Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (4): 1025-1033.doi: 10.19799/j.cnki.2095-4239.2022.0727
• Energy Storage Materials and Devices • Previous Articles Next Articles
Received:2022-12-05
Revised:2022-12-19
Online:2023-04-05
Published:2023-05-08
Contact:
Chao TAN
E-mail:826985826@qq.com
CLC Number:
Chao TAN, Chao WANG. Study on the performance of functionalized graphene oxide as positive sulfur carrier for lithium-sulfur batteries[J]. Energy Storage Science and Technology, 2023, 12(4): 1025-1033.
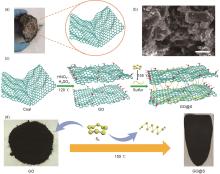
Fig. 1
(a) Schematic diagram of the synthesis of coal-based graphene oxide (GO); (b) Scanning electron microscope (SEM) diagram of coal; (c) Schematic diagram of lithium-sulfur batteries cathode material synthesis; (d) Physical picture of coal-based GO and schematic diagram of slurry synthesis route"
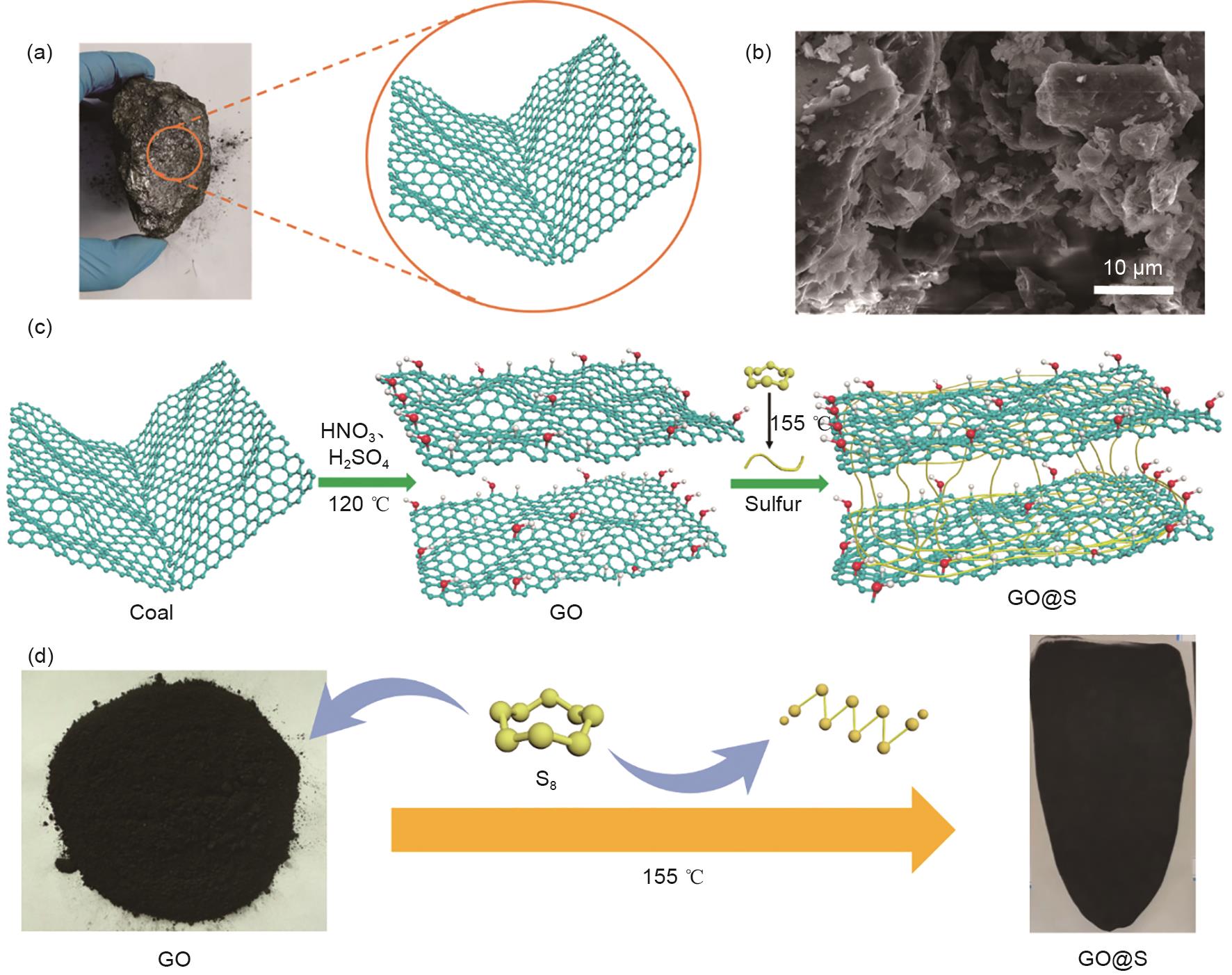

Fig. 2
(a)—(c) Scanning electron microscope (SEM) images of coal-based graphene oxide (GO) with corresponding sizes of 1 μm, 5 μm, and 20 μm, respectively; (d) Transmission electron microscopy (TEM) image of coal-based graphene oxide (GO); (e) Magnification high-power transmission electron microscope (HRTEM) images at (d) marks; (f) X-ray diffraction (XRD) diagram of coal-based GO; (g) Raman spectra of coal-based graphene (G) and coal-based graphene oxide (GO); (h) and (i) are nitrogen adsorption/desorption ratio surface (BET) and pore size profiles of coal-based GO, respectively"


Fig. 3
Electrochemical performance test of lithium sulfur batteries: (a) CV curves of lithium-sulfur batteries at GO@S and G@S cathodes at 0.1 mV/s scan rate; (b) and (c) CV curves of lithium-sulfur batteries with cathodes containing GO@S and G@S under different voltage windows. The insets are Tafel curves corresponding to (b) peaks 1 and (c) peaks 2; (d) Comparison of charge and discharge curves of lithium-sulfur batteries with GO@S and G@S cathodes at 0.1 C; (e) and (f) EIS curves of lithium-sulfur batteries with cathodes containing GO@S and G@S before and after cycling, respectively"


Fig. 4
(a) and (b) are the CV curves of G@S and GO@S lithium-sulfur batteries at different scan rates (0.1—0.5 mV/s), respectively; (c) Anodic oxidation process (peak 1); (d) The first cathodic reduction process (peak 2); (e) The second cathodic reduction process (peak 3), the relationship between CV peak current and the square root of scanning rate"
