储能科学与技术 ›› 2024, Vol. 13 ›› Issue (1): 113-129.doi: 10.19799/j.cnki.2095-4239.2023.0802
• 高比能二次电池关键材料与先进表征专刊 • 上一篇 下一篇
陈淑媛1( ), 程晨1(
), 程晨1( ), 夏啸1, 鞠焕鑫2(
), 夏啸1, 鞠焕鑫2( ), 张亮1,3(
), 张亮1,3( )
)
收稿日期:2023-11-07
修回日期:2023-11-23
出版日期:2024-01-05
发布日期:2024-01-22
通讯作者:
鞠焕鑫,张亮
E-mail:20224214077@stu.suda.edu.cn;chencheng2023@suda.edu.cn;huanxin.ju@coretechint.com;liangzhang2019@suda.edu.cn
作者简介:陈淑媛(2000—),女,硕士研究生,研究方向为钠离子电池层状氧化物正极材料,E-mail:20224214077@stu.suda.edu.cn;基金资助:
Shuyuan CHEN1( ), Chen CHENG1(
), Chen CHENG1( ), Xiao XIA1, Huanxin JU2(
), Xiao XIA1, Huanxin JU2( ), Liang ZHANG1,3(
), Liang ZHANG1,3( )
)
Received:2023-11-07
Revised:2023-11-23
Online:2024-01-05
Published:2024-01-22
Contact:
Huanxin JU, Liang ZHANG
E-mail:20224214077@stu.suda.edu.cn;chencheng2023@suda.edu.cn;huanxin.ju@coretechint.com;liangzhang2019@suda.edu.cn
摘要:
二次电池具有高能量密度和长循环寿命等特点,为储存并利用清洁能源提供了有效的解决方案。为了满足社会日益增长的能源需求,进一步研究和开发二次电池迫在眉睫,而X射线表征技术可以为二次电池的研究、设计与应用提供全方位视角。基于此,本综述通过对近几年相关文献进行归纳总结,综述了X射线谱学技术在二次电池领域的最新进展以及遇到的问题,重点介绍了X射线表征技术(主要包括X射线光电子能谱、X射线吸收谱和共振非弹性X射线散射等)的基本原理、在二次电池领域的最新研究成果和科学挑战,详细阐释了不同X射线表征手段的技术特点、适用条件和独特优势,并对未来X射线谱学在二次电池领域的应用提出展望。综合分析表明,X射线表征技术可以提供一系列电极材料晶格、电子、物相结构等基本信息,实现从宏观尺度到微观尺度的电极材料结构表征,进而系统揭示电极材料晶体结构与电子结构演变、电荷补偿机制、离子与电子输运以及表界面化学过程等信息,为二次电池性能提升和技术瓶颈突破提供支持。
中图分类号:
陈淑媛, 程晨, 夏啸, 鞠焕鑫, 张亮. 高比能二次电池正极材料的X射线谱学研究进展[J]. 储能科学与技术, 2024, 13(1): 113-129.
Shuyuan CHEN, Chen CHENG, Xiao XIA, Huanxin JU, Liang ZHANG. Research progress in the X-ray spectroscopy investigation of cathode materials for high-energy-density secondary batteries[J]. Energy Storage Science and Technology, 2024, 13(1): 113-129.
