Energy Storage Science and Technology
Received:2020-03-16
Revised:2020-04-01
Manman JIA, Long ZHANG. Recent development on sulfide solid electrolytes for solid-state sodium batteries[J]. Energy Storage Science and Technology, doi: 10.19799/j.cnki.2095-4239.2020.0108.
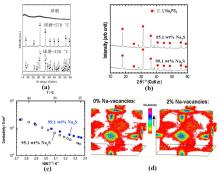
Fig.1
(a) XRD patterns of Na3PS4 powders and the powders sintered respectively at 270 °C and 420 °C. The filled circles and rhombuses represent cubic and tetragonal phases, respectively[34]; (b) XRD patterns of Na3PS4 prepared using Na2S with different purities[47]; (c) Ionic conductivity of Na3PS4 prepared using Na2S with different purities[47]; (d) Molecular dynamics simulation of Na-distribution for Na3PS4 with 0% and 2% Na vacancies[48]"
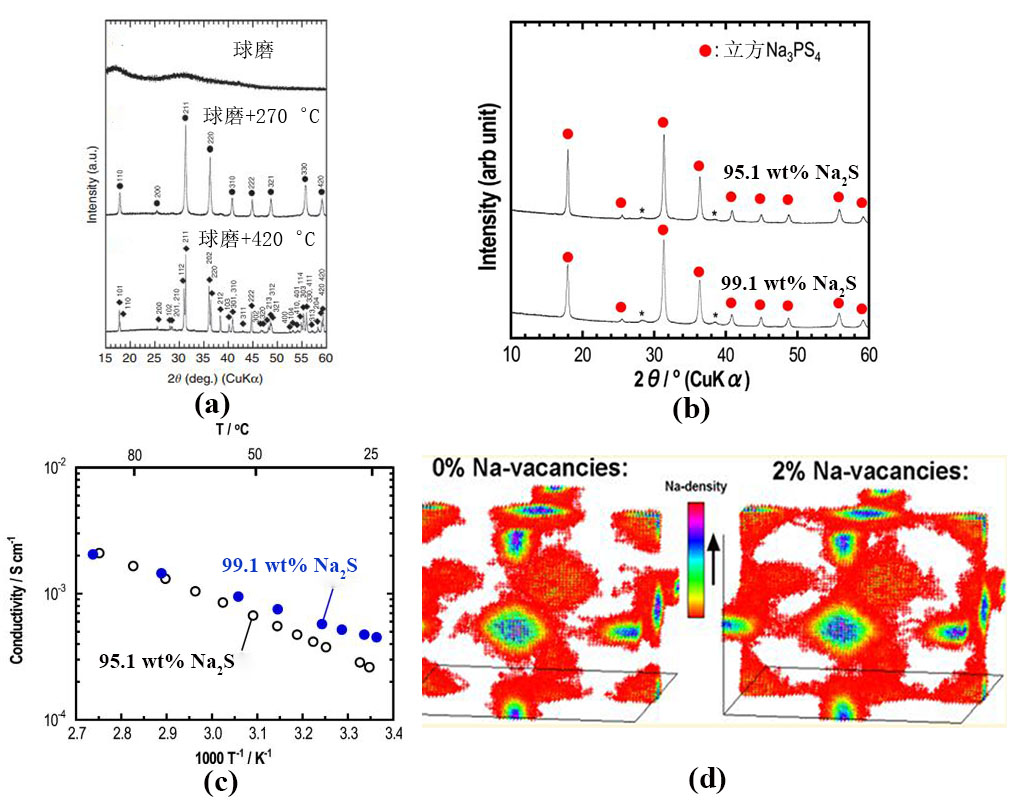

Fig.2
(a) Crystal structure of Na3PSe4. The sodium ions located at the diffusion channels formed by PSe4 tetrahedral[40]; (b) Fourier map of the difference pattern on the xy (z=0) plane[40]; (c) Room temperature ionic conductivity and lattice parameters with various S contents in Na3PSe4-xSx[40]; (d) Schematic diagram if Na+ diffusion channels of different electrolytes[53]; (e) Arrhenius plots of Na3PSxSe4-x (x=0,1,2,3,4)[55]; (f) X-ray diffraction pattern of Na3PSxSe4-x (x=0,1,2,3,4)[55]"
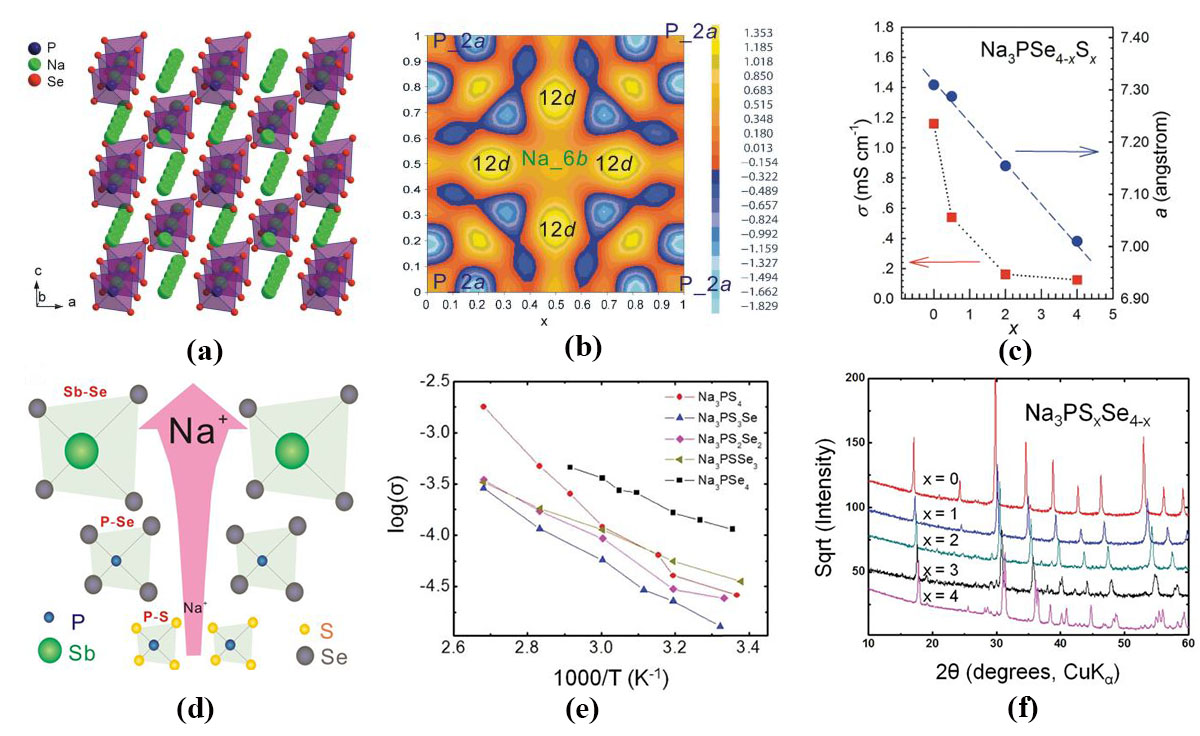

Fig.3
(a) Room temperature ion conductivity of (100-x)Na3PS4?xNa4SiS4 glass-ceramic[58]; (b) Crystal structure of cubic Na3PS4 (left panel) and the interstitial sodium ions (right panel)[59]; (c) Room temperature ion conductivity of Na3P0.62As0.38S4 (i) and Arrhenius plots and the corresponding impedance plots of Na3P0.62As0.38S4 (ii)[36]; (d) Room temperature ion conductivity and activation energy of cubic Na3-2xCaxPS4 as a function of x[61]"