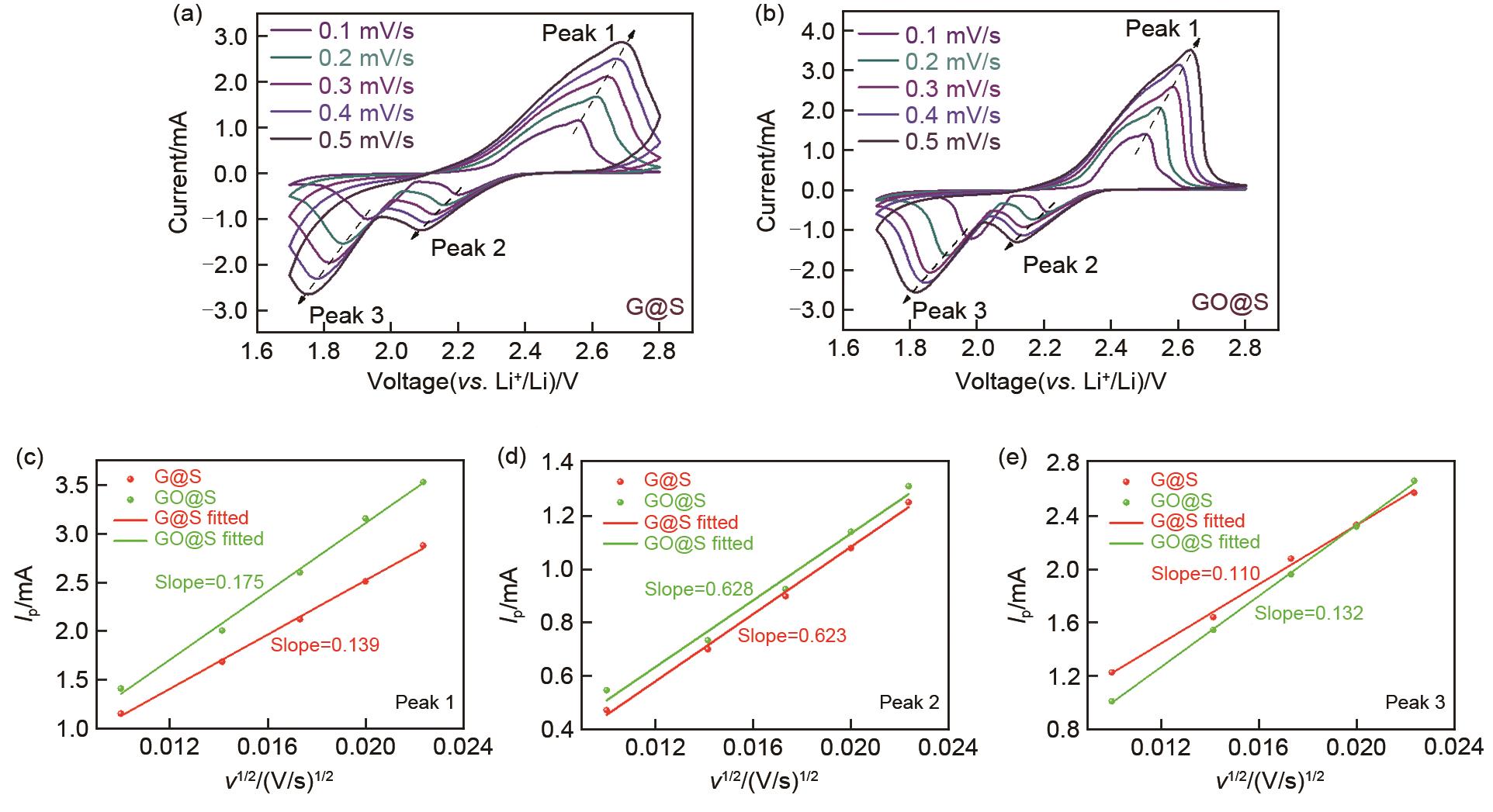
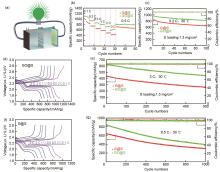
Fig. 5
(a) Schematic diagram of Lithium-sulfur batteries structure; (b) Performance comparison of lithium-sulfur batteries with cathodes containing GO@S and G@S at different magnifications from 0.1 C to 3 C; (c) Comparison of cycle performance of lithium-sulfur batteries with cathodes containing GO@S and G@S at 0.2 C; (d) and (f) The cathode contains lithium sulfur electricity GO@S and G@S comparison of charging and discharging curves of the cell at different rates of 0.1—3 C; (e) The lithium-sulfur batteries containing GO@S and G@S were compared 500 times at a rate of 3 C; (g) The lithium-sulfur batteries with cathodes containing GO@S and G@S were compared 1000 times at a rate of 0.5 C"

| 1 | PENG H J, HUANG J Q, ZHANG Q. A review of flexible lithium–sulfur and analogous alkali metal-chalcogen rechargeable batteries[J]. Chemical Society Reviews, 2017, 46(17): 5237-5288. |
| 2 | CHONG W G, HUANG J Q, XU Z L, et al. Lithium-sulfur battery cable made from ultralight, flexible graphene/carbon nanotube/sulfur composite fibers[J]. Advanced Functional Materials, 2017, 27(4): doi: 10.1002/adfm.201604815. |
| 3 | WANG Y Z, HUANG X X, ZHANG S Q, et al. Sulfur hosts against the shuttle effect[J]. Small Methods, 2018, 2(6): doi: 10.1002/smtd.201700345. |
| 4 | WANG L, HUA W X, WAN X, et al. Design rules of a sulfur redox electrocatalyst for lithium-sulfur batteries[J]. Advanced Materials (Deerfield Beach, Fla), 2022, 34(14): doi: 10.1002/adma.202110279. |
| 5 | XU R, TANG H A, ZHOU Y Y, et al. Enhanced catalysis of radical-to-polysulfide interconversion via increased sulfur vacancies in lithium-sulfur batteries[J]. Chemical Science, 2022, 13(21): 6224-6232. |
| 6 | BAEK M, SHIN H, CHAR K, et al. New high donor electrolyte for lithium-sulfur batteries[J]. Advanced Materials (Deerfield Beach, Fla), 2020, 32(52): doi: 10.1002/adma.202005022. |
| 7 | JI X L, LEE K T, NAZAR L F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries[J]. Nature Materials, 2009, 8(6): 500-506. |
| 8 | PAN Y, YAN S, LIU Y Q, et al. Significantly enhanced electrochemical performance of 2D Ni-MOF by carbon quantum dot for high-performance supercapacitors[J]. Electrochimica Acta, 2022, 422: doi: 10.1016/j.electacta.2022.140560. |
| 9 | ZHU J Y, WANG L X, GAN X M, et al. Graphene quantum dot inlaid carbon nanofibers: Revealing the edge activity for ultrahigh rate pseudocapacitive energy storage[J]. Energy Storage Materials, 2022, 47: 158-166. |
