Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (1): 278-298.doi: 10.19799/j.cnki.2095-4239.2022.0436
• Energy Storage Test: Methods and Evaluation • Previous Articles Next Articles
Ziwei YUAN1( ), Chuyuan LIN1, Ziyan YUAN1, Xiaoli SUN1, Qingrong QIAN1,2, Qinghua CHEN1,2, Lingxing ZENG1,2(
), Chuyuan LIN1, Ziyan YUAN1, Xiaoli SUN1, Qingrong QIAN1,2, Qinghua CHEN1,2, Lingxing ZENG1,2( )
)
Received:2022-08-02
Revised:2022-08-26
Online:2023-01-05
Published:2023-02-08
Contact:
Lingxing ZENG
E-mail:ziweiyuan2001@163.com;lingxing@fjnu.edu.cn
CLC Number:
Ziwei YUAN, Chuyuan LIN, Ziyan YUAN, Xiaoli SUN, Qingrong QIAN, Qinghua CHEN, Lingxing ZENG. The research process on low temperature performance of zinc ion batteries[J]. Energy Storage Science and Technology, 2023, 12(1): 278-298.
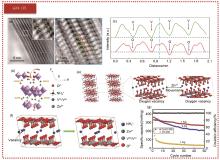
Fig. 2
(a) ABF-STEM image of NH4V4O10-x ·nH2O cathode material; (b) NVOH atom resolution image along axis [100], green sphere is V atom, blue sphere is NH4+ ion, red sphere is O atom, yellow circle represents oxygen vacancy; (c) Intensity line outlines of corresponding columns in the figure (b); (d) Schematic diagram of Zn2+ (de-intercalation) of NH4V4O10-x ·nH2O cathode material; (e) Schematic diagram of the diffusion of Zn2+ in NH4V4O10-x ·nH2O, with oxygen vacancy at the end sites, red spheres are O atoms, blue spheres are N atoms (NH4+ ions), green spheres are Zn2+ ions, and gray spheres are V atoms [35]; (f) Diffusion diagram of Zn2+ in Zn0.3(NH4)0.3V4O10·0.91H2O with NH4+ vacancy, black dotted circle indicates NH4+ vacancy [10]; (g) Capacitance comparison diagram of VO-300 and CuVO-300 at 1 A/g current density[37]"
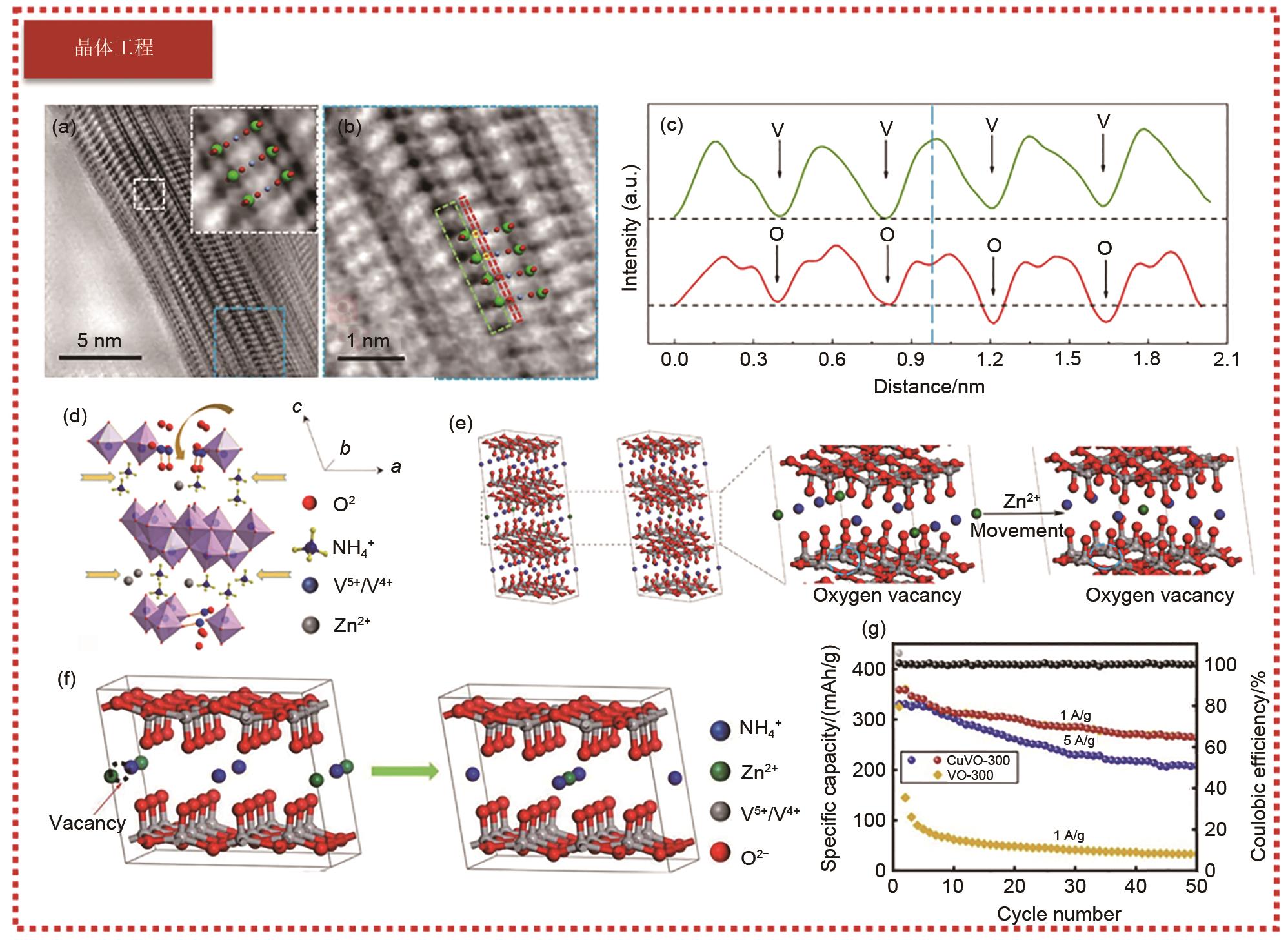

Fig. 3
(a) Schematic diagram of the rechargeable battery based on the cathode electrode of quinone, and corresponding redox chemistry character; (b) Current density and charge transfer resistance of PAQS and MmH electrodes with temperature; (c) Nyquist plots of PAQS electrodes obtained from electrochemical impedance spectroscopy measurements; (d) Micropolarization curves measured immediately after impedance measurements [41]"


Fig. 4
(a) Schematic diagram of structural evolution of water and electrolyte and design of low freezing point solution. Water networks connected by hydrogen bonds are easily converted to ice networks at 0 ℃. With the addition of ZnCl2, the strong interaction between ions and water destroys the hydrogen bond network, and the ionic interaction is enhanced. Electrolytes at criticalCZnCl2can operate at extremely low temperatures through equilibrium hydrogen bonding and ion interactions used to regulate freezing points; (b) Structure and redox mechanism of Zn||PANI batteries; (c) The interaction energy between ions and water, and the energy to form solvated Zn2+ configurations; (d) Operating temperature window of existing batteries[11]; (e) Differential scanning calorimetry (DSC) curve of 4 mol/L Zn(BF4)2 electrolyte solution; (f) Conductivity curve of 4 mol/L Zn(BF4)2 electrolyte solution with temperature change; (g) Different types of O—H…F hydrogen bond obtained from snapshots in molecular dynamics (MD) simulations; (h) The average hydrogen bond number of Zn(BF4)2 electrolyte solutions with different concentrations after 140 ns simulation time; (I) Ratio of different hydrogen bonds in Zn(BF4)2 electrolyte solutions with different concentrations[49]"


Fig. 5
(a) Negative electrostatic potential mapping of anions SO42-、 NO3-、Cl-、I-和CF3SO3-; (b) The complexes configurations and binding energies of SO42-- H2O、NO3--H2O、Cl--H2O、I--H2O和CF3SO3--H2O were obtained by DFT calculation; (c) The complexes configurations and binding energies of Zn2+- SO42-、Zn2+- NO3-、Zn2+- Cl-、Zn2+- I-和Zn2+- CF3SO3-were calculated by DFT calculation; (d) Capacity comparison between Zn|2 mol/L Zn(CF3SO3)2|V2O5 batteries and reported low temperature aqueous batteries[51]; (e) Snapshots of 4 mol/L ZnSO4 and (f) 4 mol/L Zn(TFSI)2 electrolytes during DFT-MD simulation (Red represents oxygen, gray represents zinc, yellow represents sulfur, white represents hydrogen, blue represents nitrogen, purple represents fluorine); (g) The average number of hydrogen bonds between water molecules at 4 mol/L ZnSO4 、Zn(TFSI)2 and water; (h) The variation curve of discharge capacity with the number of cycles at different coulomb rates (from 2 C to 30 C) and different temperatures (from 25 to -35 ℃)[54]"