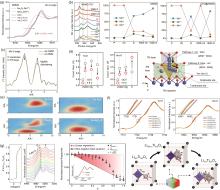
图4
(a) NMMO在不同带电状态下的归一化Mn K边XANES光谱[71];(b) NNMO和CTO@NNMO在第1次和第100次充放电状态下的Mn L边XANES光谱和定量拟合结果[73];(c) Air-NMM和Ar-NMM的Mn K边傅里叶变换EXAFS谱[75];(d) 由EXAFS分析得出的Ni-O和Mn-O配位数的变化和TM的迁移途径[76];(e) Fe-N3C2-C、Fe-N4-C、Fe2O3 和Fe箔的WT图[77];(f) Na x LMNMT的Ni、Mn K边的原位XANES光谱[78];(g) 一系列Ti K边XANES,钛酸锂中Li嵌入过程中Li4/3Ti5/3O4 频谱加权函数随光谱指纹归一化幅度的演变以及锂化驱动的结构转变的演变[79]"
| 1 | 岑官骏, 乔荣涵, 申晓宇, 等. 锂电池百篇论文点评(2023.6.1—2023.7.31)[J]. 储能科学与技术, 2023, 12(9): 3003-3018. |
| CEN G J, QIAO R H, SHEN X Y, et al. Reviews of selected 100 recent papers for lithium batteries(Jun. 1, 2023 to Jul. 31, 2023)[J]. Energy Storage Science and Technology, 2023, 12(9): 3003-3018. | |
| 2 | YU Z J, WANG W, ZHU Y, et al. Construction of double reaction zones for long-life quasi-solid aluminum-ion batteries by realizing maximum electron transfer[J]. Nature Communications, 2023, 14: 5596. |
| 3 | 郑薇, 刘琼, 卢周广. 钠离子电池层状过渡金属氧化物中阴离子氧的氧化还原反应活性调控[J]. 储能科学与技术, 2020, 9(5): 1416-1427. |
| ZHENG W, LIU Q, LU Z G. Modulating anionic redox reaction in layered transition metal oxides for sodium-ion batteries[J]. Energy Storage Science and Technology, 2020, 9(5): 1416-1427. | |
| 4 | CHU S Y, GUO S H, ZHOU H S. Advanced cobalt-free cathode materials for sodium-ion batteries[J]. Chemical Society Reviews, 2021, 50(23): 13189-13235. |
| 5 | MA X, YUAN C, LIU G L, et al. Steering the liquid-solid redox conversion of lithium-selenium batteries through ultrafine MoC catalyst[J]. Chemical Communications, 2023, 59: 11208-11. |
| 6 | YAN T R, FENG J, ZENG P, et al. Modulating eg orbitals through ligand engineering to boost the electrocatalytic activity of NiSe for advanced lithium-sulfur batteries[J]. Journal of Energy Chemistry, 2022, 74: 317-323. |
| 7 | YAN T R, WU Y, GONG F, et al. TiH2 nanodots exfoliated via facile sonication as bifunctional electrocatalysts for Li-S batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(5): 6937-6944. |
| 8 | CHU S, MAJUMDAR A. Opportunities and challenges for a sustainable energy future[J]. Nature, 2012, 488(7411): 294-303. |
| 9 | CHOW J, KOPP R J, PORTNEY P R. Energy resources and global development[J]. Science, 2003, 302(5650): 1528-1531. |
| 10 | CHU S, CUI Y, LIU N. The path towards sustainable energy[J]. Nature Materials, 2017, 16(1): 16-22. |
| 11 | DUNN B, KAMATH H, TARASCON J M. Electrical energy storage for the grid: A battery of choices[J]. Science, 2011, 334(6058): 928-935. |
| 12 | 朱晓辉, 庄宇航, 赵旸, 等. 钠离子电池层状正极材料研究进展[J]. 储能科学与技术, 2020, 9(5): 1340-1349. |
| ZHU X H, ZHUANG Y H, ZHAO Y, et al. Development of layered cathode materials for sodium-ion batteries[J]. Energy Storage Science and Technology, 2020, 9(5): 1340-1349. | |
| 13 | FUJITA T, TODA K. Microdisplacement measurement using a liquid-delay-line oscillator[J]. Japanese Journal of Applied Physics, 2003, 42(Part 1, No. 9B): 6131-6134. |
| 14 | YANG S C, HE R, ZHANG Z J, et al. CHAIN: Cyber hierarchy and interactional network enabling digital solution for battery full-lifespan management[J]. Matter, 2020, 3(1): 27-41. |