Table 1
Summary of sodium sulfide solid electrolytes"
| 组分 | 对称性 | 晶格常数 /(?) | 电导率 /(mS cm-1) | 激活能/(eV) | 原料; 制备方法 | 掺杂效果 | 文献 |
|---|---|---|---|---|---|---|---|
| Na3PS4 | Cubic I43m | 0.2-0.46 | 0.281-0.198 | Na2S+P2S5;500rpm球磨20h→270度烧结2h | [ | ||
| Na3PS4 | Tetragonal P421c | 6.6919 7.0838 | 3.39 | 0.173 | Na2S、P2S5;450度烧结8h→700度烧结12h | [ | |
| Na2.9PS3.95Se0.05 | Cubic I43m | 0.121 | 0.230 | Na2S+P2S5+Se;乙腈分散→80度干燥→270度退火 | 扩大Na+扩散通道;产生空位 | [ | |
| Na3PSe4 | Cubic I43m | 7.3094 | 1.16 | 0.210 | Na+P+Se; 800度烧结12h | 扩大晶格体积;降低Na+与骨架结合能 | [ |
| Na3PSe4 | Cubic I43m | 7.3136 | 0.11 | 0.281 | Na2Se+P+Se;300度烧结12h | 有空位形成 | [ |
| Na3SbSe4 | Cubic I43m | 7.4920 | 3.70 | 0.190 | Na+Sb+Se;700度烧结12h | 扩大晶胞体积 | [ |
| Na2.9375PS3.9375Cl0.0625 | Tetragonal P421c | 6.9704 7.0925 | 1.14 | 0.249 | Na2S+P2S5+NaCl;800度烧结4h→420度退火2h | 产生Na+空位 | [ |
| 94Na3PS4·6Na4SiS4 | Cubic I43m | 6.9978 | 0.74 | 0.260 | Na2S+P2S5+SiS2;510rpm球磨15h→220度退火2h | 提高Na2占位 | [ |
| Na3.1Ge0.1P0.9S4 | Cubic I43m | 6.9950 | 0.212 | 0.21 | Na2S+P2S5+GeS2;球磨10h→250度退火6h | 提高Na+浓度 | [ |
| Na3.1Ti0.1P0.9S4 | Cubic I43m | 6.9893 | 0.23 | 0.20 | Na2S+P2S5+TiS2;球磨10h→250度退火6h | 提高Na+浓度 | [ |
| Na3.1Sn0.1P0.9S4 | Cubic I43m | 7.0088 | 0.25 | 0.18 | Na2S+P2S5+SnS2;球磨10h→250度退火6h | 提高Na+浓度 | [ |
| Na3P0.62As0.38S4 | Tetragonal P421c | 1.46 | 0.256 | Na2S+P2S5+As2S5;510rpm球磨15h→270度退火2h | 提高离子电导率、空气稳定性 | [ | |
| Na2.730Ca0.135PS4 | Cubic I43m | 6.9768 | 1.40 | 0.346 | Na2S+P2S5+CaS;500rpm球磨5h→700度退火12h | 提高钠离子空位 | [ |
| Na3SbS4 | Tetragonal P421c | 7.1597 7.2906 | 3.00 | 0.250 | Na+Sb+S;700度烧结12h | [ | |
| Na3SbS4 | Tetragonal P421c | 7.1453 7.2770 | 1.10 | 0.200 | Na2S+Sb2S3+S;550度烧结→甲醇/水溶液法 | [ | |