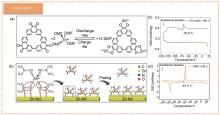
Fig. 6
(a) Reaction process diagram of Zn||PQ-MCT battery; (b) Zn2+-DMF complexes contribute to the smooth deposition of zinc in Zn(OTf)2|DMF electrolyte; (c) DSC curves of 0.5 mol/L Zn(OTf)2/DMF electrolyte and (d) pure DMF electrolyte reduced to -140 ℃ (held for 10 minutes) at a rate of 5 ℃/min while liquid nitrogen cooling, and DSC curves from -140 ℃ scanning to 20 ℃ at a rate of 5 ℃/min[59]"
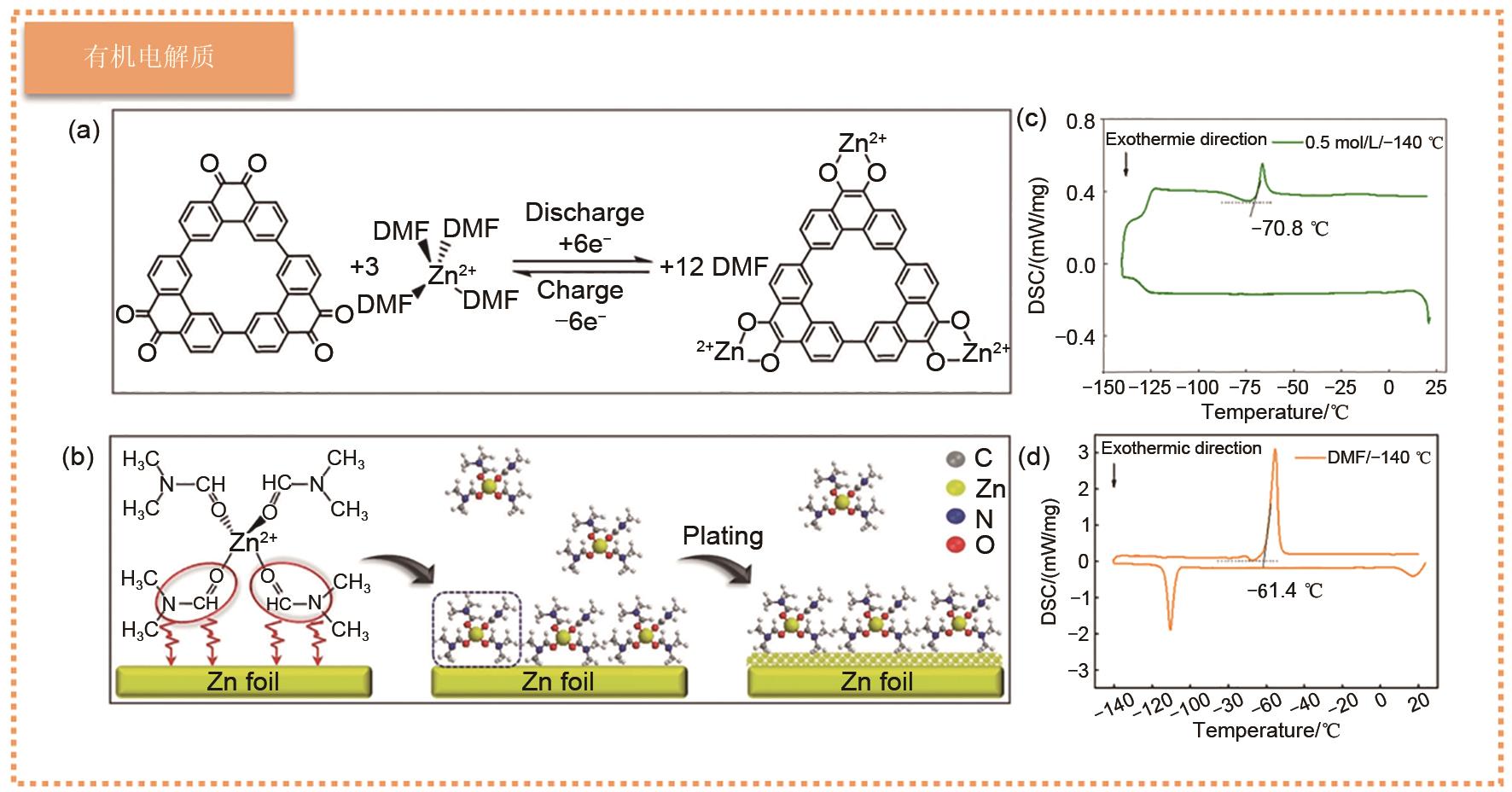
Fig. 7
(a) Molecular models for simulating interactions between water molecules and different terminal groups in polymers; (b) Formation technology of δ-MgVO battery and the schematic diagram of working principle of Zn||δ-MgVO battery; (c) SEM images of lyophilized PVA/G gel electrolyte. Ragone diagram of (d) area and (e) volume compared with previously reported energy storage devices [68]; (f) Synthesis schematic diagram of PVA-B-G; (g) Integrated 3D network of polycomplexation in PVA-B-G and photographs of PVA-B-G films; (h) Photographs of PVA-B-G that have been shaped into the desired shape; (i) SEM images of freeze-dried PVA-B-G. Ragone diagram of (j) area and (k) volume of PVA-B-G battery compared with previously reported energy storage battery [69]"
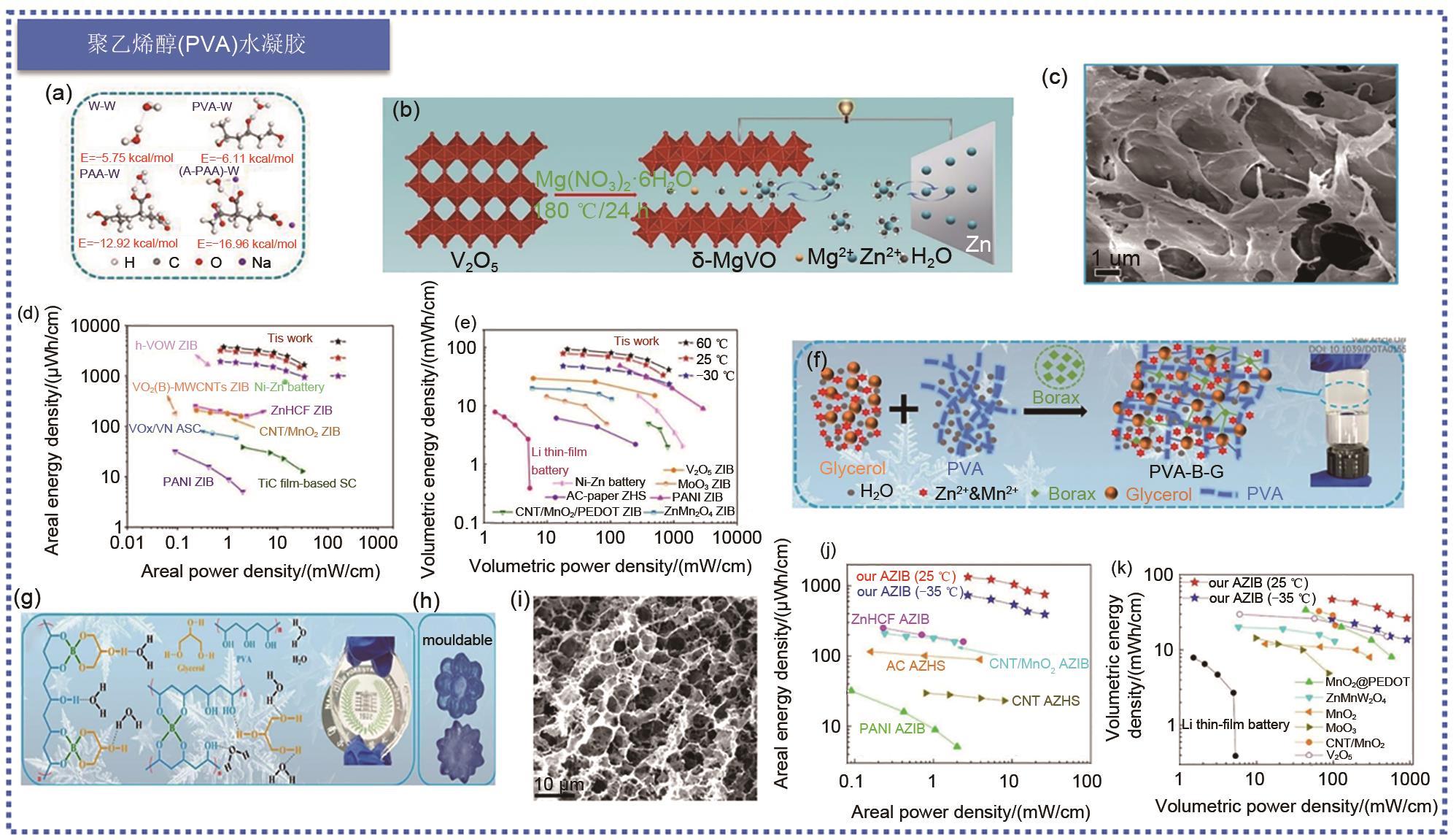

Fig. 8
(a) Schematic diagram of interfacial reactivity of Z-PAAM and ZL-PAAM (Z represents Zn2+, L represents Li+); (b) Rate performance of Zn||LiFePO4 at -20 and 25 ℃ [70]; (c) Various hydrogen bonds are formed between alginic acid, EG and water molecules; (d) Different hydrogen bond interactions are formed between alginate, EG and water molecules; (e) Cycle performance of Zn||MnO2 batteries at 1.6 A/g, 25 ℃ and -20 ℃ [44]. (f) Specific capacity and (g) cycle stability of Zn||[EMIM]PF6-PEDOT:PSS|Bi2S3 batteries compared with other reported aqueous ZIBs[45]; (h) Schematic diagram of synthesis route of hydrogel electrolyte. Electrolytes are synthesized by polymerization of sodium acrylate with ammonium persulfate (APS) initiator and then immersion in the mixed solution of 6 mol/L KOH and 0.2 mol/L Zn(CH3COO)2[75]"
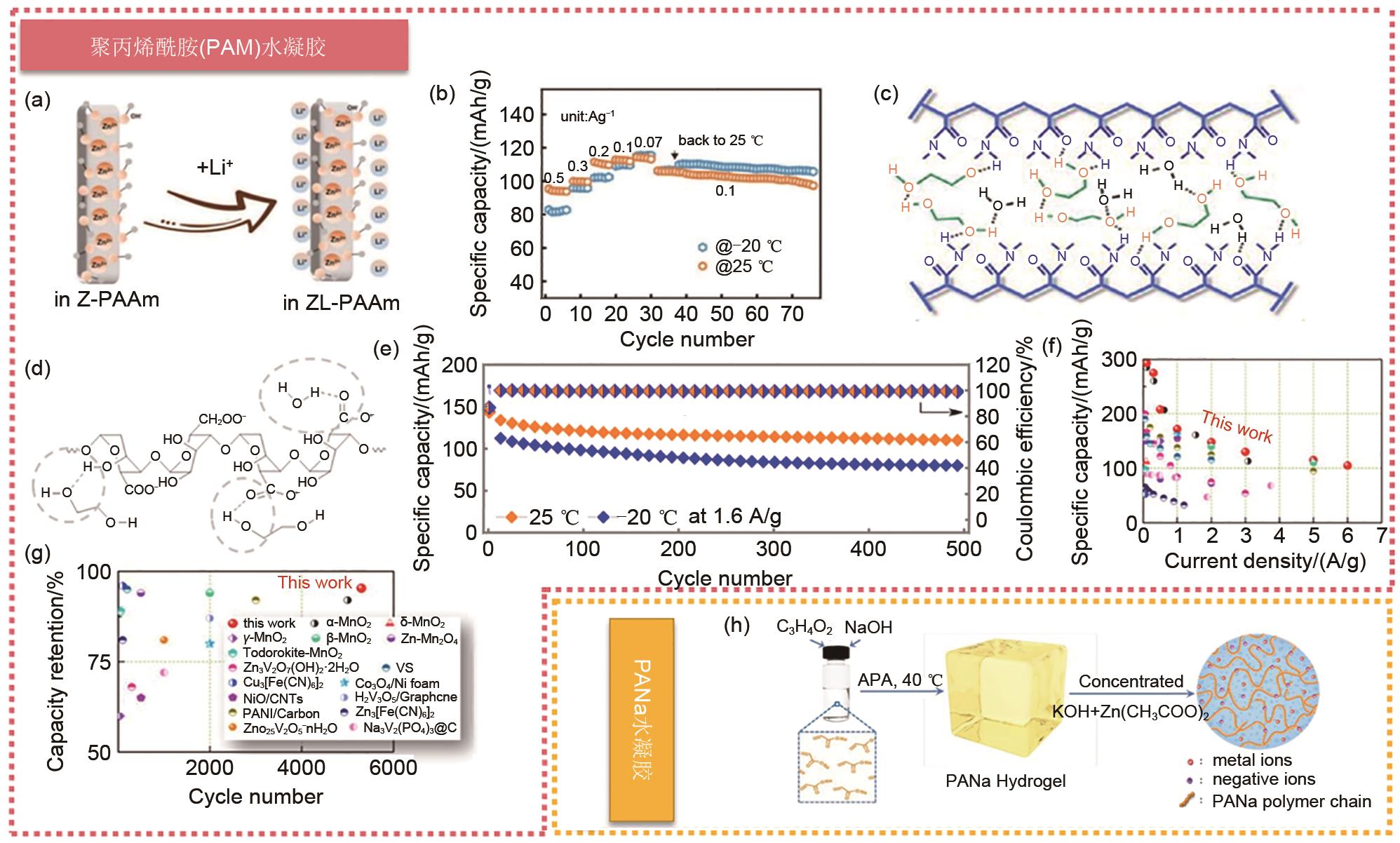
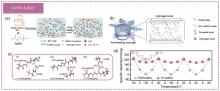
Fig. 9
(a) Schematic diagram of PAMPS-K/MC hydrogel obtained by simple solvent substitution method[79]; (b) Schematic diagram of strong hydrogen bond between EG-waPUA、H2O and PAM in EG-waPUA/PAM double-crosslinked hydrogel; (c) DFT analysis of the interaction between H2O, PAM and EG-waPUA in EG-waPUA/PAM double crosslinked hydrogel through multiple hydrogen bond; (d) Cyclic performance tests were performed on PAM and AF cells at 20, 0 and -20 ℃ at 0.3 A/g[34]"
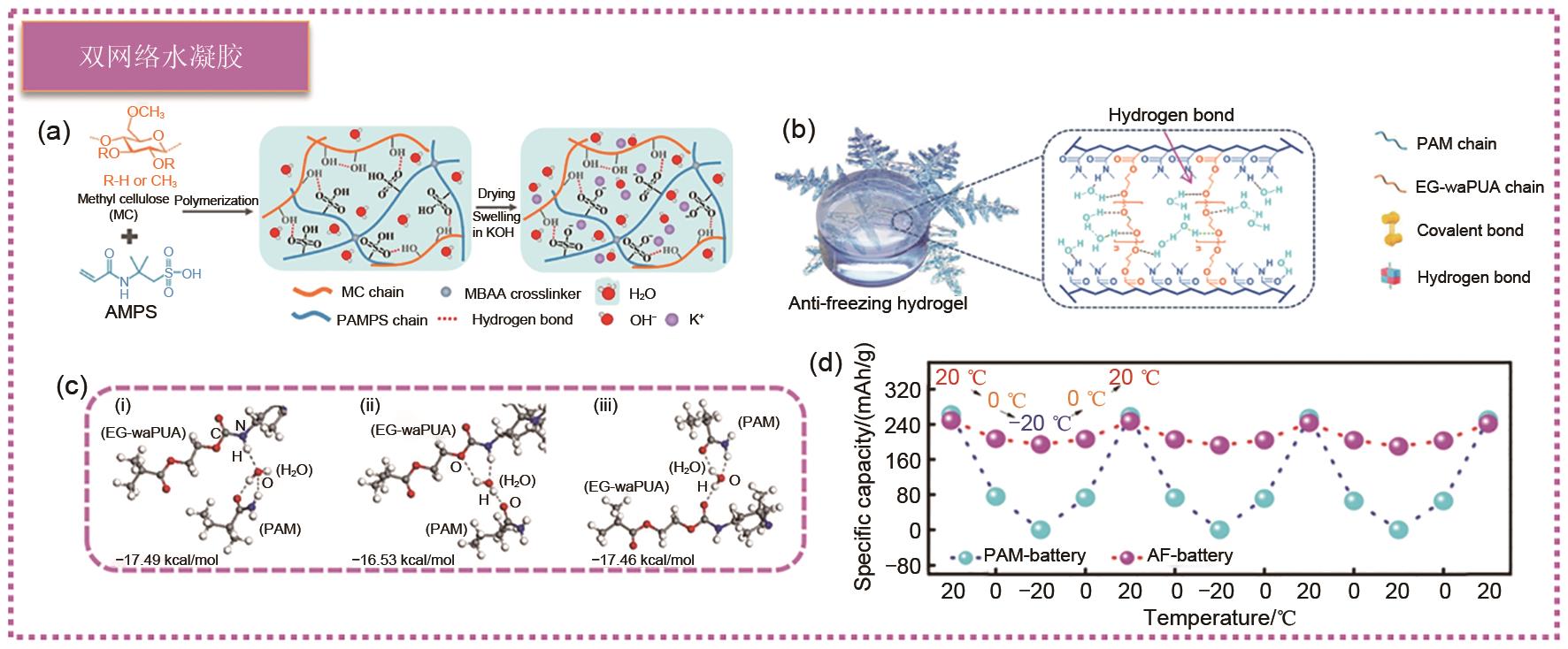

Fig. 10
(a) Comparison of the capacities of ZnCF||PANI batteries with EG and without EG at different temperatures; (b) Cycling performance of solid-state fiber-optic ZnCF||PANI batteies at -20 ℃ at 1.0 A/g[87]; Photographs and SEM images of zinc foil surface after 500 cycles with (c) unmodified electrolyte and (d) EG&Et2O modified electrolyte; (e) Cycling characteristics of Zn||MnO2 batteries without additives and electrolyte with 1% Et2O and different content of EG additives at -10 ℃ and current density of 3 A/g[88]; (f) Schematic diagram of preparation of GG/SA and GG/SA/EG hydrogel electrolytes; (g) Discharge capacity of hydrogel electrolytes containing GG, GG/SA and GG/SA/EG at 25, 0 and -20 ℃[90]; (h) Schematic diagram of synthesis of CT3G30 hydrogel electrolyte; (i) SEM images of freeze-dried CT3G30; (j) Energy density diagrams of area and (k) volume of CT3G30 hydrogel Zn||MnO2 battery compared with those previously reported at different temperatures[91]"
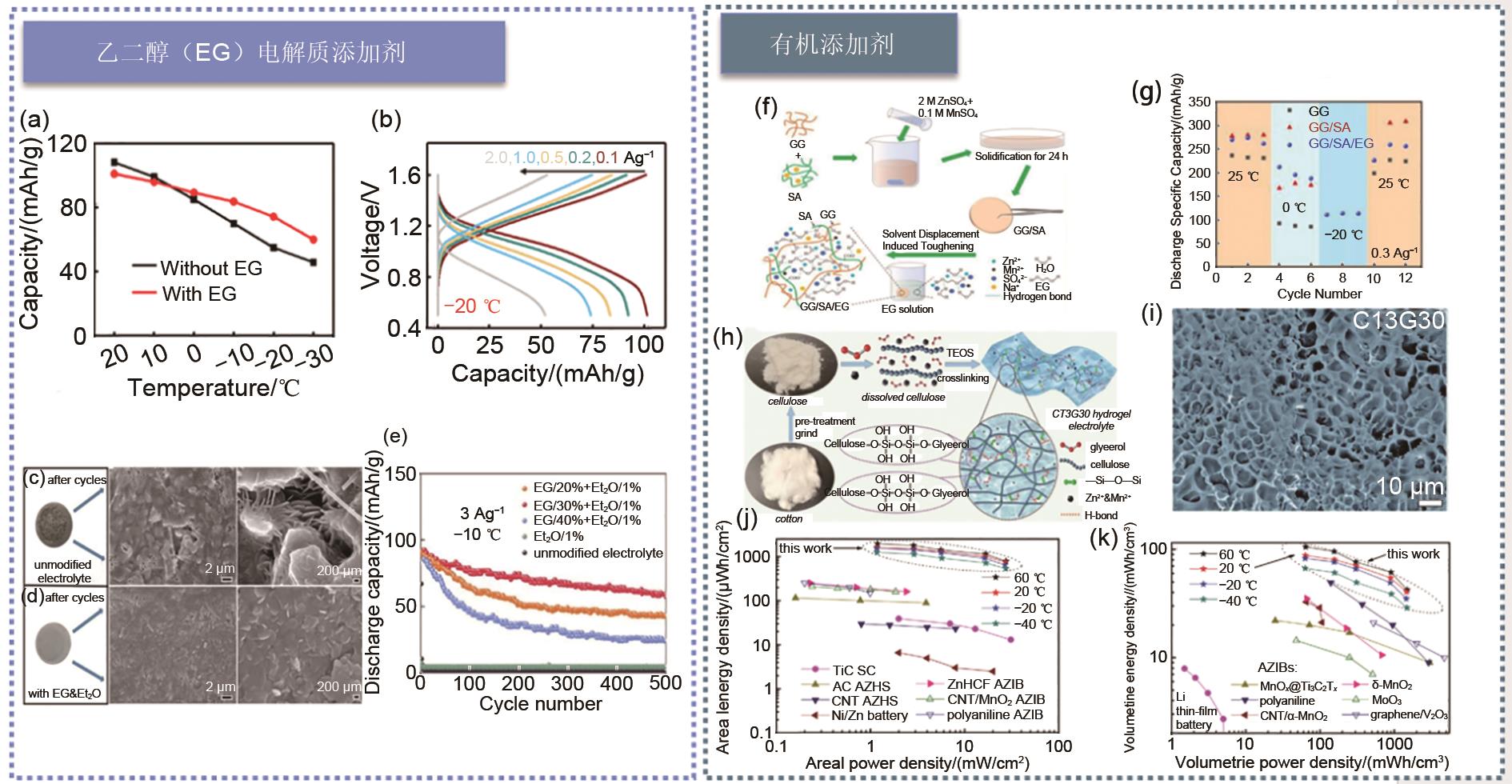
Fig. 11