| 10 | PARK J, MOON J, RI V, et al. Nitrogen-doped graphene quantum dots: Sulfiphilic additives for the high-performance Li-S cells[J]. ACS Applied Energy Materials, 2021, 4(4): 3518-3525. |
| 11 | LI S, LUO Z, TU H Y, et al. N, S-codoped carbon dots as deposition regulating electrolyte additive for stable lithium metal anode[J]. Energy Storage Materials, 2021, 42: 679-686. |
| 12 | XU L Q, LI J Y, LI L, et al. Carbon dots evoked Li ion dynamics for solid state battery[J]. Small (Weinheim an Der Bergstrasse, Germany), 2021, 17(39): doi: 10.1002/smll.202102978. |
| 13 | WANG A N, HONG W W, LI L, et al. Hierarchical bismuth composite for fast lithium storage: Carbon dots tuned interfacial interaction[J]. Energy Storage Materials, 2022, 44: 145-155. |
| 14 | GAO Z W, WANG Y, LIU H, et al. Tailoring the interface in FAPbI3 planar perovskite solar cells by imidazole-graphene-quantum-dots[J]. Advanced Functional Materials, 2021, 31(27): doi: 10.1002/adfm.202101438. |
| 15 | HAN Y D, WU J, LI Y, et al. Carbon dots enhance the interface electron transfer and photoelectrochemical kinetics in TiO2 photoanode[J]. Applied Catalysis B: Environmental, 2022, 304: doi: 10.1016/j.apcatb.2021.120983. |
| 16 | CHENG R Q, JIANG M, LI K Q, et al. Dimensional engineering of carbon dots derived sulfur and nitrogen co-doped carbon as efficient oxygen reduction reaction electrocatalysts for aluminum-air batteries[J]. Chemical Engineering Journal, 2021, 425: doi: 10.1016/j.cej.2021.130603. |
| 17 | LIN X R, YANG C Y, HAN T L, et al. A graphene oxide scaffold-encapsulated microcapsule for polysulfide-immobilized long life lithium-sulfur batteries[J]. Lab on a Chip, 2022, 22(11): 2185-2191. |
| 18 | HAKIMI M, SANAEE Z, GHASEMI S, et al. Graphene oxide interlayered in binder-free sulfur vapor deposited cathode for lithium-sulfur battery[J]. Journal of Physics D: Applied Physics, 2022, 55(16): doi: 10.1088/1361-6463/ac4b55. |
| 19 | HU Z J, YAN G J, ZHAO J C, et al. Covalent organic framework wrapped by graphene oxide as an efficient sulfur host for high performance lithium-sulfur batteries[J]. Nanotechnology, 2022, 33(22): doi: 10.1088/1361-6528/ac54e0. |
| 20 | HE X Z, JI X, ZHANG B, et al. Tuning interface lithiophobicity for lithium metal solid-state batteries[J]. ACS Energy Letters, 2022, 7(1): 131-139. |