| Na3SbS4 | Tetragonal P421c | 0.10-0.20 | 0.270-0.350 | Na2S+Sb2S3+S;溶于水→干燥后200度退火 | [ | ||
| Na3SbS4 | Cubic I43m | 7.1570 7.2874 | 1.05 | 0.220 | Na3SbS4·9H2O;150度1h脱水 | [ | |
| Na2.88Sb0.88W0.12S4 | Cubic I43m | 7.1920 | 32 | 0.177 | Na2S+S +Sb2S3+WS2;510rpm球磨5h/30h→275度退火 | 增大空位浓度,稳定立方相 | [ |
| Na10SnP2S12 | Tetragonal P42/nmc | 9.6800 13.6300 | 0.4 | 0.356 | Na2S+P2S5+SnS2;380rpm球磨17h→700度退火 | [ | |
| Na10GeP2S12 | Tetragonal P42/nmc | 9.5700 13.4300 | 0.024 | 0.4160 | Na2S+P2S5+SnS2;510rpm球磨5h→235度退火1h | [ | |
| Na11Sn2PS12 | Tetragonal I41/acd | 13.6148 27.2244 | 1.40 | 0.250 | Na2S+P2S5+SnS2;700度烧结2h并缓慢降温 | [ | |
| Na11Sn2PS12 | Tetragonal I41/acd | 13.6436 27.2715 | 3.70 | 0. 387 | Na3PS4+Na4SnS4;420度烧结24h | [ | |
| Na11Sn2PSe12 | Tetragonal I41/acd | 14.2741 28.5113 | 1.00 | Na+P+Sn+Se;450rpm球磨15h→550度退火6h | [ |
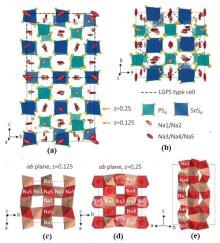
Fig.5
Crystal structure of Na11Sn2PS12[76]. (a) The Na1、Na2 sites are fractional occupancy while the Na3、Na4、Na5 ion sites are almost full occupied sites; (b) The small tetragonal cell (a×a×c) equivalent to Li10GeP2S12 is related to the actual tetragonal cell (a′×a′×c′) of Na11Sn2PS12 as follows: a=2a′,c=2c′; (c)、(d) View of the ab planes consisting of interconnected NaS6 octahedron; (e) view of the sodium atoms chains that run along c"


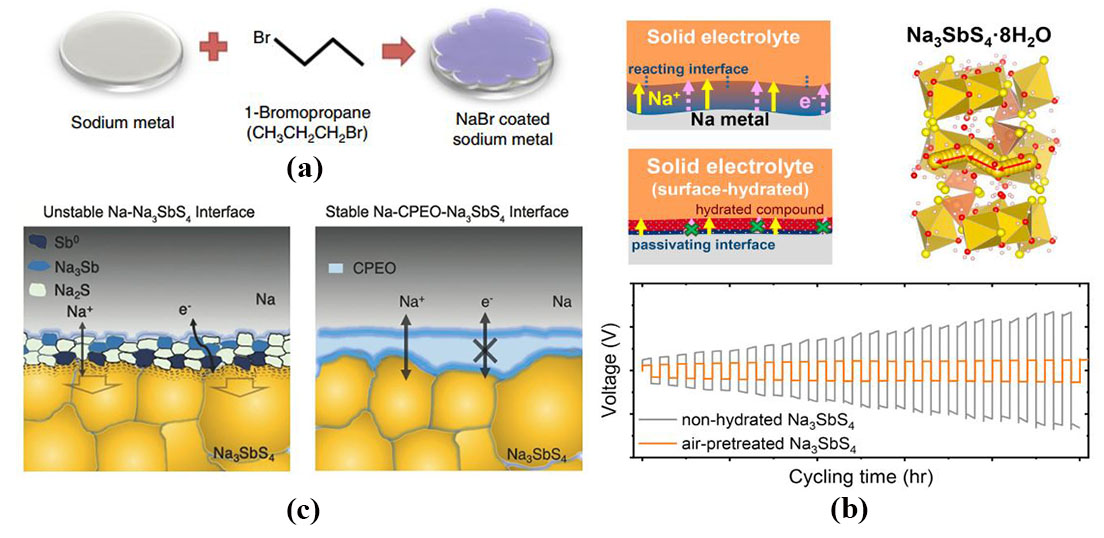
Fig.7
(a) Schematic diagram of NaBr coating on sodium disk[98]; (b) Schematic diagram of ion and electron transport before and after the passivation layer formed at the interface between Na3SbS4 electrolyte and Na electrode (top panel). The galvanostatic cycling of the symmetric cells with and without surface hydration[99]; (c) Schematic diagram of electron transport with and without CPEO at the interface between Na3SbS4 electrolyte and Na electrode[100]"