(a) Schematic diagram of zinc electrolyte surface evolution with or without additive CH3COONH4; (b) Long life cycle performance of Zn||Zn symmetrical batteries in ZnSO4: CH3COONH4 and ZnSO4 electrolytes at -10 ℃. (c) Cycling performance diagram of Zn||Ac capacitor in ZnSO4: CH3COONH4 electrolyte at -10 ℃ [96]"
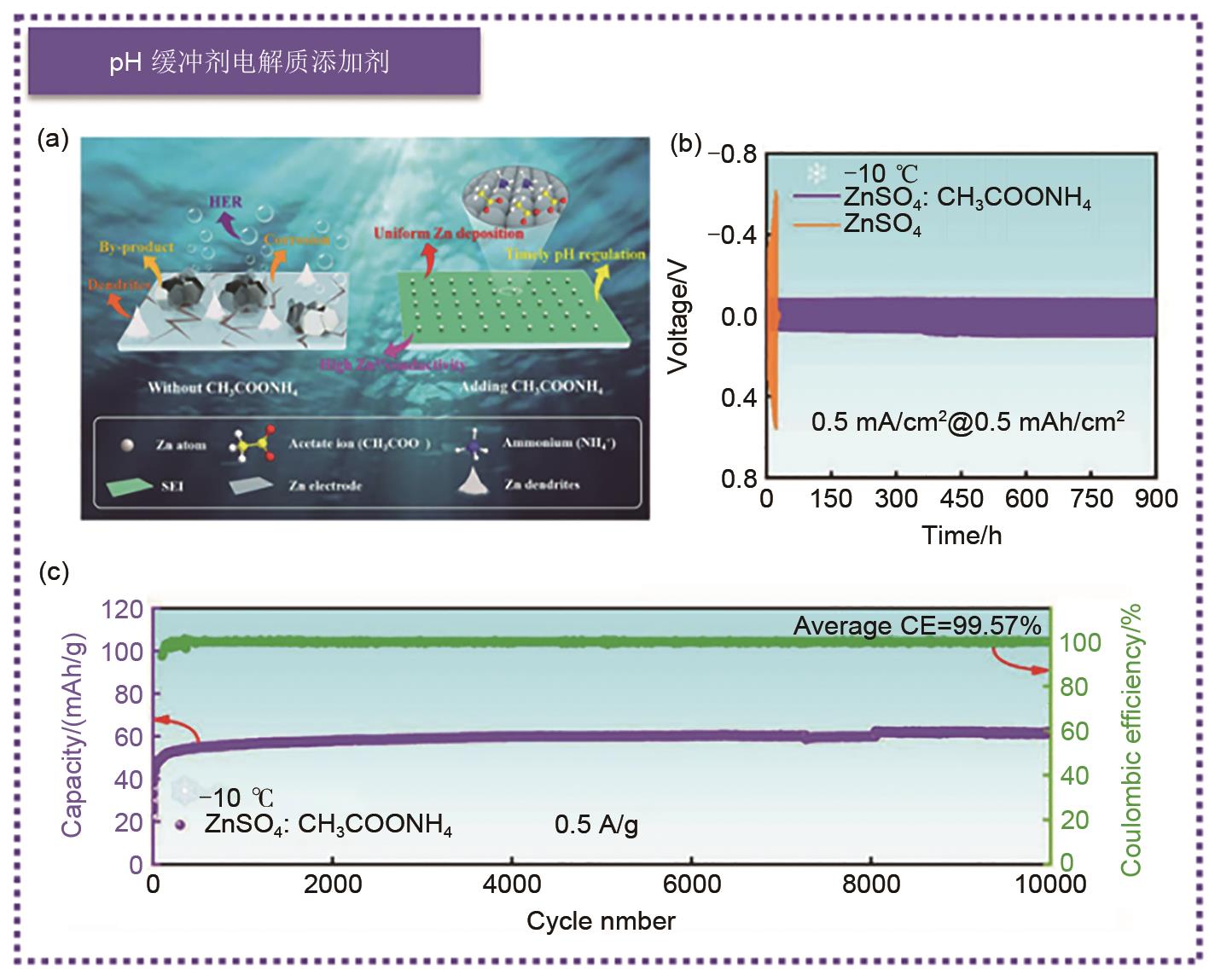

Fig. 12
(a) Schematic diagrams of electrolytes and electrolyte-electrode-interphase structures; (b) A snapshot of the system evolution at -70 ℃, with red, white, green and dark gray spheres representing O, H, Cl and Zn atoms, respectively; (c) Coulomb efficiency of Zn||Ti battery galvanizing/stripping at 1 mA/cm2 and 0.5 mAh/cm2[102]"
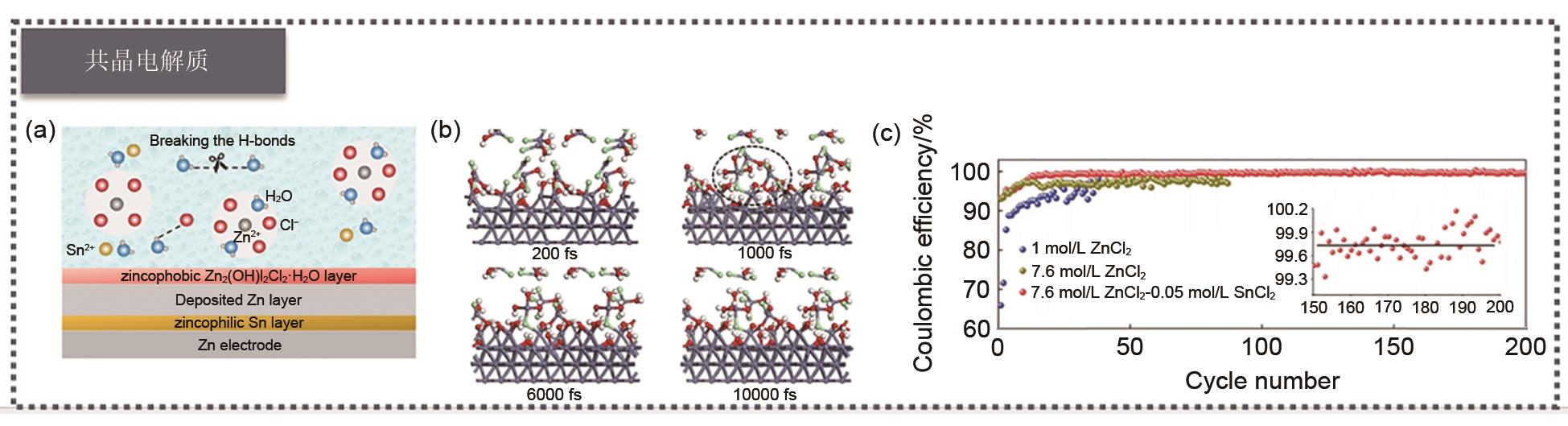
Table 1
Comparison of physicochemical properties and performances of low-temperature zinc ion batteries"
| 电解质 | 分类 | 正极材料 | 负极材料 | 离子电导率/[(mS/cm)/℃] | 运行温度范围/℃ | 凝固点/℃ | 容量/[(mAh/g)/(A/g)] | 年份 |
|---|---|---|---|---|---|---|---|---|
| 7.5 mol/L ZnCl2 | 高浓度 电解质 | 聚苯胺(PANI) | Zn | 1.79/-60 | -90~60 | -114 | -90 ℃:50.6/0.01 | 2020[ |
| 4 mol/L Zn(BF4)2 | 高浓度 电解质 | 四氯苯醌(TCBQ) | Zn | 1.47/-70 | -95~25 | -122 | -95 ℃:63.5/0.022 | 2021[ |
| 2 mol/L Zn(CF3SO3)2 | 高浓度 电解质 | V2O5 | Zn | 4.47/-30 | -30~25 | -34.1 | -30 ℃:194.1/1 | 2021[ |
| 4 mol/L Zn(TFSI)2 | 高浓度 电解质 | 聚(邻苯二酚)氧化还原共聚物 | Zn | 90/25 | -35~ 25 | -38 | -35 ℃:178/2C | 2021[ |
| ZnOTf2-DMF | 有机电解质 | 菲醌大环三聚体(PQ-MCT) | Zn | 18.9/25 | -70~150 | -70.8 | -70 ℃:31.3/0.2 | 2020[ |
| PVA/G | 凝胶电解质 | δ-MgVO | Zn | 10.7/-30 | -30~60 | -30 | -30 ℃:136.7/5 | 2020[ |
| PVA-B-G | 凝胶电解质 | MnO2 | Zn | 10.1/-30 | -35~25 | -60 | -35 ℃:133.8/0.5 | 2020[ |
| 21 mol/L LiTFSI+3 mol/L ZnOTf2+PVA | 高浓度凝胶电解质 | V2O5/GO | Zn | 2.1/20 | 0~40 | — | 0 ℃:325/0.02 | 2020[ |
| 2 mol/L ZnSO4+4 mol/L LiCl+PAM | 高浓度凝胶电解质 | LiFePO4 | Zn | — | -20~25 | -45 | -20 ℃:104/0.1 | 2019[ |
| 海藻酸锌/PAM | 水凝胶电解质 | MnO2 | Zn | 14.1/-20 | -20~80 | -30~-20 | -20 ℃:165/0.2 | 2019[ |
1 mol/L Zn(TFSI)2+ 21 mol/L LiTFSI +PAM | 高浓度凝胶电解质 | [EMIM]PF6-PEDOT:PSS/Bi2S3 | Zn | — | — | — | 0 ℃:73/1 | 2019[ |
| 6 mol/L OH-/ PANa | 高浓度凝胶电解质 | NiCo | Zn | 5.7/-20 | -20~50 | — | -20 ℃:172/1.9C | 2018[ |
| PAMPS-K/ MC | 双网络水凝胶电解质 | 空气 | Zn | 18.1/-20 | -20~25 | -30 | -20 ℃:754.2/824.6 mWh/g | 2020[ |
| EG-waPUA+PAM | 双交联水凝胶电解质 | α-MnO2/CNT | Zn | 14.6/-20 | -20~20 | -24 | -20 ℃:196/0.3 | 2019[ |
| EG+PVA | 电解质添加剂 | PANI | Zn | 2.89/-30 | -30~20 | — | -20 ℃:101.2/0.1 | 2021[ |
| Et2O+EG+2 mol/L ZnSO4+0.2 mol/L MnSO4 | 电解质添加剂 | MnO2 | Zn | 0.42/-10 | -10~25 | — | -10 ℃:65.1/3 | 2020[ |
| 2 mol/L ZnSO4 +0.1 mol/L MnSO4 +GG/SA/EG | 电解质添加剂 | MnO2 | Zn | 6.19/-20 | -20~25 | — | -20 ℃:181.5/0.1 | 2020[ |
| 2 mol/L ZnSO4/0.2 mol/L MnSO4/甘油 /纤维素/ TEOS (CT3G30) | 电解质添加剂 | rGO/MnO2 | Zn | 19.4/-40 | -40~60 | -64.6 | -20 ℃:181.5/0.1 | 2021[ |
| ZnSO4:CH3COONH4 | 电解质添加剂 | Zn | Zn | — | -10~25 | — | — | 2022[ |
| 0.05 mol/L SnCl2-7.5 mol/L ZnCl2 | 共晶电解质 | VOPO4 | Zn | 0.8/-70 | -70~ 20 | — | -70 ℃:48.7/— | 2021[ |
| 1 | 陈海生, 李泓, 马文涛, 等. 2021年中国储能技术研究进展[J]. 储能科学与技术, 2022, 11(3): 1052-1076. |
| CHEN H S, LI H, MA W T, et al. Research progress of energy storage technology in China in 2021[J]. Energy Storage Science and Technology, 2022, 11(3): 1052-1076. | |
| 2 | 张强, 李泓. 乘势而上, 奋发有为 努力推动化工与储能高质量发展[J]. 储能科学与技术, 2022, 11(6): 1682. |
| ZHANG Q, LI H. Riding on the trend and striving to be promising_Strive to promote the high-quality development of chemical industry and energy storage [J]. Energy Storage Science and Technology, 2022, 11(6): 1682. | |
| 3 | ARMAND M, TARASCON J M. Building better batteries[J]. Nature, 2008, 451(7179): 652-657. |
| 4 | DUNN B, KAMATH H, TARASCON J M. Electrical energy storage for the grid: A battery of choices[J]. Science, 2011, 334(6058): 928-935. |
| 5 | LUO F, FENG X, ZENG L, et al. In situ simultaneous encapsulation of defective MoS2 nanolayers and sulfur nanodots into SPAN fibers for high rate sodium-ion batteries[J]. Chemical Engineering Journal, 2021, 404: doi:10.1016/j.cej.2020.126430. |
| 6 | SUN Y, GUO S H, ZHOU H S. Exploration of advanced electrode materials for rechargeable sodium-ion batteries[J]. Advanced Energy Materials, 2019, 9(23): doi: 10.1002/aenm.201800212. |
