Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (10): 3112-3122.doi: 10.19799/j.cnki.2095-4239.2022.0067
• Energy Storage Materials and Devices • Previous Articles Next Articles
Yonggang LOU1,2( ), Dayong WU1, Boran CAI1,2, Weihua LIANG1, Luye YANG1,2, Lei HE1,2, Jianhua CAO1(
), Dayong WU1, Boran CAI1,2, Weihua LIANG1, Luye YANG1,2, Lei HE1,2, Jianhua CAO1( )
)
Received:2022-02-15
Revised:2022-03-02
Online:2022-10-05
Published:2022-10-10
Contact:
Jianhua CAO
E-mail:louyonggang18@mails.ucas.ac.cn;caojh@mail.ipc.ac.cn
CLC Number:
Yonggang LOU, Dayong WU, Boran CAI, Weihua LIANG, Luye YANG, Lei HE, Jianhua CAO. Study on preparation and performance of poly(m-phenylene isophthalamide)/solid-state ionic conductor composite membrane[J]. Energy Storage Science and Technology, 2022, 11(10): 3112-3122.
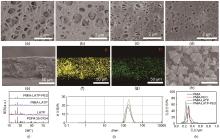
Fig. 1
The surface SEM images of membranes (a) PMIA, (b) PMIA-LATP, (c) PMIA-PEO, (d) PMIA-LATP-PEO; (e) The cross-section SEM image of PMIA-LATP-PEO membrane; The EDS mapping of (f) P, (g) Ti in the cross-section of PMIA-LATP-PEO membrane; (h) The SEM image of LATP powder; (i) The XRD pattern of LATP powder, PMIA-LATP and PMIA-LATP-PEO membranes; (j) The particle size distribution diagram of LATP particle; (k) The diagram of pore size distribution of membranes"
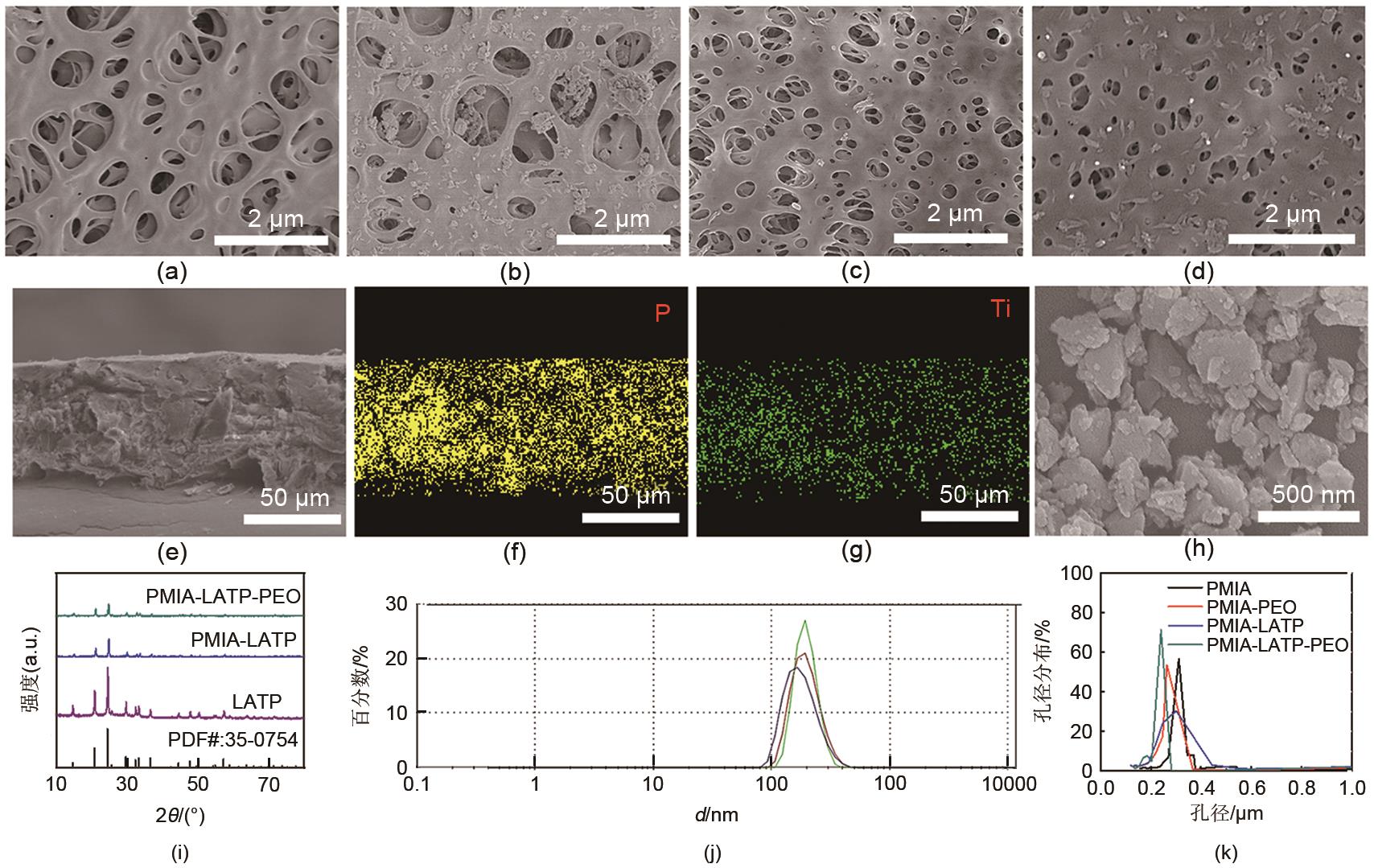
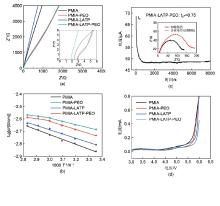
Fig. 3
(a) The Nyquist plots of four membranes at 25 ℃; (b) Arrhenius plots of membranes as a function of different temperatures; (c) Current variation with time during polarization of the Li||PMIA-LATP-PEO||Li battery at a potential step of 10 mV at room temperature (in inset: Nyquist profiles corresponding to the EIS measurement performed at the initial and the steady state condition) The chronoamperometric profile of Li||separator||Li cell, insets measure of AC impedance of symmetric cell before and after the DC polarization measurement; (d) The LSV curves of the membranes"
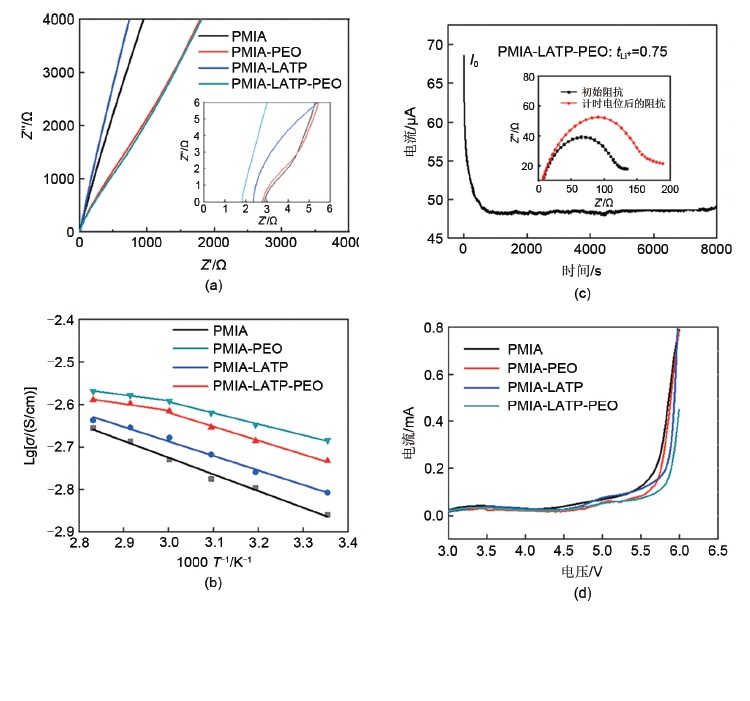
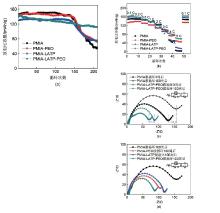
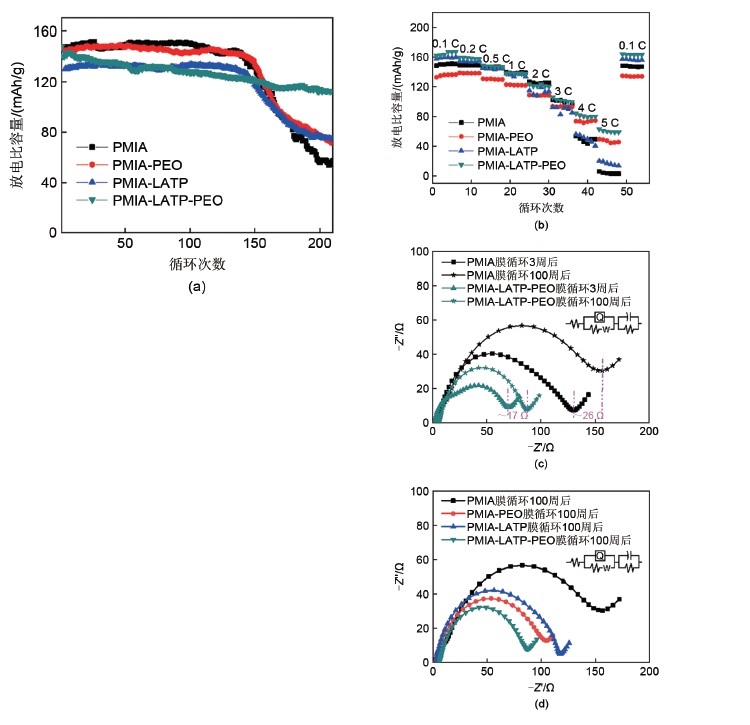
Fig. 4
