Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (10): 3100-3111.doi: 10.19799/j.cnki.2095-4239.2022.0211
• Energy Storage Materials and Devices • Previous Articles Next Articles
Received:2022-04-19
Revised:2022-05-07
Online:2022-10-05
Published:2022-10-10
CLC Number:
Lifeng FANG. Application of aliphatic-crown ethers in battery electrolytes[J]. Energy Storage Science and Technology, 2022, 11(10): 3100-3111.
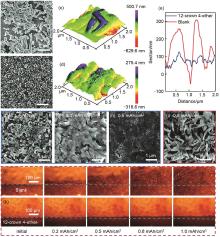
Fig. 3
Comparison of Li deposition morphology in 12-crown-4 added and CrEt -free electrolytes. SEM images of Li deposits at a deposition capacity of 0.05 mAh/cm2 and a current density of 0.5 mA/cm2 in CrEt-free (a) and 12-crown-4 added (b) electrolytes. 3D atomic force microscopy (AFM) images (2 μm×2 μm scan size) of Li deposition morphology in CrEt-free (c) and 12-crown-4added (d) electrolytes. (e)( Height profiles of these two deposits. (f)-(h) Morphologies at increased deposition capacities (0.1~0.5 mAh/cm2) in a 12-crown-4added electrolyte. (i) Morphology at a deposition capacity of 0.5 mAh/cm2 in a CrEt-free electrolyte. Optical microscopy observations of Li electrochemical deposition in CrEt-free (j) and 12-crown-4added (k) electrolytes at a current density of 3 mA/cm2[26]"

| 1 | 吴凯, 张耀, 曾毓群, 等. 锂离子电池安全性能研究[J]. 化学进展, 2011, 23(S1): 401-409. |
| WU K, ZHANG Y, ZENG Y Q, et al. Safety performance of lithium-ion battery[J]. Progress in Chemistry, 2011, 23(S1): 401-409. | |
| 2 | 张丽娟. 锂离子电池宽温电解液体系的构建与性能研究[D]. 西宁: 中国科学院大学(中国科学院青海盐湖研究所), 2018. |
| ZHANG L J. Construction and properties study of wide temperature electrolyte system for lithium ion batteries[D]. Xining: Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, 2018. | |
| 3 | 野上隆, 名和政良, 化學電池: 日本, 特開昭58-35874[P].1983-03-02. |
| NOGAMI T, NAWA M.Chemical cell: JP58035874A[P]. 1983-03-02. | |
| 4 | PEDERSEN C J. Cyclic polyethers and their complexes with metal salts[J]. Journal of the American Chemical Society, 1967, 89(26): 7017-7036. |
| 5 | PEDERSEN C J. Macrocyclic polyether compounds and ionic complexes thereof:US3562295[P].1971-02-09. |
| 6 | PEDERSEN C J. Macrocyclic polyether compounds: US3687978[P]. 1972-08-29. |
| 7 | PEDERSEN C J. Macrocyclic polyether compounds: US3987061[P]. 1972-10-19. |
| 8 | DALE J, KRISTIANSEN P O, HENRIKSEN L, et al. Macrocyclic oligo-ethers related to ethylene oxide[J]. Acta Chemica Scandinavica, 1972, 26: 1471-1478. |
| 9 | 朱玉岚, 黄险峰, 宋国强. 氟代碳酸乙烯酯的合成工艺研究[J]. 广州化工, 2012, 40(6): 97-98. |
| ZHU Y L, HUANG X F, SONG G Q. Study on the synthesis of fluoroethylene carbonate[J]. Guangzhou Chemical Industry, 2012, 40(6): 97-98. | |
| 10 | 吴茂祥, 方桂煌, 潘荧, 等. 一种相转移催化合成氟代碳酸乙烯酯的方法: CN101870687A[P]. 2010-10-27. |
| FANG G H, HUANG J Y, LU B Q, et al. Method for synthesizing fluoroethylene carbonate by phase-transfer catalysis: CN101870687A[P]. 2010-10-27. | |