| 7 | XU Q, SUN J K, LI J Y, et al. Scalable synthesis of spherical Si/C granules with 3D conducting networks as ultrahigh loading anodes in lithium-ion batteries[J]. Energy Storage Materials, 2018, 12: 54-60. |
| 8 | ZENG L X, FANG Y X, XU L H, et al. Rational design of few-layer MoSe2 confined within ZnSe-C hollow porous spheres for high-performance lithium-ion and sodium-ion batteries[J]. Nanoscale, 2019, 11(14): 6766-6775. |
| 9 | LIU Z X, LUO X, QIN L, et al. Progress and prospect of low-temperature zinc metal batteries[J]. Advanced Powder Materials, 2022, 1(2): doi: 10.1016/j.apmate.2021.10.002. |
| 10 | TAMTÖGL A, BAHN E, SACCHI M, et al. Motion of water monomers reveals a kinetic barrier to ice nucleation on graphene[J]. Nature Communications, 2021, 12: 3120. |
| 11 | ZHANG Q, MA Y L, LU Y, et al. Modulating electrolyte structure for ultralow temperature aqueous zinc batteries[J]. Nature Communications, 2020, 11: 4463. |
| 12 | MATSUMOTO M, SAITO S, OHMINE I. Molecular dynamics simulation of the ice nucleation and growth process leading to water freezing[J]. Nature, 2002, 416(6879): 409-413. |
| 13 | DENG T, ZHANG W, ZHANG H B, et al. Anti-freezing aqueous electrolyte for high-performance co(OH)2 supercapacitors at -30 ℃[J]. Energy Technology, 2018, 6(4): 605-612. |
| 14 | ZHENG J X, BOCK D C, TANG T, et al. Regulating electrodeposition morphology in high-capacity aluminium and zinc battery anodes using interfacial metal-substrate bonding[J]. Nature Energy, 2021, 6(4): 398-406. |
| 15 | HAO J N, LI X L, ZENG X H, et al. Deeply understanding the Zn anode behaviour and corresponding improvement strategies in different aqueous Zn-based batteries[J]. Energy & Environmental Science, 2020, 13(11): 3917-3949. |
| 16 | ZHENG J X, YIN J F, ZHANG D H, et al. Spontaneous and field-induced crystallographic reorientation of metal electrodeposits at battery anodes[J]. Science Advances, 2020, 6(25): eabb1122. |
| 17 | SU J R, YIN X X, ZHAO H N, et al. Temperature-dependent nucleation and electrochemical performance of Zn metal anodes[J]. Nano Letters, 2022, 22(4): 1549-1556. |
| 18 | LI F Y, HU X L. Zinc metal energy storage devices under extreme conditions of low temperatures[J]. Batteries & Supercaps, 2021, 4(3): 389-406. |
| 19 | NIAN Q S, SUN T, LIU S, et al. Issues and opportunities on low-temperature aqueous batteries[J]. Chemical Engineering Journal, 2021, 423: do: 10.1016/j.cej.2021.130253. |
| 20 | RAMANUJAPURAM A, YUSHIN G. Understanding the exceptional performance of lithium-ion battery cathodes in aqueous electrolytes at subzero temperatures[J]. Advanced Energy Materials, 2018, 8(35): doi: 10.1002/aenm.201802624. |
| 21 | XU L H, CHEN X C, GUO W T, et al. Co-construction of sulfur vacancies and carbon confinement in V5S8/CNFs to induce an ultra-stable performance for half/full sodium-ion and potassium-ion batteries[J]. Nanoscale, 2021, 13(9): 5033-5044. |
| 22 | XU L, GUO W, ZENG L, et al. V3Se4 embedded within N/P co-doped carbon fibers for sodium/potassium ion batteries[J]. Chemical Engineering Journal, 2021, 419: doi: 10.1016/j.cej.2021.129607. |
| 23 | WANG Y Y, CHEN X, CHEN X C, et al. Stabilizing intermediate phases via the efficient confinement effects of the SnS2-SPAN fibre composite for ultra-stable half/full sodium/potassium-ion batteries[J]. Journal of Materials Chemistry A, 2022, 10(21): 11449-11457. |
| 24 | WANG Y, LIU J, CHEN X, et al. Structural engineering of tin sulfides anchored on nitrogen/phosphorus dual-doped carbon nanofibres in sodium/potassium-ion batteries[J]. Carbon, 2022, 189: 46-56. |
| 25 | GUO Z W, HUANG J H, DONG X L, et al. An organic/inorganic electrode-based hydronium-ion battery[J]. Nature Communications, 2020, 11: 959. |
| 26 | RUFFO R, LA MANTIA F, WESSELLS C, et al. Electrochemical characterization of LiCoO2 as rechargeable electrode in aqueous LiNO3 electrolyte[J]. Solid State Ionics, 2011, 192(1): 289-292. |
| 27 | ZHANG Y, XU J, LI Z, et al. All-climate aqueous Na-ion batteries using "water-in-salt" electrolyte[J]. Science Bulletin, 2022, 67(2): 161-170. |
