Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (1): 42-50.doi: 10.19799/j.cnki.2095-4239.2022.0429
• Energy Storage Materials and Devices • Previous Articles Next Articles
Yelong ZHANG1( ), Qi MIAO2,3, Pengfei SONG1, Linghua TAN2,3, Yi JIN1(
), Qi MIAO2,3, Pengfei SONG1, Linghua TAN2,3, Yi JIN1( ), Yulong DING4
), Yulong DING4
Received:2022-08-03
Revised:2022-08-12
Online:2023-01-05
Published:2023-02-08
Contact:
Yi JIN
E-mail:ZYL1988219@163.com;yi.jin@jinhe-energy.com
CLC Number:
Yelong ZHANG, Qi MIAO, Pengfei SONG, Linghua TAN, Yi JIN, Yulong DING. Preparation and performance evaluation of mineral-based magnesium sulfate thermochemical adsorption materials[J]. Energy Storage Science and Technology, 2023, 12(1): 42-50.

Fig. 1
Preparation process of mineral-based magnesium sulfate composites(a) The drying process of mineral carriers; (b) Impregnation of mineral carriers; (c) The drying process of the impregnation; (d) The grinding process of mineral-based magnesium sulfate composites; (e) Final powdery product"
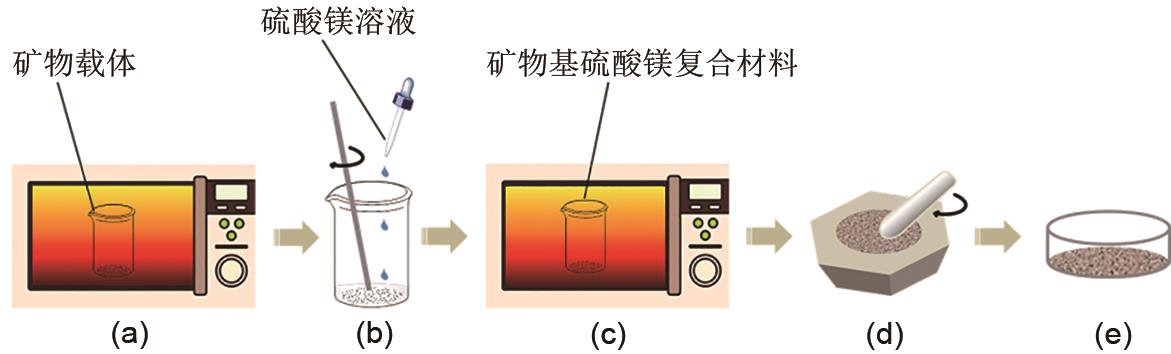

Fig. 6
SEM images of mineral-based magnesium sulfate composites (a), (b), (c) SEM images of AM samples at 2000 magnification and 10000 magnification and their element distribution, respectively; (d), (e), (f) SEM images of DM samples at 2000 magnification and 10000 magnification and their element distribution, respectively; (g), (h), (i) SEM images of EVM samples with 2000 magnification and 10000 magnification and their element distribution, respectively"

| 1 | YANG Q, ZHOU H W, BARTOCCI P, et al. Prospective contributions of biomass pyrolysis to China's 2050 carbon reduction and renewable energy goals[J]. Nature Communications, 2021, 12: 1698. |
| 2 | RAZMJOO A, GAKENIA K L, VAZIRI R M A, et al. A Technical analysis investigating energy sustainability utilizing reliable renewable energy sources to reduce CO2 emissions in a high potential area[J]. Renewable Energy, 2021, 164: 46-57. |
| 3 | RAHMAN M, ONI A, GEMECHU E, et al. Assessment of energy storage technologies: A review[J]. Energy Conversion and Management, 2020, 223: doi: 10.1016/j.enconman.2020.113295. |
| 4 | LIU J, HU C, KIMBER A, et al. Uses, cost-benefit analysis, and markets of energy storage systems for electric grid applications[J]. Journal of Energy Storage, 2020, 32: doi: 10.1016/j.est.2020.101731. |
| 5 | ARBABZADEH M, SIOSHANSI R, JOHNSON J X, et al. The role of energy storage in deep decarbonization of electricity production[J]. Nature Communications, 2019, 10: 3413. |
| 6 | GAUTAM A, SAINI R. A review on sensible heat based packed bed solar thermal energy storage system for low temperature applications[J]. Solar Energy, 2020, 207: 937-956. |
| 7 | WOODS J, MAHVI A, GOYAL A, et al. Rate capability and Ragone plots for phase change thermal energy storage[J]. Nature Energy, 2021, 6(3): 295-302. |
| 8 | DESAI F, SUNKU P J, MUTHUKUMAR P, et al. Thermochemical energy storage system for cooling and process heating applications: A review[J]. Energy Conversion and Management, 2021, 229: doi: 10.1016/j.enconman.2020.113617. |
| 9 | KOÇAK B, FERNANDEZ A I, PAKSOY H. Review on sensible thermal energy storage for industrial solar applications and sustainability aspects[J]. Solar Energy, 2020, 209: 135-169. |
| 10 | LIN J, ZHAO Q, HUANG H, et al. Applications of low-temperature thermochemical energy storage systems for salt hydrates based on material classification: A review[J]. Solar Energy, 2021, 214: 149-178. |
| 11 | N'TSOUKPOE K, SCHMIDT T, RAMMELBERG H, et al. A systematic multi-step screening of numerous salt hydrates for low temperature thermochemical energy storage[J]. Applied Energy, 2014, 124: 1-16. |
| 12 | HONGOIS S, KUZNIK F, STEVENS P, et al. Development and characterisation of a new MgSO4-zeolite composite for long-term thermal energy storage[J]. Solar Energy Materials and Solar Cells, 2011, 95(7): 1831-1837. |