(a) Cycling performance of LiFePO4||Li cells with different membranes at 0.2 C; (b) Rate performance of LiFePO4||Li cells with membranes; (c) Variation in the AC impedance spectra of cells with PMIA and PMIA-LATP-PEO membrane after 3 cycles and after the 100 cycles at 0.2 C; (d) The AC impedance spectra of different cells after 100 cycles at 0.2 C"
| 1 | 李文俊, 徐航宇, 杨琪, 等. 高能量密度锂电池开发策略[J]. 储能科学与技术, 2020, 9(2): 448-478. |
| LI W J, XU H Y, YANG Q, et al. Development of strategies for high-energy-density lithium batteries[J]. Energy Storage Science and Technology, 2020, 9(2): 448-478. | |
| 2 | 田孟羽, 詹元杰, 闫勇, 等. 锂离子电池补锂技术[J]. 储能科学与技术, 2021, 10(3): 800-812. |
| TIAN M Y, ZHAN Y J, YAN Y, et al. Replenishment technology of the lithium ion battery[J]. Energy Storage Science and Technology, 2021, 10(3): 800-812. | |
| 3 | LI T, LIU H, SHI P, et al. Recent progress in carbon/lithium metal composite anode for safe lithium metal batteries[J]. Rare Metals, 2018, 37(6): 449-458. |
| 4 | LIU H, PENG D C, XU T Y, et al. Porous conductive interlayer for dendrite-free lithium metal battery[J]. Journal of Energy Chemistry, 2021, 53: 412-418. |
| 5 | COSTA C M, LEE Y H, KIM J H, et al. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes[J]. Energy Storage Materials, 2019, 22: 346-375. |
| 6 | CAI B R, CAO J H, LIANG W H, et al. Ultraviolet-cured Al2O3-polyethylene terephthalate/polyvinylidene fluoride composite separator with asymmetric design and its performance in lithium batteries[J]. ACS Applied Energy Materials, 2021, 4(5): 5293-5303. |
| 7 | LIANG T, CAO J H, LIANG W H, et al. Asymmetrically coated LAGP/PP/PVDF-HFP composite separator film and its effect on the improvement of NCM battery performance[J]. RSC Advances, 2019, 9(70): 41151-41160. |
| 8 | HE L, CAO J H, LIANG T, et al. Effect of monomer structure on properties of polyimide as LIB separator and its mechanism study[J]. Electrochimica Acta, 2020, 337: doi: 10.1016/j.electacta.2020.135838. |
| 9 | NUNES-PEREIRA J, COSTA C M, LANCEROS-MÉNDEZ S. Polymer composites and blends for battery separators: State of the art, challenges and future trends[J]. Journal of Power Sources, 2015, 281: 378-398. |
| 10 | ZHANG H, ZHANG Y, XU T G, et al. Poly(m-phenylene isophthalamide) separator for improving the heat resistance and power density of lithium-ion batteries[J]. Journal of Power Sources, 2016, 329: 8-16. |