| 15 | WHITTINGHAM M S. Lithium batteries and cathode materials[J]. Chemical Reviews, 2004, 104(10): 4271-4302. |
| 16 | ZENG P, SU B, WANG X L, et al. In situ reconstruction of electrocatalysts for lithium-sulfur batteries: Progress and prospects[J]. Advanced Functional Materials, 2023, 33(33): 2301743. |
| 17 | XIANG X D, ZHANG K, CHEN J. Recent advances and prospects of cathode materials for sodium-ion batteries[J]. Advanced Materials (Deerfield Beach, Fla), 2015, 27(36): 5343-5364. |
| 18 | KIM S W, SEO D H, MA X H, et al. Electrode materials for rechargeable sodium-ion batteries: Potential alternatives to current lithium-ion batteries[J]. Advanced Energy Materials, 2012, 2(7): 710-721. |
| 19 | FANG K, TANG Y L, LIU J J, et al. Injecting excess Na into a P2-type layered oxide cathode to achieve presodiation in a Na-ion full cell[J]. Nano Letters, 2023, 23(14): 6681-6688. |
| 20 | ZHAO C L, WANG Q D, YAO Z P, et al. Rational design of layered oxide materials for sodium-ion batteries[J]. Science, 2020, 370(6517): 708-711. |
| 21 | ZHOU X, DING M L, CHENG C, et al. Covalency modulation enables stable Na-rich layered oxide cathodes for Na-ion batteries[J]. Electronic Structure, 2023, 5(1): 014004. |
| 22 | YABUUCHI N, KUBOTA K, DAHBI M, et al. Research development on sodium-ion batteries[J]. Chemical Reviews, 2014, 114(23): 11636-11682. |
| 23 | YUAN C, SONG X C, ZENG P, et al. Precisely optimizing polysulfides adsorption and conversion by local coordination engineering for high-performance Li-S batteries[J]. Nano Energy, 2023, 110: 108353. |
| 24 | DING L Y, WANG L, GAO J C, et al. Facile Zn2+ desolvation enabled by local coordination engineering for long-cycling aqueous zinc-ion batteries[J]. Advanced Functional Materials, 2023, 33(32): 2301648. |
| 25 | RATNER B D, CASTNER D G. Electron spectroscopy for chemical analysis[M]. Surface analysis - the principal techniques. 2009: 47-112. |
| 26 | SMITH J W, SAYKALLY R J. Soft X-ray absorption spectroscopy of liquids and solutions[J]. Chemical Reviews, 2017, 117(23): 13909-13934. |
| 27 | BRUNDLE C R, CHUANG T J, RICE D W. X- ray photoemission study of the interaction of oxygen and air with clean cobalt surfaces[J]. Surface Science, 1976, 60(2): 286-300. |
| 28 | LIN F, LIU Y J, YU X Q, et al. Synchrotron X-ray analytical techniques for studying materials electrochemistry in rechargeable batteries[J]. Chemical Reviews, 2017, 117(21): 13123-13186. |
| 29 | SIOL S, MANN J, NEWMAN J, et al. Concepts for chemical state analysis at constant probing depth by lab-based XPS/HAXPES combining soft and hard X-ray sources[J]. Surface and Interface Analysis, 2020, 52(12): 802-810. |
| 30 | HASHIMOTO S. Changes of XPS spectra from oxides by ion bombardment[J]. Journal of surface analysis, 2003, 10: 136-43. |
| 31 | TIMOSHENKO J, ROLDAN CUENYA B. in situ/Operando electrocatalyst characterization by X-ray absorption spectroscopy[J]. Chemical Reviews, 2021, 121(2): 882-961. |