| 13 | VAN ESSEN V M, ZONDAG H A, GORES J C, et al. Characterization of MgSO4 hydrate for thermochemical seasonal heat storage[J]. Journal of Solar Energy Engineering, 2009, 131(4): doi:10.1115/1.4000275. |
| 14 | POSERN K, LINNOW K, NIERMANN M, et al. Thermochemical investigation of the water uptake behavior of MgSO4 hydrates in host materials with different pore size[J]. Thermochimica Acta, 2015, 611: 1-9. |
| 15 | LINNOW K, NIERMANN M, BONATZ D, et al. Experimental studies of the mechanism and kinetics of hydration reactions[J]. Energy Procedia, 2014, 48: 394-404. |
| 16 | STEIGER M, LINNOW K, JULING H, et al. Hydration of MgSO4 ·H2O and generation of stress in porous materials[J]. Crystal Growth & Design, 2008, 8(1): 336-343. |
| 17 | MAHON D, CLAUDIO G, EAMES P C. An experimental investigation to assess the potential of using MgSO4 impregnation and Mg2+ ion exchange to enhance the performance of 13X molecular sieves for interseasonal domestic thermochemical energy storage[J]. Energy Conversion and Management, 2017, 150: 870-877. |
| 18 | XU S, WANG R, WANG L, et al. Performance characterizations and thermodynamic analysis of magnesium sulfate-impregnated zeolite 13X and activated alumina composite sorbents for thermal energy storage[J]. Energy, 2019, 167: 889-901. |
| 19 | WHITING G, GRONDIN D, BENNICI S, et al. Heats of water sorption studies on zeolite-MgSO4 composites as potential thermochemical heat storage materials[J]. Solar Energy Materials and Solar Cells, 2013, 112: 112-119. |
| 20 | QIN Y, LENG G, YU X, et al. Sodium sulfate-diatomite composite materials for high temperature thermal energy storage[J]. Powder Technology, 2015, 282: 37-42. |
| 21 | XU B, MA H, LU Z, et al. Paraffin/expanded vermiculite composite phase change material as aggregate for developing lightweight thermal energy storage cement-based composites[J]. Applied Energy, 2015, 160: 358-367. |
| 22 | SARı A. Thermal energy storage characteristics of bentonite-based composite PCMs with enhanced thermal conductivity as novel thermal storage building materials[J]. Energy Conversion and Management, 2016, 117: 132-141. |
| 23 | SHI J, LI M. Surface modification effects in phase change material-infiltrated attapulgite[J]. Materials Chemistry and Physics, 2020, 254: doi: 10.1016/j.matchemphys.2020.123521. |
| 24 | YU H, LI C, ZHANG K, et al. Preparation and thermophysical performance of diatomite-based composite PCM wallboard for thermal energy storage in buildings[J]. Journal of Building Engineering, 2020, 32: doi: 10.1016/j.jobe.2020.101753. |
| 25 | FENG J, LIU M, MO W, et al. Heating temperature effect on the hygroscopicity of expanded vermiculite[J]. Ceramics International, 2021, 47(18): 25373-25380. |
| 26 | WANG Q, XIE Y, DING B, et al. Structure and hydration state characterizations of MgSO4-zeolite 13X composite materials for long-term thermochemical heat storage[J]. Solar Energy Materials and Solar Cells, 2019, 200: doi: 10.1016/j.solmat.2019.110047. |
| 27 | BRANCATO V, GORDEEVA L, SAPIENZA A, et al. Experimental characterization of the LiCl/vermiculite composite for sorption heat storage applications[J]. International Journal of Refrigeration, 2019, 105: 92-100. |
| 28 | KORHAMMER K, DRUSKE M M, FOPAH-LELE A, et al. Sorption and thermal characterization of composite materials based on chlorides for thermal energy storage[J]. Applied Energy, 2016, 162: 1462-1472. |
| 29 | MEHRABADI A, FARID M. New salt hydrate composite for low-grade thermal energy storage[J]. Energy, 2018, 164: 194-203. |
| 30 | SHERE L, TRIVEDI S, ROBERTS S, et al. Synthesis and characterization of thermochemical storage material combining porous zeolite and inorganic salts[J]. Heat Transfer Engineering, 2019, 40(13/14): 1176-1181. |
| 31 | XU C, YU Z, XIE Y, et al. Study of the hydration behavior of zeolite-MgSO4 composites for long-term heat storage[J]. Applied Thermal Engineering, 2018, 129: 250-259. |