| 11 | 李云峰, 王永勤. 氟代碳酸乙烯酯的合成工艺研究[J]. 河南化工, 2018, 35(7): 29-31. |
| LI Y F, WANG Y Q. Study on the synthetic process of fluoroethylene carbonate[J]. Henan Chemical Industry, 2018, 35(7): 29-31. | |
| 12 | ANET F A L, KRANE J, DALE J, et al. The conformation of 1, 4, 7, 10-tetraoxacyclododecane and its 1: 1 lithium salt complexes[J]. Acta Chemica Scandinavica, 1973, 27: 3395-3402. |
| 13 | Takehara Z, 胡行仁. 日本锂电池发展史[J]. 电池, 1989, 19(6): 48-50, 64. |
| TAKEHARA Z, HU X R. Lithium batteries history of Japan[J]. Battery Bimonthly, 1989, 19(6): 48-50, 64. | |
| 14 | 鸢岛真一, 山木凖一, 山路昭彦.リチウム二次電池用電解液: 日本, 特開昭57-141878[P].1982-09-02. |
| TOBISHIMA S, YAMAKI J, YAMAJI A. Electrolyte for lithium secondary battery: JP57141878A[P]. 1982-09-02. | |
| 15 | 小林征男, 武内正隆, 獅々倉 利一, 等. 非水二次電池: 日本, 特開昭61-284071[P]. 1986-12-15. |
| KOBAYASHI M, TAKEUCHI M, SHISHIKURA RIICHI, et al.Nonaqueous secondary battery: JP6128407A[P]. 1986-12-15. | |
| 16 | 宫林光孝, 唐沢環江, 安川栄起. 非水溶媒二次電池: 日本, 特開平5-290888[P]. 1993-11-05. |
| MIYABAYASHI M, KARASAWA T, YASUKAWA S. Nonaqueous solvent secondary battery: JP05290888A[P]. 1993-11-05. | |
| 17 | 奥田昌久, 原贤二, 真下清孝. 非水電解液二次電池: 日本, 特開2000-195548[P]. 2000-07-14. |
| OKUDA M, HARA K, MASHITA K. Nonaqueous electrolyte secondary battery: JP2000195548A [P]. 2000-07-14. | |
| 18 | 藤田茂, 明石寛之, 足立百惠. Secondary cell: 世界, 0122519[P]. 2001-03-29. |
| FUJITA S, AKASHI H, ADACHI M. Secondary cell: WO0122519[P]. 2001-03-29. | |
| 19 | XU W, WANG J L, DING F, et al. Lithium metal anodes for rechargeable batteries[J]. Energy & Environmental Science, 2014, 7(2): 513-537. |
| 20 | LIN D C, LIU Y Y, CUI Y. Reviving the lithium metal anode for high-energy batteries[J]. Nature Nanotechnology, 2017, 12(3): 194-206. |
| 21 | ZHANG H, ESHETU G G, JUDEZ X, et al. Electrolyte additives for lithium metal anodes and rechargeable lithium metal batteries: Progress and perspectives[J]. Angewandte Chemie (International Ed in English), 2018, 57(46): 15002-15027. |
| 22 | WU F, YUAN Y X, CHENG X B, et al. Perspectives for restraining harsh lithium dendrite growth: Towards robust lithium metal anodes[J]. Energy Storage Materials, 2018, 15: 148-170. |
| 23 | WANG H P, HE J, LIU J D, et al. Electrolytes enriched by crown ethers for lithium metal batteries[J]. Advanced Functional Materials, 2021, 31(2): doi: 10.1002/adfm.202002578. |
| 24 | ZHAO J, LI Q L, PANG Y D, et al. Introducing crown ether as a functional additive for high-performance dendrite-free Li metal batteries[J]. ACS Applied Energy Materials, 2021, 4(8): 7829-7838. |
| 25 | HUANG X L, ZHUANG D M, CHEN Z H, et al. The investigation for electrodeposition behavior of lithium metal in a crown ether/propylene carbonate electrolyte[J]. Journal of Electroanalytical Chemistry, 2021, 887: doi: 10.1016/j.jelechem.2021.115156. |