| 32 | SONG Z X, LI J J, DAVIS K D, et al. Emerging applications of synchrotron radiation X-ray techniques in single atomic catalysts[J]. Small Methods, 2022, 6(11): e2201078. |
| 33 | GIORGETTI M. A review on the structural studies of batteries and host materials by X-ray absorption spectroscopy[J]. ISRN Materials Science, 2013, 2013: 1-22. |
| 34 | HARKS P P R M L, MULDER F M, NOTTEN P H L. in situ methods for Li-ion battery research: A review of recent developments[J]. Journal of Power Sources, 2015, 288: 92-105. |
| 35 | GONG Z L, YANG Y. The application of synchrotron X-ray techniques to the study of rechargeable batteries[J]. Journal of Energy Chemistry, 2018, 27(6): 1566-1583. |
| 36 | ZHANG L, GUO J H. Understanding the reaction mechanism of lithium-sulfur batteries by in situ/operando X-ray absorption spectroscopy[J]. Arabian Journal for Science and Engineering, 2019, 44(7): 6217-6229. |
| 37 | YANG W L, DEVEREAUX T P. Anionic and cationic redox and interfaces in batteries: Advances from soft X-ray absorption spectroscopy to resonant inelastic scattering[J]. Journal of Power Sources, 2018, 389: 188-197. |
| 38 | BAKER M L, MARA M W, YAN J J, et al. K- and L-edge X-ray absorption spectroscopy (XAS) and resonant inelastic X-ray scattering (RIXS) determination of differential orbital covalency (DOC) of transition metal sites[J]. Coordination Chemistry Reviews, 2017, 345: 182-208. |
| 39 | GEL'MUKHANOV F, ÅGREN H. Resonant X-ray Raman scattering[J]. Physics Reports, 1999, 312(3/4/5/6): 87-330. |
| 40 | KOTANI A, SHIN S. Resonant inelastic X-ray scattering spectra for electrons in solids[J]. Reviews of Modern Physics, 2001, 73(1): 203-246. |
| 41 | BENKERT A, MEYER F, HAUSCHILD D, et al. Isotope effects in the resonant inelastic soft X-ray scattering maps of gas-phase methanol[J]. The Journal of Physical Chemistry A, 2016, 120(14): 2260-2267. |
| 42 | LIU Y S, GLANS P A, CHUANG C H, et al. Perspectives of in situ/operando resonant inelastic X-ray scattering in catalytic energy materials science[J]. Journal of Electron Spectroscopy and Related Phenomena, 2015, 200: 282-292. |
| 43 | KUNNUS K, ZHANG W K, DELCEY M G, et al. Viewing the valence electronic structure of ferric and ferrous hexacyanide in solution from the Fe and cyanide perspectives[J]. The Journal of Physical Chemistry B, 2016, 120(29): 7182-7194. |
| 44 | MATSUBARA M, UOZUMI T, KOTANI A, et al. Polarization dependence of resonant X-ray emission spectra in 3dn transition metal compounds with n = 0, 1, 2, 3[J]. Journal of the Physical Society of Japan, 2002, 71(1): 347-356. |
| 45 | GHIRINGHELLI G, MATSUBARA M, DALLERA C, et al. Resonant inelastic X-ray scattering of MnO: L2, 3 edge measurements and assessment of their interpretation[J]. Physical Review B, 2006, 73(3): 035111. |