| 11 | LI C, HUANG Y, CHEN C, et al. A high-performance solid electrolyte assisted with hybrid biomaterials for lithium metal batteries[J]. Journal of Colloid and Interface Science, 2022, 608: 313-321. |
| 12 | YANG L Y, CAO J H, CAI B R, et al. Electrospun MOF/PAN composite separator with superior electrochemical performances for high energy density lithium batteries[J]. Electrochimica Acta, 2021, 382: doi: 10.1016/j.electacta.2021.138346. |
| 13 | LIN C E, WANG J, ZHOU M Y, et al. Poly(m-phenylene isophthalamide) (PMIA): A potential polymer for breaking through the selectivity-permeability trade-off for ultrafiltration membranes[J]. Journal of Membrane Science, 2016, 518: 72-78. |
| 14 | ZHANG H N, XIE Y X, SONG Y, et al. Preparation of high-temperature resistant poly (m-phenylene isophthalamide)/polyacrylonitrile composite nanofibers membrane for air filtration[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 624: doi: 10.1016/j.colsurfa.2021.126831. |
| 15 | LI Y. A sandwich-structure composite membrane as separator with high wettability and thermal properties for advanced lithium-ion batteries[J]. International Journal of Electrochemical Science, 2019: 7088-7103. |
| 16 | HU S Y, LIN S D, TU Y Y, et al. Novel aramid nanofiber-coated polypropylene separators for lithium ion batteries[J]. Journal of Materials Chemistry A, 2016, 4(9): 3513-3526. |
| 17 | ZHAI Y Y, WANG N, MAO X, et al. Sandwich-structured PVDF/PMIA/PVDF nanofibrous separators with robust mechanical strength and thermal stability for lithium ion batteries[J]. J Mater Chem A, 2014, 2(35): 14511-14518. |
| 18 | WANG L Y, DENG N P, JU J G, et al. A novel core-shell structured poly-m-phenyleneisophthalamide@polyvinylidene fluoride nanofiber membrane for lithium ion batteries with high-safety and stable electrochemical performance[J]. Electrochimica Acta, 2019, 300: 263-273. |
| 19 | TUNG S O, HO S, YANG M, et al. A dendrite-suppressing composite ion conductor from aramid nanofibres[J]. Nature Communications, 2015, 6: 6152. |
| 20 | SHENG L, LI Z L, HSUEH C H, et al. Suppression of lithium dendrite by aramid nanofibrous aerogel separator[J]. Journal of Power Sources, 2021, 515: doi: 10.1016/j.jpowsour.2021.230608. |