| 26 | LU Z Y, GUO Y, ZHANG S W, et al. Crowning metal ions by supramolecularization as a general remedy toward a dendrite-free alkali-metal battery[J]. Advanced Materials (Deerfield Beach, Fla), 2021, 33(31): doi: 10.1002/adma.202101745. |
| 27 | PELED E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—The solid electrolyte interphase model[J]. Journal of the Electrochemical Society, 1979, 126(12): 2047-2051. |
| 28 | 足立百惠, 電池: 日本, 特開2003-187864[P]. 2003-07-04. |
| ADACHI M. Battery: JP2003187864A[P]. 2003-07-04. | |
| 29 | 江田信夫, 饭岛孝志, 電池: 日本, 特開昭53-76322[P]. 1978-07-06. |
| EDA N, IIJIMA T. Battery: JP53076322A[P]. 1978-07-06. | |
| 30 | SOFFER A. Electrochemical cells with non-aqueous electrolytes containing macroheterocyclic compounds: US4132837[P]. 1979-01-02. |
| 31 | WILKINSON D P, DAHN J R. Electrolyte solution sequestering agents for electrochemical cells having carbonaceous electrodes: US5130211[P]. 1992-07-14. |
| 32 | 芦伟. 锂离子电池宽温电解液的设计与性能研究[D]. 长沙: 国防科学技术大学, 2015. |
| LU W. Research on the electrolyte designed for lithium-ion batteries with wide operating temperature range[D]. Changsha: National University of Defense Technology, 2015. | |
| 33 | 森垣健一, 大河内正也, 美濃辰治. リチウム二次電池: 日本, 特開2000-243446[P]. 2000-09-08. |
| MORIGAKI K, OKOCHI M, MINO T. Lithium secondary battery: JP2000243446A[P]. 2000-09-08. | |
| 34 | 村冈宪树, 木下一成, 鵜木重幸, 等. リチウム二次電池: 日本, 特開2000-306602[P]. 2000-11-02. |
| MURAOKA N, KINOSHITA K, UNOKI S, et al. Lithium secondary battery: JP2000306602A[P]. 2000-11-02. | |
| 35 | Sakurai Takahiro, Kado Hiroyasu. etc. Nonaqueous electrolyte secondary battery,method for producing same and nonaqueous electrolyte: WO2015136855[P]. 2015-09-17. |
| 36 | HOU C, HAN J H, LIU P, et al. Operando observations of SEI film evolution by mass-sensitive scanning transmission electron microscopy[J]. Advanced Energy Materials, 2019, 9(45): doi: 10.1002/aenm.201902675. |
| 37 | 胡华坤, 薛文东, 霍思达, 等. 锂离子电池电解液SEI成膜添加剂的研究进展[J]. 化工学报, 2022, 73(4): 1436-1454. |
| HU H K, XUE W D, HUO S D, et al. Review of SEI film forming additives for electrolyte of lithium ion battery[J]. CIESC Journal, 2022, 73(4): 1436-1454. | |
| 38 | GU S C, ZHANG S W, HAN J W, et al. Nitrate additives coordinated with crown ether stabilize lithium metal anodes in carbonate electrolyte[J]. Advanced Functional Materials, 2021, 31(28): doi: 10.1002/adfm.202102128. |
| 39 | 江田信夫, 守田彰克, 中井正树. 電池: 日本, 特開昭59-60970[P]. 1984-04-07. |
| EDA N, MORITA T, NAKAI M. Battery: JP59060970A[P]. 1984-04-07. | |
| 40 | 由光一三, 梶田耕三,清水明夫, リチウム有機二次電池: 日本, 特開昭59-151779[P]. 1984-08-30. |
| YOSHIMITSU K, KAJITA K, SHIMIZU A. Lithium organic secondary battery: JP59151779A[P]. 1984-08-30. | |
| 41 | HEINZE J, MORTENSEN J. Electrochemical cell containing electrode made of polymeric compound and electrolyte containing organic complex ligand: US4609600[P]. 1986-09-02. |