| 46 | KUNNUS K, JOSEFSSON I, SCHRECK S, et al. From ligand fields to molecular orbitals: Probing the local valence electronic structure of Ni2+ in aqueous solution with resonant inelastic X-ray scattering[J]. The Journal of Physical Chemistry B, 2013, 117(51): 16512-16521. |
| 47 | GLATZEL P, SINGH J, KVASHNINA K O, et al. In situ characterization of the 5d density of states of Pt nanoparticles upon adsorption of CO[J]. Journal of the American Chemical Society, 2010, 132(8): 2555-2557. |
| 48 | KVASHNINA K O, BUTORIN S M, GLATZEL P. Direct study of the f-electron configuration in lanthanide systems[J]. Journal of Analytical Atomic Spectrometry, 2011, 26(6): 1265-1272. |
| 49 | WU J P, SHEN Z X, YANG W L. Redox mechanism in Na-ion battery cathodes probed by advanced soft X-ray spectroscopy[J]. Frontiers in Chemistry, 2020, 8: 816. |
| 50 | DE GROOT F M F, GRIONI M, FUGGLE J C, et al. Oxygen 1sx-ray-absorption edges of transition-metal oxides[J]. Physical Review B, 1989, 40(8): 5715-5723. |
| 51 | AMENT L J P, VAN VEENENDAAL M, DEVEREAUX T P, et al. Resonant inelastic X-ray scattering studies of elementary excitations[J]. Reviews of Modern Physics, 2011, 83(2): 705-767. |
| 52 | DE GROOT F. High-resolution X-ray emission and X-ray absorption spectroscopy[J]. Chemical Reviews, 2001, 101(6): 1779-1808. |
| 53 | ÅGREN H, LUO Y, GELMUKHANOV F, et al. Screening in resonant X-ray emission of molecules[J]. Journal of Electron Spectroscopy and Related Phenomena, 1996, 82(1/2): 125-134. |
| 54 | FÖHLISCH A, HASSELSTRÖM J, BENNICH P, et al. Ground-state interpretation of X-ray emission spectroscopy on adsorbates: CO adsorbed on Cu(100)[J]. Physical Review B, 2000, 61(23): 16229-16240. |
| 55 | SHUTTHANANDAN V, NANDASIRI M, ZHENG J M, et al. Applications of XPS in the characterization of battery materials[J]. Journal of Electron Spectroscopy and Related Phenomena, 2019, 231: 2-10. |
| 56 | ATKINS D, AYERBE E, BENAYAD A, et al. Understanding battery interfaces by combined characterization and simulation approaches: Challenges and perspectives[J]. Advanced Energy Materials, 2022, 12(17): 2102687. |
| 57 | WOOD K N, TEETER G. XPS on Li-battery-related compounds: Analysis of inorganic SEI phases and a methodology for charge correction[J]. ACS Applied Energy Materials, 2018, 1(9): 4493-4504. |
| 58 | YU W L, YU Z A, CUI Y, et al. Degradation and speciation of Li salts during XPS analysis for battery research[J]. ACS Energy Letters, 2022, 7(10): 3270-3275. |
| 59 | HAN J G, BIN LEE J, CHA A M, et al. Unsymmetrical fluorinated malonatoborate as an amphoteric additive for high-energy-density lithium-ion batteries[J]. Energy & Environmental Science, 2018, 11(6): 1552-1562. |
| 60 | ZHENG Y, HE Y B, QIAN K, et al. Effects of state of charge on the degradation of LiFePO4/graphite batteries during accelerated storage test[J]. Journal of Alloys and Compounds, 2015, 639: 406-414. |
| 61 | LU L L, ZHU Z X, MA T, et al. Superior fast-charging lithium-ion batteries enabled by the high-speed solid-state lithium transport of an intermetallic Cu6Sn5 network[J]. Advanced Materials, 2022, 34(32): 2202688. |