| 32 | CAMMARATA A, VERDA V, SCIACOVELLI A, et al. Hybrid strontium bromide-natural graphite composites for low to medium temperature thermochemical energy storage: Formulation, fabrication and performance investigation[J]. Energy Conversion and Management, 2018, 166: 233-240. |
| 33 | MIAO Q, ZHANG Y, JIA X, et al. MgSO4-expanded graphite composites for mass and heat transfer enhancement of thermochemical energy storage[J]. Solar Energy, 2021, 220: 432-439. |
| 34 | PALOMBA V, FRAZZICA A. Recent advancements in sorption technology for solar thermal energy storage applications[J]. Solar Energy, 2019, 192: 69-105. |
| [1] | Zhu JIANG, Boyang ZOU, Lin CONG, Chunping XIE, Chuan LI, Geng QIAO, Yanqi ZHAO, Binjian NIE, Tongtong ZHANG, Zhiwei GE, Hongkun MA, Yi JIN, Yongliang LI, Yulong DING. Recent progress and outlook of thermal energy storage technologies [J]. Energy Storage Science and Technology, 2022, 11(9): 2746-2771. |
| [2] | Zhongbo LI, Jingxiao HAN, Chengcheng WANG, Hui YANG, Na YANG, Shaowu YIN, Li WANG, Lige TONG, Zhiwei TANG, Yulong DING. Simulation and the parameter influence relationship of the discharging process in a thermochemical reactor [J]. Energy Storage Science and Technology, 2022, 11(7): 2133-2140. |
| [3] | Na YANG, Chengcheng WANG, Hui YANG, Zhihao HU, Lige TONG, Zhongbo LI, Li WANG, Yulong DING, Na LI. Non-isothermal kinetics calculation and heat storage performance analysis of silica gel based on thermochemical reaction [J]. Energy Storage Science and Technology, 2022, 11(5): 1331-1338. |
| [4] | Yachao MO, Jun YAN, Changying ZHAO. Preparation and thermal storage properties of CaO/Ca(OH) 2 core-shell-structured particles [J]. Energy Storage Science and Technology, 2022, 11(12): 3828-3835. |
| [5] | Youqiang LINGHU, Dehou XU, Xiuyan YUE, Xuezhi ZHOU, Yujie XU, Yong SHENG, Zhitao ZUO, Haisheng CHEN. Study on characteristics of the discharge process for zeolite-liquid water adsorption heat storage system [J]. Energy Storage Science and Technology, 2021, 10(3): 1103-1108. |
| [6] | Likui WENG, Yelong ZHANG, Lin JIANG, Yixuan JIA, Linghua TAN, Yi JIN, Yulong DING. Research progress on thermochemical adsorption heat storage technology based on hydrate [J]. Energy Storage Science and Technology, 2020, 9(6): 1729-1736. |
| [7] | HAO Maosen, LIU Hongzhi, WANG Wantong, LYU Jing. Research progress of thermochemical heat storage materials of hydrated salts [J]. Energy Storage Science and Technology, 2020, 9(3): 791-796. |
| [8] | LING Haoshu, HE Jingdong, XU Yujie, WANG Liang, CHEN Haisheng. Status and prospect of thermal energy storage technology for clean heating [J]. Energy Storage Science and Technology, 2020, 9(3): 861-868. |
| [9] | DENG Chang, PAN Zhihao, YAN Jun, ZHAO Changying. Numerical study on exothermic process of a CaO-Ca(OH)2 thermochemical heat storage system [J]. Energy Storage Science and Technology, 2018, 7(2): 248-254. |
| [10] | TANG Xiaonan1,2, SUN Zhenhua1, CHEN Ke1, YANG Huicong1, ZHUO Shuping2, LI Feng1. Cathode hybrid materials for lithium-sulfur battery: The interaction between the host and polysulfide [J]. Energy Storage Science and Technology, 2017, 6(3): 345-359. |
| [11] | SHANG Yongliang1, WANG Chengwen1, LIU Bin1, LIU Jun1,2, KE Xi1,2, LIU Liying1,2, SHI Zhicong1,2. Preparation and properties of manganese dioxide coated carbon nanotubes-sulfur composite cathode material [J]. Energy Storage Science and Technology, 2017, 6(3): 411-417. |
| [12] | ZHENG Xingang, DING Yulong. Recent progress in the adsorption heat pump technology [J]. Energy Storage Science and Technology, 2014, 3(5): 495-508. |
| [13] | MA Hongyun, JIA Zhijun, WU Xuran, LIAO Sida, WANG Baoguo. Fundamentals of electrochemistry (Ⅳ) --Electrode kinetics [J]. Energy Storage Science and Technology, 2013, 2(3): 267-271. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