| 21 | KANG W M, DENG N P, MA X M, et al. A thermostability gel polymer electrolyte with electrospun nanofiber separator of organic F-doped poly-m-phenyleneisophthalamide for lithium-ion battery[J]. Electrochimica Acta, 2016, 216: 276-286. |
| 22 | ZHAO H J, DENG N P, YAN J, et al. Effect of octaphenyl polyhedral oligomeric silsesquioxane on the electrospun poly-m-phenylene isophthalamid separators for lithium-ion batteries with high safety and excellent electrochemical performance[J]. Chemical Engineering Journal, 2019, 356: 11-21. |
| 23 | LI J L, TIAN W T, YAN H C, et al. Preparation and performance of aramid nanofiber membrane for separator of lithium ion battery[J]. Journal of Applied Polymer Science, 2016, 133(30): 43623. |
| 24 | ZHU C Q, ZHANG J X, XU J, et al. Aramid nanofibers/polyphenylene sulfide nonwoven composite separator fabricated through a facile papermaking method for lithium ion battery[J]. Journal of Membrane Science, 2019, 588: doi: 10.1016/j.memsci.2019.117169. |
| 25 | DONG J M, ZHANG Y F, WANG J Y, et al. Highly porous single ion conducting polymer electrolyte for advanced lithium-ion batteries via facile water-induced phase separation process[J]. Journal of Membrane Science, 2018, 568: 22-29. |
| 26 | MIAO L, WU Y, HU J W, et al. Hierarchical aramid nanofibrous membranes from a nanofiber-based solvent-induced phase inversion process[J]. Journal of Membrane Science, 2019, 578: 16-26. |
| 27 | LI Y H, LIN Q F, CHEN Z, et al. Phase separation-induced hierarchical porous PVDF/PMIA blended separator with high wettability and thermal stability for lithium-ion batteries[J]. Journal of the Electrochemical Society, 2021, 168(4): 040510. |
| 28 | LIU L H, MO J S, LI J R, et al. Comprehensively-modified polymer electrolyte membranes with multifunctional PMIA for highly-stable all-solid-state lithium-ion batteries[J]. Journal of Energy Chemistry, 2020, 48: 334-343. |
| 29 | BACHMAN J C, MUY S, GRIMAUD A, et al. Inorganic solid-state electrolytes for lithium batteries: Mechanisms and properties governing ion conduction[J]. Chemical Reviews, 2016, 116(1): 140-162. |