| 62 | OTTO S K, MORYSON Y, KRAUSKOPF T, et al. In-depth characterization of lithium-metal surfaces with XPS and ToF-SIMS: Toward better understanding of the passivation layer[J]. Chemistry of Materials, 2021, 33(3): 859-867. |
| 63 | BENAYAD A, MORALES-UGARTE J E, SANTINI C C, et al. Operando XPS: A novel approach for probing the lithium/electrolyte interphase dynamic evolution[J]. The Journal of Physical Chemistry A, 2021, 125(4): 1069-1081. |
| 64 | CHO D H, JO C H, CHO W, et al. Effect of residual lithium compounds on layer Ni-rich Li[Ni0.7Mn0.3]O2[J]. Journal of the Electrochemical Society, 2014, 161(6): A920-A926. |
| 65 | YIN Y C, YANG J T, LUO J D, et al. A LaCl3-based lithium superionic conductor compatible with lithium metal[J]. Nature, 2023, 616(7955): 77-83. |
| 66 | CHEN J, FAN X L, LI Q, et al. Electrolyte design for LiF-rich solid-electrolyte interfaces to enable high-performance microsized alloy anodes for batteries[J]. Nature Energy, 2020, 5(5): 386-397. |
| 67 | YIN Y C, WANG Q, YANG J T, et al. Metal chloride perovskite thin film based interfacial layer for shielding lithium metal from liquid electrolyte[J]. Nature Communications, 2020, 11: 1761. |
| 68 | MALMGREN S, CIOSEK K, HAHLIN M, et al. Comparing anode and cathode electrode/electrolyte interface composition and morphology using soft and hard X-ray photoelectron spectroscopy[J]. Electrochimica Acta, 2013, 97: 23-32. |
| 69 | QIAN Y X, CHU Y L, ZHENG Z T, et al. A new cyclic carbonate enables high power/low temperature lithium-ion batteries[J]. Energy Storage Materials, 2022, 45: 14-23. |
| 70 | IIDA S I, TERASHIMA M, MAMIYA K, et al. Characterization of cathode-electrolyte interface in all-solid-state batteries using TOF-SIMS, XPS, and UPS/LEIPS[J]. Journal of Vacuum Science & Technology B, 2021, 39(4): doi: 10.1116/6.0001044. |
| 71 | WU Z H, NI Y X, TAN S, et al. Realizing high capacity and zero strain in layered oxide cathodes via lithium dual-site substitution for sodium-ion batteries[J]. Journal of the American Chemical Society, 2023, 145(17): 9596-9606. |
| 72 | ZOU P C, YAO L B, WANG C Y, et al. Regulating cation interactions for zero-strain and high-voltage P2-type Na2/3Li1/6Co1/6Mn2/3O2 layered oxide cathodes of sodium-ion batteries[J]. Angewandte Chemie International Edition, 2023, 62(28): e202304628. |
| 73 | XIA X, LIU T, CHENG C, et al. Suppressing the dynamic oxygen evolution of sodium layered cathodes through synergistic surface dielectric polarization and bulk site-selective Co-doping[J]. Advanced Materials, 2023, 35: e2209556. |
| 74 | HU H L, HE H C, XIE R K, et al. Achieving reversible Mn2+/Mn4+ double redox couple through anionic substitution in a P2-type layered oxide cathode[J]. Nano Energy, 2022, 99: 107390. |
| 75 | JIN J T, LIU Y C, ZHAO X D, et al. Annealing in argon universally upgrades the Na-storage performance of Mn-based layered oxide cathodes by creating bulk oxygen vacancies[J]. Angewandte Chemie (International Ed in English), 2023, 62(15): e202219230. |