| 28 | LIU S L, ZHANG R Z, MAO J F, et al. From room temperature to harsh temperature applications: Fundamentals and perspectives on electrolytes in zinc metal batteries[J]. Science Advances, 2022, 8(12): doi: 10.1126/sciadv.abn5097. |
| 29 | WU X Y, QIU S, XU Y K, et al. Hydrous nickel-iron turnbull's blue as a high-rate and low-temperature proton electrode[J]. ACS Applied Materials & Interfaces, 2020, 12(8): 9201-9208. |
| 30 | YAN L, HUANG J H, GUO Z W, et al. Solid-state proton battery operated at ultralow temperature[J]. ACS Energy Letters, 2020, 5(2): 685-691. |
| 31 | WEI T T, PENG Y Q, MO L E, et al. Modulated bonding interaction in propanediol electrolytes toward stable aqueous zinc-ion batteries[J]. Science China Materials, 2022, 65(5): 1156-1164. |
| 32 | CHEN S, ZHANG Y, GENG H, et al. Zinc ions pillared vanadate cathodes by chemical pre-intercalation towards long cycling life and low-temperature zinc ion batteries[J]. Journal of Power Sources, 2019, 441: doi: 10.1016/j.jpowsour.2019.227192. |
| 33 | YANG Y Q, TANG Y, FANG G Z, et al. Li+ intercalated V2O5 · nH2O with enlarged layer spacing and fast ion diffusion as an aqueous zinc-ion battery cathode[J]. Energy & Environmental Science, 2018, 11(11): 3157-3162. |
| 34 | MO F N, LIANG G J, MENG Q Q, et al. A flexible rechargeable aqueous zinc manganese-dioxide battery working at -20 ℃[J]. Energy & Environmental Science, 2019, 12(2): 706-715. |
| 35 | HE T, YE YS, LI H, et al. Oxygen-deficient ammonium vanadate for flexible aqueous zinc batteries with high energy density and rate capability at -30 ℃[J]. Materials Today, 2021, 43: 53-61. |
| 36 | HE T. Cation-deficient Zn0.3(NH4)0.3V4O101·0.91H2O for rechargeable aqueous zinc battery with superior low- temperature performance[J]. Energy Storage Materials, 2021, 38: 389-396. |
| 37 | YANG Y, TANG Y, LIANG S, et al.Transition metal ion-preintercalated V2O5 as high-performance aqueous zinc-ion battery cathode with broad temperature adaptability[J]. Nano Energy, 2019, 61: 617-625. |
| 38 | CHUAI M, YANG J, WANG M, et al. High-performance Zn battery with transition metal ions co-regulated electrolytic MnO2[J]. eScience, 2021, 1(2): 178-185. |
| 39 | 熊小琳, 岳金明, 周安行, 等. 尖晶石锰酸锂正极在Water-in-salt电解液中的电化学性能[J]. 储能科学与技术, 2020, 9(2): 375-384. |
| XIONG X L, YUE J M, ZHOU A X, et al. Electrochemical performance of spinel LiMn2O4 in water-in-salt aqueous electrolyte[J]. Energy Storage Science and Technology, 2020, 9(2): 375-384. | |
| 40 | ZHENG S B, SHI D J, YAN D, et al. Orthoquinone-based covalent organic frameworks with ordered channel structures for ultrahigh performance aqueous zinc-organic batteries[J]. Angewandte Chemie International Edition, 2022, 61(12): doi: 10.1002/anie. 202117511. |
| 41 | LIANG Y L, JING Y, GHEYTANI S, et al. Universal quinone electrodes for long cycle life aqueous rechargeable batteries[J]. Nature Materials, 2017, 16(8): 841-848. |
| 42 | MA L T, LI N, LONG C B, et al. Achieving both high voltage and high capacity in aqueous zinc-ion battery for record high energy density[J]. Advanced Functional Materials, 2019, 29(46): doi: 10.1002/adfm.201906142. |
| 43 | ZHANG H, LIU X, LI H H, et al. High-voltage operation of a V2O5 cathode in a concentrated gel polymer electrolyte for high-energy aqueous zinc batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(13): 15305-15312. |
| 44 | MO F N, LIANG G J, WANG D H, et al. Biomimetic organohydrogel electrolytes for high-environmental adaptive energy storage devices[J]. EcoMat, 2019, 1(1): e12008. |
| 45 | ZHAO Y W, MA L T, ZHU Y B, et al. Inhibiting grain pulverization and sulfur dissolution of bismuth sulfide by ionic liquid enhanced poly(3, 4-ethylenedioxythiophene): Poly(styrenesulfonate) for high-performance zinc-ion batteries[J]. ACS Nano, 2019, 13(6): 7270-7280. |
| 46 | LUKATSKAYA M R, FELDBLYUM J I, MACKANIC D G, et al. Concentrated mixed cation acetate "water-in-salt" solutions as green and low-cost high voltage electrolytes for aqueous batteries[J]. Energy & Environmental Science, 2018, 11(10): 2876-2883. |
| 47 | HU P, YAN M Y, ZHU T, et al. Zn/V2O5 aqueous hybrid-ion battery with high voltage platform and long cycle life[J]. ACS Applied Materials & Interfaces, 2017, 9(49): 42717-42722. |