| 30 | KEY B, SCHROEDER D J, INGRAM B J, et al. Solution-based synthesis and characterization of lithium-ion conducting phosphate ceramics for lithium metal batteries[J]. Chemistry of Materials, 2012, 24(2): 287-293. |
| 31 | SIYAL S H, LI M J, LI H, et al. Ultraviolet irradiated PEO/LATP composite gel polymer electrolytes for lithium-metallic batteries (LMBs)[J]. Applied Surface Science, 2019, 494: 1119-1126. |
| 32 | YAO L R, LEE C, KIM J. Fabrication of electrospun meta-aramid nanofibers in different solvent systems[J]. Fibers and Polymers, 2010, 11(7): 1032-1040. |
| 33 | ALLOIN F, D'APREA A, KISSI N E, et al. Nanocomposite polymer electrolyte based on whisker or microfibrils polyoxyethylene nanocomposites[J]. Electrochimica Acta, 2010, 55(18): 5186-5194. |
| 34 | CHEN L, LI Y T, LI S P, et al. PEO/garnet composite electrolytes for solid-state lithium batteries: From "ceramic-in-polymer" to "polymer-in-ceramic"[J]. Nano Energy, 2018, 46: 176-184. |
| 35 | XU J L, YUAN L, LIANG G Z, et al. Achieving superiorly high heat-dimensional stability, high strength, and good electrochemical performance for electrospun separators in power lithium-ion battery through building unique condensed structure based on polyimide and poly (m-phenylene isophthalamide)[J]. Journal of Applied Polymer Science, 2021, 138(42): 51233. |
| 36 | WATANABE T, INAFUNE Y, TANAKA M, et al. Development of all-solid-state battery based on lithium ion conductive polymer nanofiber framework[J]. Journal of Power Sources, 2019, 423: 255-262. |
| 37 | LI H Y, LIU W, YANG X D, et al. Fluoroethylene carbonate-Li-ion enabling composite solid-state electrolyte and lithium metal interface self-healing for dendrite-free lithium deposition[J]. Chemical Engineering Journal, 2021, 408: doi: 10.1016/j.cej.2020.127254. |
| 38 | PAN F S, QIAO L N, YUAN B, et al. Polydopamine coated poly(m-phenylene isophthalamid) membrane as heat-tolerant separator for lithium-ion batteries[J]. Ionics, 2020, 26(11): 5471-5480. |
| [1] | Jie WU, Xiaobiao JIANG, Yang YANG, Yongmin WU, Lei ZHU, Weiping TANG. Progress of NASICON-structured Li1+xAlxTi2-x(PO4)3 (0 ≤x≤ 0.5) solid electrolyte [J]. Energy Storage Science and Technology, 2020, 9(5): 1472-1488. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