| 76 | ZHANG B D, ZHANG Y M, WANG X T, et al. Role of substitution elements in enhancing the structural stability of Li-rich layered cathodes[J]. Journal of the American Chemical Society, 2023: 8700-8713. |
| 77 | LIU G L, WANG W M, ZENG P, et al. Strengthened d–p orbital hybridization through asymmetric coordination engineering of single-atom catalysts for durable lithium-sulfur batteries[J]. Nano Letters, 2022, 22(15): 6366-6374. |
| 78 | CHENG Z W, ZHAO B, GUO Y J, et al. Mitigating the large-volume phase transition of P2-type cathodes by synergetic effect of multiple ions for improved sodium-ion batteries[J]. Advanced Energy Materials, 2022, 12(14): 2103461. |
| 79 | ZHANG W, TOPSAKAL M, CAMA C, et al. Multi-stage structural transformations in zero-strain lithium titanate unveiled by in situ X-ray absorption fingerprints[J]. Journal of the American Chemical Society, 2017, 139(46): 16591-16603. |
| 80 | HU H L, KAO C W, CHENG C, et al. Local construction of Mn-based layered cathodes through covalency modulation for sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2023, 15(25): 30332-30341. |
| 81 | XU C L, HUA W B, ZHANG Q H, et al. Sufficient utilization of Mn2+/Mn3+/Mn4+ redox in NASICON phosphate cathodes towards high-energy Na-ions batteries[J]. Advanced Functional Materials, 2023, 33(33): 2302810. |
| 82 | ZHAO G, CHEN Q J, WANG L, et al. Self-standing sulfur cathodes enabled by a single Fe site decorated fibrous membrane for durable lithium-sulfur batteries[J]. Journal of Materials Chemistry A, 2022, 10(37): 19893-19902. |
| 83 | DU Z Z, CHEN X J, HU W, et al. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium–sulfur batteries[J]. Journal of the American Chemical Society, 2019, 141(9): 3977-3985. |
| 84 | CHENG C, CHEN C, CHU S Y, et al. Enhancing the reversibility of lattice oxygen redox through modulated transition metal-oxygen covalency for layered battery electrodes[J]. Advanced Materials, 2022, 34(20): e2201152. |
| 85 | XIAO B W, LIU H S, CHEN N, et al. Size-mediated recurring spinel sub-nanodomains in Li- and Mn-rich layered cathode materials[J]. Angewandte Chemie International Edition, 2020, 59(34): 14313-14320. |
| 86 | FIROUZI A, QIAO R M, MOTALLEBI S, et al. Monovalent manganese based anodes and co-solvent electrolyte for stable low-cost high-rate sodium-ion batteries[J]. Nature Communications, 2018, 9: 861. |
| 87 | CHENG C, LI S Y, LIU T F, et al. Elucidation of anionic and cationic redox reactions in a prototype sodium-layered oxide cathode[J]. ACS Applied Materials & Interfaces, 2019, 11(44): 41304-41312. |
| 88 | XU J, SUN M L, QIAO R M, et al. Elucidating anionic oxygen activity in lithium-rich layered oxides[J]. Nature Communications, 2018, 9: 947. |
| 89 | DAI K H, SHAO W W, ZHAO B B, et al. Precisely quantifying bulk transition metal valence evolution in conventional battery electrode by inverse partial fluorescence yield[J]. Journal of Energy Chemistry, 2022, 69: 363-368. |