| 42 | 岸井豊,村田修平, 喜井敬介等. いイオン伝導体: 日本, 特開2007-194151[P]. 2007-08-02. |
| KISHII Y, MURATA S, YOSHII K.Ion conductor: JP2007194151A[P]. 2007-08-02. | |
| 43 | 大坪亮二, 安部武志, 小久见善八. 二次電池用電解液: 日本, 特開2017-117592[P]. 2017-06-29. |
| OTSUBO R, ABE T, OKUMI Z. Electrolyte for secondary battery:JP2017117592A[P].2017-06-29. | |
| 44 | 大道馨, Christopher Brooks,Ryan Mckenney. バッテリー用の液体电解质: 日本, 特開2018-101621[P]. 2018-06-28. |
| OMICHI K, CHRISTOPHER B, RYAN M. Liquid electrolyte for battery: JP2018101621A[P]. 2018-06-28. | |
| 45 | NAGASUBRAMANIAN G, DI STEFANO S. 12-crown-4 ether-assisted enhancement of ionic conductivity and interfacial kinetics in polyethylene oxide electrolytes[J]. Journal of the Electrochemical Society, 1990, 137(12): 3830-3835. |
| 46 | MORITA M, TANAKA H, ISHIKAWA M, et al. Effects of crown ethers on the electrochemical properties of polymeric solid electrolytes consisting of poly (ethylene oxide)-grafted poly (methylmethacrylates) [J]. Solid State Ionics, 1996, 86/87/88: 401-405. |
| 47 | 三川礼, 野上隆, 藤本雅治. 固体状陰イオン導電池: 日本, 特開昭58-48302[P]. 1983-03-22. |
| MIKAWA R,NOGAMI T, FUJIMOTO M. Solid anion conductor: JP58048302A[P]. 1983-03-22. | |
| 48 | 藤波达雄, 青木孝浩. リチウムイオン導電性材料及びリチウム二次電池: 日本, 特開2005-276509[P]. 2005-10-06. |
| FUJINAMI T, AOKI T.Lithium ion conductive material and lithium secondary battery: JP2005276509A[P]. 2005-10-06. | |
| 49 | 松田好晴, 森田昌行. 固体電解質電池: 日本, 特開平5-315007[P]. 1993-11-26. |
| MATSUDA Y, MORITA M.Solid electrolyte cell: JP05315007A[P]. 1993-11-26. | |
| 50 | 梶谷芳男, 增田誠治. リチウム二次電池: 日本, 特開平8-106920[P]. 1996-04-23. |
| KAJITANI Y, MASUDA S. Lithium secondary battery: JP08106920A[P]. 1996-04-23. | |
| 51 | NAGASUBRAMANIAN G, DISTEFANO S. Secondary Li battery incorporating 12-crown-4 ether: US5110694[P]. 1992-05-05. |
| 52 | YARMOLENKO O V, BELOV D, EFIMOV O. Effect of crown ethers on the conduction of plasticized polyacrylonitrile-based electrolytes[J]. Russian Journal of Electrochemistry, 2001, 37: 280-286. |
| 53 | BASKAKOVA Y V, YARMOLENKO O V, SHUVALOVA N I, et al. Effect of 15-crown-5 on the charge transfer resistance at the polymer electrolyte/modified Li-electrode interface[J]. Russian Journal of Electrochemistry, 2006, 42(9): 949-953. |
| 54 | 西原禎文, 井上克也, 金野大輻, イオン伝導性結晶およびそれを用いた固体電解質、電池用セパレー夕、電池: 日本, 特開2012-182060[P]. 2012-09-20. |
| NISHIHARA S,INOUE K, KONNO D.Ion conductive crystal,and solid electrolyte,cell separator and battery using the same:JP2012182060A[P].2012-09-20. | |
| 55 | 吉村精司, 井町直希, 最相圭司, 等.リチウム二次電池: 日本, 特開2005-63871[P]. 2005-03-10. |
| YOSHIMURA S, IMACHI N,SAISHO K. Lithium secondary battery: JP2005063871A[P]. 2005-03-10. | |
| 56 | 吉本信子, 山吹一大, 福井一輝, 等. マグネシウム二次電池用の絶縁抑制電解液及び絶縁抑制方法: 日本, 特開2021-77459[P]. 2021-05-20. |
| YOSHIMOTO N, YAMABUKI K, FUKUI K. Insulation suppression electrolyte and insulation suppression method for magnesium secondary battery: JP2021077459A[P]. 2021-05-20. | |