| 48 | HUANG Y, LI Z, PEI Z X, et al. Solid-state rechargeable Zn||NiCo and Zn-air batteries with ultralong lifetime and high capacity: The role of a sodium polyacrylate hydrogel electrolyte[J]. Advanced Energy Materials, 2018, 8(31): doi: 10.1002/aenm.201802288. |
| 49 | SUN T J, YUAN X M, WANG K, et al. An ultralow-temperature aqueous zinc-ion battery[J]. Journal of Materials Chemistry A, 2021, 9(11): 7042-7047. |
| 50 | ZHANG Y J, CREMER P S. Interactions between macromolecules and ions: The hofmeister series[J]. Current Opinion in Chemical Biology, 2006, 10(6): 658-663. |
| 51 | ZHANG Q, XIA K X, MA Y L, et al. Chaotropic anion and fast-kinetics cathode enabling low-temperature aqueous Zn batteries[J]. ACS Energy Letters, 2021, 6(8): 2704-2712. |
| 52 | GUAN C, HU F, YU X, et al. High performance of HNaV6O16 ·4H2O nanobelts for aqueous zinc-ion batteries with in situ phase transformation by Zn(CF3SO3)2 electrolyte[J]. Rare Metals, 2022, 41(2): 448-456. |
| 53 | ZHANG N, CHENG F Y, LIU Y C, et al. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery[J]. Journal of the American Chemical Society, 2016, 138(39): 12894-12901. |
| 54 | PATIL N, DE LA CRUZ C, CIURDUC D, et al. An ultrahigh performance zinc-organic battery using poly(catechol) cathode in Zn(TFSI)2-based concentrated aqueous electrolytes[J]. Advanced Energy Materials, 2021, 11(26): doi: 10.1002/aenm.202100939. |
| 55 | ZHANG Y, ZHAO L, LIANG Y, et al. Effect of electrolyte anions on the cycle life of a polymer electrode in aqueous batteries[J]. eScience, 2022, 2(1): 110-115. |
| 56 | BANIK S J, AKOLKAR R. Suppressing dendrite growth during zinc electrodeposition by PEG-200 additive[J]. Journal of the Electrochemical Society, 2013, 160(11): D519-D523. |
| 57 | ZHAO Z M, ZHAO J W, HU Z L, et al. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase[J]. Energy & Environmental Science, 2019, 12(6): 1938-1949. |
| 58 | MORÓN L E. Electrodeposition and corrosion behavior of Zn coatings formed using as brighteners arene additives of different structure[J]. Surface and Coatings Technology, 2011, 205(21/22): 4985-4992. |
| 59 | WANG N, DONG X L, WANG B L, et al. Zinc-organic battery with a wide operation-temperature window from -70 to 150 ℃[J]. Angewandte Chemie International Edition, 2020, 59(34): 14577-14583. |
| 60 | RODRIGUES M T F, BABU G, GULLAPALLI H, et al. A materials perspective on Li-ion batteries at extreme temperatures[J]. Nature Energy, 2017, 2: 17108. |
| 61 | YUE F, TIE Z W, DENG S Z, et al. An ultralow temperature aqueous battery with proton chemistry[J]. Angewandte Chemie International Edition, 2021, 60(25): 13882-13886. |
| 62 | JIANG H, SHIN W, MA L, et al. A high-rate aqueous proton battery delivering power below -78 ℃ via an unfrozen phosphoric acid[J]. Advanced Energy Materials, 2020, 10(28): doi: 10.1002/aenm.202000968. |
| 63 | HUANG S, WAN F, BI S S, et al. A self-healing integrated all-in-one zinc-ion battery[J]. Angewandte Chemie International Edition, 2019, 58(13): 4313-4317. |
| 64 | LIU Z, WANG D, TANG Z, et al. A mechanically durable and device-level tough Zn-MnO2 battery with high flexibility[J]. Energy Storage Materials, 2019, 23: 636-645. |
| 65 | LI C, LI P, YANG S, et al. Recently advances in flexible zinc ion batteries[J]. Journal of Semiconductors, 2021, 42(10): doi: 10.1088/1674-4926/42/10/101603. |
| 66 | LIANG G J, WANG Y L, HUANG Z D, et al. Initiating hexagonal MoO3 for superb-stable and fast NH4 + storage based on hydrogen bond chemistry[J]. Advanced Materials, 2020, 32(14): doi: 10.1002/adma.201907802. |
| 67 | PEI Z X, YUAN Z W, WANG C J, et al. A flexible rechargeable zinc-air battery with excellent low-temperature adaptability[J]. Angewandte Chemie International Edition, 2020, 59(12): 4793-4799. |
| 68 | ZHOU W J, CHEN J Z, CHEN M F, et al. An environmentally adaptive quasi-solid-state zinc-ion battery based on magnesium vanadate hydrate with commercial-level mass loading and anti-freezing gel electrolyte[J]. Journal of Materials Chemistry A, 2020, 8(17): 8397-8409. |