| 90 | JI H W, WU J P, CAI Z J, et al. Ultrahigh power and energy density in partially ordered lithium-ion cathode materials[J]. Nature Energy, 2020, 5(3): 213-221. |
| 91 | WU J P, ZHUO Z Q, RONG X H, et al. Dissociate lattice oxygen redox reactions from capacity and voltage drops of battery electrodes[J]. Science Advances, 2020, 6(6): eaaw3871. |
| 92 | DAI K H, MAO J, ZHUO Z Q, et al. Negligible voltage hysteresis with strong anionic redox in conventional battery electrode[J]. Nano Energy, 2020, 74: 104831. |
| 93 | DAI K H, WU J P, ZHUO Z Q, et al. High reversibility of lattice oxygen redox quantified by direct bulk probes of both anionic and cationic redox reactions[J]. Joule, 2019, 3(2): 518-541. |
| 94 | CHEN M Z, CHOU S L, DOU S X. Understanding challenges of cathode materials for sodium-ion batteries using synchrotron-based X-ray absorption spectroscopy[J]. Batteries & Supercaps, 2019, 2(10): 842-851. |
| 95 | GHIGNA P, QUARTARONE E. Operando X-ray absorption spectroscopy on battery materials: A review of recent developments[J]. Journal of Physics: Energy, 2021, 3(3): 032006. |
| [1] | 杨建航, 冯文婷, 韩俊伟, 魏欣茹, 马晨宇, 毛常明, 智林杰, 孔德斌. 锂/钠-氯二次电池的最新进展——从材料构建到性能评估[J]. 储能科学与技术, 2024, 13(6): 1824-1834. |
| [2] | 李万瑞, 李文俊, 王小青, 路胜利, 徐喜连. 锌离子电池用锰/钒基氧化物异质结构正极的研究进展[J]. 储能科学与技术, 2024, 13(5): 1496-1515. |
| [3] | 缪胤宝, 张文华, 刘伟昊, 王帅, 陈哲, 彭望, 曾杰. 富锂正极材料Li1.2Ni0.13Co0.13Mn0.54O2 的制备及性能[J]. 储能科学与技术, 2024, 13(5): 1427-1434. |
| [4] | 朱璟, 郝峻丰, 孙蔷馥, 张新新, 申晓宇, 岑官骏, 乔荣涵, 田孟羽, 金周, 詹元杰, 闫勇, 贲留斌, 俞海龙, 刘燕燕, 黄学杰. 锂电池百篇论文点评(2024.2.1—2024.3.31)[J]. 储能科学与技术, 2024, 13(5): 1398-1416. |
| [5] | 孙蔷馥, 申晓宇, 岑官骏, 乔荣涵, 朱璟, 郝峻丰, 张新新, 田孟羽, 金周, 詹元杰, 闫勇, 贲留斌, 俞海龙, 刘燕燕, 黄学杰. 锂电池百篇论文点评(2023.12.1—2024.1.31)[J]. 储能科学与技术, 2024, 13(3): 725-741. |
| [6] | 彭可, 张志成, 胡有章, 张旭辉, 周稼辉, 李彬. 基于有限元的热力耦合场匣钵运动分析与优化[J]. 储能科学与技术, 2024, 13(2): 634-642. |
| [7] | 郭秀丽, 周小龙, 邹才能, 唐永炳. 水系双离子电池的研究进展与展望[J]. 储能科学与技术, 2024, 13(2): 462-479. |
| [8] | 李顺, 黄建国, 何桂金. 木质素基碳/硫纳米球复合材料作为高性能锂硫电池正极材料[J]. 储能科学与技术, 2024, 13(1): 270-278. |
| [9] | 杜文, 王君雷, 徐运飞, 李世龙, 王昆. 火焰喷雾热解法生产锂离子电池高镍三元正极材料的技术经济分析[J]. 储能科学与技术, 2024, 13(1): 345-357. |
| [10] | 王盼晴, 黄彦杰, 何一芃, 陈祁恒, 尹提, 陈伟豪, 谭磊, 宁天翔, 邹康宇, 李灵均. 高镍正极材料表面锂残渣的研究进展[J]. 储能科学与技术, 2024, 13(1): 92-112. |
| [11] | 张新新, 申晓宇, 岑官骏, 乔荣涵, 朱璟, 郝峻丰, 孙蔷馥, 田孟羽, 金周, 詹元杰, 武怿达, 闫勇, 贲留斌, 俞海龙, 刘燕燕, 黄学杰. 锂电池百篇论文点评(2023.10.1—2023.11.30)[J]. 储能科学与技术, 2024, 13(1): 252-269. |
| [12] | 詹世英, 李欢欢, 胡方. 水系锌离子电容器正极材料的研究进展[J]. 储能科学与技术, 2023, 12(9): 2799-2810. |
| [13] | 岑官骏, 乔荣涵, 申晓宇, 朱璟, 郝峻丰, 孙蔷馥, 张新新, 田孟羽, 金周, 詹元杰, 武怿达, 闫勇, 贲留斌, 俞海龙, 刘燕燕, 黄学杰. 锂电池百篇论文点评(2023.6.1—2023.7.31)[J]. 储能科学与技术, 2023, 12(9): 3003-3018. |
| [14] | 张吉禄, 董育辰, 宋强, 袁思鸣, 郭孝东. 多晶及单晶高镍三元材料LiNi0.9Co0.05Mn0.05O2 的可控制备及其电化学储锂特性[J]. 储能科学与技术, 2023, 12(8): 2382-2389. |
| [15] | 张梓楠, 陈剑. Nb掺杂Na3V2O2 (PO4 ) 2F空心微球钠离子电池正极材料的制备与性能[J]. 储能科学与技术, 2023, 12(8): 2370-2381. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