| 57 | YANG Y F, CHIOU C Y, LIU C W, et al. Crown ethers as electrolyte additives to modulate the electrochemical potential of lithium organic batteries[J]. The Journal of Physical Chemistry C, 2019, 123(36): 21950-21958. |
| 58 | WANG X C, WANG Y M, LIU W, et al. Influence of 12-crown-4 on oxygen electrode of aprotic Li-O2 battery[J]. Acta Physico-Chimica Sinica, 2016, 32(1): 343-348. |
| 59 | XU W, XIAO J, WANG D Y, et al. Crown ethers in nonaqueous electrolytes for lithium/air batteries[J]. Electrochemical and Solid-State Letters, 2010, 13(4): A48. |
| 60 | HASENKOX U. Polymer-ionophore separator: US20140057156[P]. 2014-02-27. |
| 61 | 한동협, 손권남, 박창훈,김일토. 리튬-황 이차전지: 韩国, 10-2021-0112026[P].2021-09-14. |
| HAN D, SOHN K,PARK C,KIM I. Lithium-sulfur secondary battery: KR1020210112026A[P]. 2021-09-14. | |
| 62 | CAO Y, PEI Q B, ANDERSSON M R, et al. Light-emitting electrochemical cells with crown ether as solid electrolyte[J]. Journal of the Electrochemical Society, 1997, 144(12): L317-L320. |
| 63 | GAO M D, WANG Y, YI Q H, et al. A novel solid-state electrolyte based on a crown ether lithium salt complex[J]. Journal of Materials Chemistry A, 2015, 3(41): 20541-20546. |
| 64 | 徐仲榆, 郑洪河. 锂离子蓄电池碳负极/电解液相容性研究进展Ⅰ碳电极界面化学与碳负极/电解液的相容性[J]. 电源技术, 2000, 24(3): 171-177. |
| XU Z Y, ZHENG H H. Research progress on compatibility of electrolyte with carbon negative electrode in lithium ion batteries Part Ⅰ Surface chemistry of carbon electrode and the compatibility of electrolyte with carbon negative electrode[J]. Chinese Journal of Power Sources, 2000, 24(3): 171-177. | |
| 65 | 刘建生, 左晓希, 贺云鹏, 等. 高倍率锂离子电池用电解液: CN1925207A[P]. 2007-03-07. |
| LIU J S, ZUO X X, HE Y P, et al High rate electrolyte for lithium ion battery: CN1925207A[P]. 2007-03-07. | |
| 66 | 叶丽萍. 一种钦酸哩电池用电解液及其铁酸理电池: 中国, 104752768B[P]. 2018-01-09. |
| YE L P. A titanate electrolyte for lithium cells and lithium titanate: CN104752768B[P]. 2018-01-09. | |
| 67 | 陈果, 刘立炳, 惠怀兵, 等. 一种磷酸铁锂电池低温电解液: CN106129472A[P]. 2016-11-16. |
| CHEN G, LIU L B, HUI H B, et al. Low-temperature electrolyte for lithium iron phosphate battery: CN106129472A[P]. 2016-11-16. | |
| 68 | 陈大波, 田维坚. Graphene lithium titanate battery: CN108878955B[P]. 2020-11-13. |
| 陈大波, 田维坚. 一种石墨烯钛酸锂电池: CN108878955B[P]. 2020-11-13. | |
| 69 | E·基泽, C·格雷, H·格拉斯, 等. 镁盐: CN107086323A[P]. 2017-08-22. |
| 70 | 金超, 杨瑞枝, 潘晓伟, 等. 锂离子固态电解质薄膜及其应用: CN109921089B[P]. 2021-02-19. |
| 71 | 段寅琦, 李先锋, 张华民, 等. 一种碱性锌铁液流电池用正极电解液及应用: CN108123174A[P]. 2018-06-05. |
| DUAN Y Q, LI X F, ZHANG H M, et al. Positive electrode electrolytic solution for alkaline zinc-iron flow battery, and applications thereof: CN108123174A[P]. 2018-06-05. | |
| 72 | 康树森, 魏彦存, 李营, 等. 一种电解质材料及其制备方法、固体电解质及电池: CN112117484A[P]. 2020-12-22. |