| 21 | LI J, YANG Z F, HUANG X F, et al. Interfacial reinforcement of composites by the electrostatic self-assembly of graphene oxide and NH3 plasma-treated carbon fiber[J]. Applied Surface Science, 2022, 585: doi: 10.1016/j.apsusc.2022.152717. |
| 22 | PALLAVOLU M R, PRABHU S, NALLAPUREDDY R R, et al. Bio-derived graphitic carbon quantum dot encapsulated S-and N-doped graphene sheets with unusual battery-type behavior for high-performance supercapacitor[J]. Carbon, 2023, 202: 93-102. |
| 23 | ZHONG J, WANG T, WANG L, et al. A silicon monoxide lithium-ion battery anode with ultrahigh areal capacity[J]. Nano-Micro Letters, 2022, 14(1): doi: 10.1007/s40820-022-00790-z. |
| 24 | ZHANG J, JIA L J, LIN H Z, et al. Advances and prospects of 2D graphene-based materials/hybrids for lithium metal-sulfur full battery: From intrinsic property to catalysis modification[J]. Advanced Energy and Sustainability Research, 2022, 3(4): doi: 10.1002/aesr.202100187. |
| 25 | ZHANG S S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions[J]. Journal of Power Sources, 2013, 231: 153-162. |
| 26 | YANG X F, LI X, ADAIR K, et al. Structural design of lithium-sulfur batteries: From fundamental research to practical application[J]. Electrochemical Energy Reviews, 2018, 1(3): 239-293. |
| [1] | Xiaofei WANG, Dawei LAN, Daoming ZHANG, Haoliang XUE, Sifei ZHOU, Chuang LIU, Jun LI, Zhendong WANG. High-performance lithium-sulfur batteries enabled by a separator modified by lithium-doped zeolite [J]. Energy Storage Science and Technology, 2022, 11(11): 3447-3454. |
| [2] | Xinxin ZHU, Wei JIANG, Zhengwei WAN, Shu ZHAO, Zeheng LI, Liguang WANG, Wenbin NI, Min LING, Chengdu LIANG. Research progress in electrolyte and interfacial issues of solid lithium sulfur batteries [J]. Energy Storage Science and Technology, 2021, 10(3): 848-862. |
| [3] | Mengdie YAN, Hui LI, Min LING, Huilin PAN, Qiang ZHANG. Brief review of progress in lithium-sulfur batteries based on dissolution-deposition reactions [J]. Energy Storage Science and Technology, 2020, 9(6): 1606-1613. |
| [4] | Yun LU, Jianing LIANG, Yong ZHU, Zhengrong LI, Yezhou HU, Ke CHEN, Deli WANG. Recent progress in organics derived cathode materials for lithium sulfur batteries [J]. Energy Storage Science and Technology, 2020, 9(5): 1454-1466. |
| [5] | SHEN Jinran, GUO Cuijing, CHEN He, ZHOU Shuqin, XU Bin, GUAN Yibiao. Synthesis and lithium storage property of high-performance N-doped reduced graphene oxide [J]. Energy Storage Science and Technology, 2019, 8(6): 1137-1144. |