| 69 | CHEN M F, ZHOU W J, WANG A R, et al. Anti-freezing flexible aqueous Zn-MnO2 batteries working at -35 ℃ enabled by a borax-crosslinked polyvinyl alcohol/glycerol gel electrolyte[J]. Journal of Materials Chemistry A, 2020, 8(14): 6828-6841. |
| 70 | ZHU M S, WANG X J, TANG H M, et al. Antifreezing hydrogel with high zinc reversibility for flexible and durable aqueous batteries by cooperative hydrated cations[J]. Advanced Functional Materials, 2020, 30(6): doi: 10.1002/adfm.201907218. |
| 71 | LIAO H, GUO X L, WAN P B, et al. Conductive MXene nanocomposite organohydrogel for flexible, healable, low-temperature tolerant strain sensors[J]. Advanced Functional Materials, 2019, 29(39): doi: 10.1002/adfm.201904507. |
| 72 | ZHANG S, ZHANG Y H, LI B, et al. One-Step preparation of a highly stretchable, conductive, and transparent poly(vinyl alcohol)-phytic acid hydrogel for casual writing circuits[J]. ACS Applied Materials & Interfaces, 2019, 11(35): 32441-32448. |
| 73 | FANG L Y, CAI Z F, DING Z Q, et al. Skin-inspired surface-microstructured tough hydrogel electrolytes for stretchable supercapacitors[J]. ACS Applied Materials & Interfaces, 2019, 11(24): 21895-21903. |
| 74 | ZHOU D, CHEN F, HANDSCHUH-WANG S, et al. Biomimetic extreme-temperature- and environment-adaptable hydrogels[J]. ChemPhysChem, 2019, 20(17): 2139-2154. |
| 75 | WANG H, LIU J, WANG J Q, et al. Concentrated hydrogel electrolyte-enabled aqueous rechargeable NiCo// Zn battery working from -20 to 50 ℃[J]. ACS Applied Materials & Interfaces, 2019, 11(1): 49-55. |
| 76 | APPEL E A, DEL BARRIO J, LOH X J, et al. Supramolecular polymeric hydrogels[J]. Chemical Society Reviews, 2012, 41: 6195-6214. |
| 77 | DENG Y, HUANG M, SUN D, et al. Dual physically cross-linked κ-carrageenan-based double network hydrogels with superior self-healing performance for biomedical application[J]. ACS Applied Materials & Interfaces, 2018, 10(43): 37544-37554. |
| 78 | GONG J P, KATSUYAMA Y, KUROKAWA T, et al. Double-network hydrogels with extremely high mechanical strength[J]. Advanced Materials, 2003, 15(14): 1155-1158. |
| 79 | SUN N, LU F, YU Y, et al. Alkaline double-network hydrogels with high conductivities, superior mechanical performances, and antifreezing properties for solid-state zinc-air batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(10): 11778-11788. |
| 80 | NIAN Q S, WANG J Y, LIU S, et al. Aqueous batteries operated at -50 ℃[J]. Angewandte Chemie, 2019, 131(47): 17150-17155. |
| 81 | LIU S, LI G R, GAO X P. Lanthanum nitrate as electrolyte additive to stabilize the surface morphology of lithium anode for lithium-sulfur battery[J]. ACS Applied Materials & Interfaces, 2016, 8(12): 7783-7789. |
| 82 | ZHENG J M, ENGELHARD M H, MEI D H, et al. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries[J]. Nature Energy, 2017, 2: 17012. |
| 83 | WANG R, LI X, WANG Z, et al. Electrochemical analysis graphite/electrolyte interface in lithium-ion batteries: P-Toluenesulfonyl isocyanate as electrolyte additive[J]. Nano Energy, 2017, 34: 131-140. |
| 84 | SUN P, MA L, ZHOU W H, et al. Simultaneous regulation on solvation shell and electrode interface for dendrite-free Zn ion batteries achieved by a low-cost glucose additive[J]. Angewandte Chemie International Edition, 2021, 60(33): 18247-18255. |
| 85 | CAO L S, LI D, HU E Y, et al. Solvation structure design for aqueous Zn metal batteries[J]. Journal of the American Chemical Society, 2020, 142(51): 21404-21409. |
| 86 | CHANG N N, LI T Y, LI R, et al. An aqueous hybrid electrolyte for low-temperature zinc-based energy storage devices[J]. Energy & Environmental Science, 2020, 13(10): 3527-3535. |
| 87 | CONG Z F, GUO W B, ZHANG P P, et al. Wearable antifreezing fiber-shaped Zn|PANI batteries with suppressed Zn dendrites and operation in sweat electrolytes[J]. ACS Applied Materials & Interfaces, 2021, 13(15): 17608-17617. |
| 88 | WANG A, ZHOU W, HUANG A, et al. Developing improved electrolytes for aqueous zinc-ion batteries to achieve excellent cyclability and antifreezing ability[J]. Journal of Colloid and Interface Science, 2021, 586: 362-370. |
| 89 | DING Y Y, ZHONG X, YUAN C M, et al. Sodium alginate binders for bivalency aqueous batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(17): 20681-20688. |