| KANG S S, WEI Y C, LI Y, et al. Electrolyte material and preparation method thereof, solid electrolyte and battery: CN112117484A[P]. 2020-12-22. | |
| 73 | 刘艳侠, 刘景博, 王恩阳, 等. 一种适用于硅碳负极材料的电解液: CN112331919A[P]. 2021-02-05. |
| LIU Y X, LIU J B, WANG E Y, et al. Electrolyte suitable for silicon-carbon negative electrode material: CN112331919A[P]. 2021-02-05. | |
| 74 | 吕伟, 谷思辰, 康飞宇, 杨全红. 一种碳酸醋类电解液及金属理电池: 中国, 113131000A[P]. 2021-07-16. |
| LV W, GU S,KANG F, YANG Q. Carbonic ester electrolyte and metal lithium battery: CN113131000A[P]. 2021-07-16. | |
| 75 | LIOTTA C L. Macrocyclic polyether/nitrile complexes: US3997562[P]. 1976-12-14. |
| 76 | 项飞勇. 一种18-冠醚-6的制备方法: CN103275059A[P]. 2013-09-04. |
| XIANG F Y. Method for preparing 18-crown ether-6: CN103275059A[P]. 2013-09-04. | |
| 77 | KUO P L, KAWAMURA N, MIKI M, et al. The synthesis of unsubstituted crown ethers by the reaction of oligoethylene glycols with arenesulfonyl or alkanesulfonyl chlorides[J]. Bulletin of the Chemical Society of Japan, 1980, 53(6): 1689-1693. |
| 78 | GAMON N. Method of isolating macrocyclic polyethers: US4656295[P]. 1987-04-07. |
| 79 | DE JONG F, REINHOUDT D N. Macrocyclic polyether complexes: US4199513[P]. 1980-04-22. |
| 80 | KRIJNEN W J, GROTENHUIS P A M. Manufacture of macrocyclic polyethers: US4435582[P]. 1984-03-06. |
| 81 | JOHANNES D.Process for selective preparation of macrocyclic polyethers: US3928386[P]. 1975-12-23. |
| 82 | DALE J, DAASVATN K. Process for selective preparation of macrocyclic polyethers: US3997563[P]. 1976-12-14. |
| 83 | 张治国, 尹红, 陈志荣. 环氧乙烷均聚反应机理的理论研究[J]. 化学学报, 2004, 62(20): 1988-1992. |
| ZHANG Z G,YIN H,CHEN Z R.Investigations on polymerization mechanism of ethylene oxide[J]. Acta Chimica Sinica, 2004, 62(20): 1988-1992. | |
| 84 | 方黎锋, 郑琪, 王高, 等. 一种环氧乙烷低聚合成脂肪族冠醚的制备方法: CN114702472A[P]. 2022-07-05. |
| 85 | IGNATOVA A A, YARMOLENKO O V, TULIBAEVA G Z, et al. Influence of 15-crown-5 additive to a liquid electrolyte on the performance of Li/CFx-Systems at temperatures up to -50 ℃[J]. Journal of Power Sources, 2016, 309: 116-121. |
| [1] | Pengbo ZHAI, Dongmei CHANG, Zhijie BI, Ning ZHAO, Xiangxin GUO. Research progress on key interfacial issues in lithium lanthanum zirconium oxide-based solid-state [J]. Energy Storage Science and Technology, 2022, 11(9): 2847-2865. |
| [2] | Qunbin ZHANG, Tao DONG, Jingjing LI, Yanxia LIU, Haitao ZHANG. Research progress on the recovery and high-value utilization of spent electrolyte from lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2798-2810. |
| [3] | Jun ZHANG, Qi LI, Ying TAO, Quanhong YANG. Sieving carbons for sodium-ion batteries: Origin and progress [J]. Energy Storage Science and Technology, 2022, 11(9): 2825-2833. |