| [6] | SONG Yuguo, XU Jiaohui, LIU Mingyu, CHEN Xiao, JIN Bo, JIANG Qing. Study on properties of polypyrrole-coated sulfur-based composite prepared by drop addition method [J]. Energy Storage Science and Technology, 2018, 7(3): 502-511. |
| [7] | LIU Jinyu,LI Dan, WANG Lihua, HAN Xutong, HUANG Qinglin. SPEEK/ SGO proton exchange membranes with superior proton selectivity for vanadium redox battery [J]. Energy Storage Science and Technology, 2018, 7(1): 66-. |
| [8] | SUN Huajun1,2, HONG Tingting1, LIU Xiaofang3, SUI Huiting2, LIU Pengdong1. Improvement of photovoltaic properties of bismuth ferrite film based solar cell using organic and inorganic interface layers [J]. Energy Storage Science and Technology, 2017, 6(6): 1340-. |
| [9] | TANG Xiaonan1,2, SUN Zhenhua1, CHEN Ke1, YANG Huicong1, ZHUO Shuping2, LI Feng1. Cathode hybrid materials for lithium-sulfur battery: The interaction between the host and polysulfide [J]. Energy Storage Science and Technology, 2017, 6(3): 345-359. |
| [10] | SHI Kai, AN Decheng, HE Yanbing, LI Baohua, KANG Feiyu. Research progress and future trends of solid state lithium-sulfur batteries based on polymer electrolytes [J]. Energy Storage Science and Technology, 2017, 6(3): 479-492. |
| [11] | CHEN Xiang, HOU Tingzheng, PENG Hongjie, CHENG Xinbing, HUANG Jiaqi, ZHANG Qiang. Review on the applications of first-principles calculation in lithium-sulfur batteries [J]. Energy Storage Science and Technology, 2017, 6(3): 500-521. |
| [12] | ZHANG Weidong, FAN Lei, ZHU Shoupu, LU Yingying. Recent developments in high-energy lithium-sulfur batteries [J]. Energy Storage Science and Technology, 2017, 6(3): 534-549. |
| [13] | CHEN Yuqing1,2, YANG Xiaofei1,2, YU Ying1,2, LI Xianfeng1,3, ZHANG Hongzhang1,3, ZHANG Huamin1,3. Key materials and technology research progress of lithium-sulfur batteries [J]. Energy Storage Science and Technology, 2017, 6(2): 169-189. |
| [14] | JING Minghua, FAN Xinzhuang, LIU Jianguo, YAN Chuanwei. Electrochemical behavior of graphene oxide modified carbon felt as the positive electrode for vanadium flow battery#br# [J]. Energy Storage Science and Technology, 2017, 6(2): 263-269. |
| [15] | MA Qiang1,2, QI Xinguo1, RONG Xiaohui1, HU Yongsheng1, ZHOU Zhibin2, LI Hong1, . Novel solid polymer electrolytes for all-solid-state lithium-sulfur batteries#br# [J]. Energy Storage Science and Technology, 2016, 5(5): 713-718. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