| 90 | WANG J, HUANG Y, LIU B, et al. Flexible and anti-freezing zinc-ion batteries using a guar-gum/sodium-alginate/ethylene-glycol hydrogel electrolyte[J]. Energy Storage Materials, 2021, 41: 599-605. |
| 91 | CHEN M F, CHEN J Z, ZHOU W J, et al. Realizing an all-round hydrogel electrolyte toward environmentally adaptive dendrite-free aqueous Zn-MnO2 batteries[J]. Advanced Materials, 2021, 33(9): doi: 10.1002/adma.202007559. |
| 92 | HUANG C, ZHAO X, HAO Y S, et al. Highly reversible zinc metal anodes enabled by protonated melamine[J]. Journal of Materials Chemistry A, 2022, 10(12): 6636-6640. |
| 93 | ZENG X H, MAO J F, HAO J N, et al. Electrolyte design for in situ construction of highly Zn2+-conductive solid electrolyte interphase to enable high-performance aqueous Zn-ion batteries under practical conditions[J]. Advanced Materials, 2021, 33(11): doi: 10.1002/adma.202007416. |
| 94 | YANG Q, LI L, HUSSAIN T, et al. Stabilizing interface pH by N-modified graphdiyne for dendrite-free and high-rate aqueous Zn-ion batteries[J]. Angewandte Chemie International Edition, 2022, 61(6): doi: 10.1002/anie.202112304. |
| 95 | HAN D L, WANG Z X, LU H T, et al. A self-regulated interface toward highly reversible aqueous zinc batteries[J]. Advanced Energy Materials, 2022, 12(9): doi: 10.1002/aenm.202102982. |
| 96 | LIN C Y, YANG X H, XIONG P X, et al. High-rate, large capacity, and long life dendrite-free Zn metal anode enabled by trifunctional electrolyte additive with a wide temperature range[J]. Advanced Science, 2022, 9(21): doi: 10.1002/advs.202201433. |
| 97 | CAO L S, LI D, DENG T, et al. Hydrophobic organic-electrolyte-protected zinc anodes for aqueous zinc batteries[J]. Angewandte Chemie International Edition, 2020, 59(43): 19292-19296. |
| 98 | YANG H J, QIAO Y, CHANG Z, et al. Reducing water activity by zeolite molecular sieve membrane for long-life rechargeable zinc battery[J]. Advanced Materials, 2021, 33(38): doi: 10.1002/adma. 202102415. |
| 99 | XIE X S, LIANG S Q, GAO J W, et al. Manipulating the ion-transfer kinetics and interface stability for high-performance zinc metal anodes[J]. Energy & Environmental Science, 2020, 13(2): 503-510. |
| 100 | ZENG Y X, ZHANG X Y, MENG Y, et al. Achieving ultrahigh energy density and long durability in a flexible rechargeable quasi-solid-state Zn-MnO2 battery[J]. Advanced Materials, 2017, 29(26): doi: 10.1002/adma.201700274. |
| 101 | AH-LUNG G, FLAMME B, GHAMOUSS F, et al. Guidelines for designing highly concentrated electrolytes for low temperature applications[J]. Chemical Communications (Cambridge, England), 2020, 56(68): 9830-9833. |
| 102 | CAO L S, LI D, SOTO F A, et al. Highly reversible aqueous zinc batteries enabled by zincophilic-zincophobic interfacial layers and interrupted hydrogen-bond electrolytes[J]. Angewandte Chemie International Edition, 2021, 60(34): 18845-18851. |
| 103 | WANG D D, LV D, PENG H L, et al. Site-selective adsorption on ZnF2/Ag coated Zn for advanced aqueous zinc-metal batteries at low temperature[J]. Nano Letters, 2022, 22(4): 1750-1758. |
| 104 | SONG C L, GONG Z S, BAI C, et al. High performance Zn-I2 battery with acetonitrile electrolyte working at low temperature[J]. Nano Research, 2022, 15(4): 3170-3177. |
| 105 | MA Z, CHEN J, VATAMANU J, et al. Expanding the low-temperature and high-voltage limits of aqueous lithium-ion battery[J]. Energy Storage Materials, 2022, 45: 903-910. |
| [1] | Xinyi WANG, Weijie LI, Chao HAN, Huakun LIU, Shixue DOU. Challenges and optimization strategies of the anode of aqueous zinc-ion battery [J]. Energy Storage Science and Technology, 2022, 11(4): 1211-1225. |
| [2] | Yongli HENG, Zhenyi GU, Jinzhi GUO, Xinglong WU. Na3V2(PO4)3@C cathode material for aqueous zinc-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(3): 938-944. |
| [3] | LI Fangfang, CHEN Shimou. Research progress on electrolyte additives for high voltage lithium-ion batteries [J]. Energy Storage Science and Technology, 2016, 5(4): 436-442. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