| [4] | Jinghua WU, Jing YANG, Gaozhan LIU, Zhiyan WANG, Zhihua ZHANG, Hailong YU, Xiayin YAO, Xuejie HUANG. Review and prospective of solid-state lithium batteries in the past decade (2011—2021) [J]. Energy Storage Science and Technology, 2022, 11(9): 2713-2745. |
| [5] | Jing ZHU, Yida WU, Junfeng HAO, Guanjun CEN, Ronghan QIAO, Xiaoyu SHEN, Mengyu TIAN, Hongxiang JI, Zhou JIN, Yuanjie ZHAN, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Xuejie HUANG. Reviews of selected 100 recent papers for lithium batteries (Jun. 1, 2022 to Jul. 31, 2022) [J]. Energy Storage Science and Technology, 2022, 11(9): 3035-3050. |
| [6] | Sida HUO, Wendong XUE, Xinli LI, Yong LI. Visualization analysis of composite electrolytes for lithium battery based on CiteSpace [J]. Energy Storage Science and Technology, 2022, 11(7): 2103-2113. |
| [7] | Xiaoyu SHEN, Guanjun CEN, Ronghan QIAO, Jing ZHU, Hongxiang JI, Mengyu TIAN, Zhou JIN, Yong YAN, Yida WU, Yuanjie ZHAN, Hailong YU, Liubin BEN, Yanyan LIU, Xuejie HUANG. Reviews of selected 100 recent papers for lithium batteries (Apr. 1, 2022 to May 31, 2022) [J]. Energy Storage Science and Technology, 2022, 11(7): 2007-2022. |
| [8] | Yingwei PEI, Hong ZHANG, Xinghui WANG. Recent advances in the electrolytes of rechargeable zinc-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(7): 2075-2082. |
| [9] | ZHOU Weidong, HUANG Qiu, XIE Xiaoxin, CHEN Kejun, LI Wei, QIU Jieshan. Research progress of polymer electrolyte for solid state lithium batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1788-1805. |
| [10] | LI Yitao, SHEN Kaier, PANG Quanquan. Advance in organics enhanced sulfide-based solid-state batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1902-1918. |
| [11] | OU Yu, HOU Wenhui, LIU Kai. Research progress of smart safety electrolytes in lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1772-1787. |
| [12] | Ronghan QIAO, Guanjun CEN, Xiaoyu SHEN, Mengyu TIAN, Hongxiang JI, Feng TIAN, Wenbin QI, Zhou JIN, Yida WU, Yuanjie ZHAN, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Xuejie HUANG. Reviews of selected 100 recent papers for lithium batteries (Feb. 1, 2022 to Mar. 31, 2022) [J]. Energy Storage Science and Technology, 2022, 11(5): 1289-1304. |
| [13] | Maolin FANG, Ying ZHANG, Lin QIAO, Shumin LIU, Zhongqi CAO, Huamin ZHANG, Xiangkun MA. Research progress of iron-chromium flow batteries technology [J]. Energy Storage Science and Technology, 2022, 11(5): 1358-1367. |
| [14] | Chaochao WEI, Chuang YU, Zhongkai WU, Linfeng PENG, Shijie CHENG, Jia XIE. Research progress of Li3PS4 solid electrolyte [J]. Energy Storage Science and Technology, 2022, 11(5): 1368-1382. |
| [15] | Zhicheng CHEN, Zongxu LI, Ling CAI, Yisi LIU. Development status and future prospects of flexible metal-air batteries [J]. Energy Storage Science and Technology, 2022, 11(5): 1401-1410. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

