENG, Tianyu FENG, Shiwei SHU, Zifeng ZHANG, Bin GUO. Review of a fast-charging strategy and technology for lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2879-2890. |
| [2] | Kaiqiang GUO, Haiying CHE, Haoran ZHANG, Jianping LIAO, Huang ZHOU, Yunlong ZHANG, Hangda CHEN, Zhan SHEN, Haimei LIU, Zifeng MA. Preparation and characterization of B2O3-coated NaNi1/3Fe1/3Mn1/3O2 cathode materials for sodium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2980-2988. |
| [3] | Xiaoyu CHEN, Mengmeng GENG, Qiankun WANG, Jiani SHEN, Yijun HE, Zifeng MA. Electrochemical impedance feature selection and gaussian process regression based on the state-of-health estimation method for lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2995-3002. |
| [4] | Jing ZHU, Yida WU, Junfeng HAO, Guanjun CEN, Ronghan QIAO, Xiaoyu SHEN, Mengyu TIAN, Hongxiang JI, Zhou JIN, Yuanjie ZHAN, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Xuejie HUANG. Reviews of selected 100 recent papers for lithium batteries (Jun. 1, 2022 to Jul. 31, 2022) [J]. Energy Storage Science and Technology, 2022, 11(9): 3035-3050. |
| [5] | Yue ZHANG, Depeng KONG, Ping PING. Performance and design optimization of a cold plate for inhibiting thermal runaway propagation of lithium-ion battery packs [J]. Energy Storage Science and Technology, 2022, 11(8): 2432-2441. |
| [6] | Chengshan XU, Borui LU, Mengqi ZHANG, Huaibin WANG, Changyong JIN, Minggao OUYANG, Xuning FENG. Study on thermal runaway gas evolution in the lithium-ion battery energy storage cabin [J]. Energy Storage Science and Technology, 2022, 11(8): 2418-2431. |
| [7] | Liping HUO, Weiling LUAN, Zixian ZHUANG. Development trend of lithium-ion battery safety technology for energy storage [J]. Energy Storage Science and Technology, 2022, 11(8): 2671-2680. |
| [8] | Zhicheng CAO, Kaiyun ZHOU, Jiali ZHU, Gaoming LIU, Min YAN, Shun TANG, Yuancheng CAO, Shijie CHENG, Weixin ZHANG. Patent analysis of fire-protection technology of lithium-ion energy storage system [J]. Energy Storage Science and Technology, 2022, 11(8): 2664-2670. |
| [9] | Yong MA, Xiaohan LI, Lei SUN, Dongliang GUO, Jinggang YANG, Jianjun LIU, Peng XIAO, Guangjun QIAN. Parameter design of lithium-ion batteries based on a three-dimensional electrochemical thermal coupling lithium precipitation model [J]. Energy Storage Science and Technology, 2022, 11(8): 2600-2611. |
| [10] | Liang TANG, Xiaobo YIN, Houfu WU, Pengjie LIU, Qingsong WANG. Demand for safety standards in the development of the electrochemical energy storage industry [J]. Energy Storage Science and Technology, 2022, 11(8): 2645-2652. |
| [11] | Tao SUN, Tengteng SHEN, Xin LIU, Dongsheng REN, Jinhai LIU, Yuejiu ZHENG, Luyan WANG, Languang LU, Minggao OUYANG. Application of titration gas chromatography technology in the quantitative detection of lithium plating in Li-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(8): 2564-2573. |
| [12] | Yang WANG, Xu LU, Yuxin ZHANG, Long LIU. Thermal runaway exhaust strategy of power battery [J]. Energy Storage Science and Technology, 2022, 11(8): 2480-2487. |
| [13] | Qingsong ZHANG, Yang ZHAO, Tiantian LIU. Effects of state of charge and battery layout on thermal runaway propagation in lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(8): 2519-2525. |
| [14] | Wei KONG, Jingtao JIN, Xipo LU, Yang SUN. Study on cooling performance of lithium ion batteries with symmetrical serpentine channel [J]. Energy Storage Science and Technology, 2022, 11(7): 2258-2265. |
| [15] | Xiaoyu SHEN, Guanjun CEN, Ronghan QIAO, Jing ZHU, Hongxiang JI, Mengyu TIAN, Zhou JIN, Yong YAN, Yida WU, Yuanjie ZHAN, Hailong YU, Liubin BEN, Yanyan LIU, Xuejie HUANG. Reviews of selected 100 recent papers for lithium batteries (Apr. 1, 2022 to May 31, 2022) [J]. Energy Storage Science and Technology, 2022, 11(7): 2007-2022. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