| 1 | YUAN H, PENG H J, HUANG J Q, et al. Sulfur redox reactions at working interfaces in lithium-sulfur batteries: A Perspective[J]. Advanced Materials Interfaces, 2019, 6(4): 1802046. |
| 2 | GAO Z, SUN H, FU L, et al. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries[J]. Advanced Materials, 2018, 30(17): 1705702. |
| 3 | 张强, 姚霞银, 张洪周, 等. 全固态锂电池界面的研究进展[J]. 储能科学与技术, 2016, 5(5): 659-667. |
| ZHANG Q, YAO X Y, ZHANG H Z, et al. Research progress on interfaces of all solid lithium batteries[J]. Energy Storage Science and Technology, 2016, 5(5): 659-667. | |
| 4 | 方铮, 曹余良, 胡勇胜,等. 室温钠离子电池技术经济性分析[J]. 储能科学与技术, 2016, 5(2): 149-158. |
| FANG Z, CAO Y L, HU Y S, et al. Economic analysis for room-temperature sodium-ioon battery technologies[J]. Energy Storage Science and Technology, 2016, 5(2): 149-158. | |
| 5 | LIU T, ZHANG Y, JIANG Z, et al. Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage[J]. Energy & Environmental Science, 2019, 12(5): 1512-1533. |
| 6 | 容晓晖, 陆雅翔, 戚兴国, 等. 钠离子电池:从基础研究到工程化探索[J]. 储能科学与技术, 2020, 9(2): 515-522. |
| RONG X H, LU Y X, QI X G, et al. Na-ion batteries: From fundamental research to engineering exploration[J]. Energy Storage Science and Technology, 2020, 9(2): 515-522. | |
| 7 | VAALMA C, BUCHHOLZ D, WEIL M, et al. A cost and resource analysis of sodium-ion batteries.[J]. Nature Reviews Materials, 2018, 3(18013): |
| 8 |
FENG X, REN D, HE X, et al. Mitigating thermal runaway of lithium-ion batteries[J]. Joule, 2020, doi:10.1016/j.joule.2020.02.010:
doi: 10.1016/j.joule.2020.02.010: |
| 9 | LU Y, RONG X, HU Y S, et al. Research and development of advanced battery materials in China[J]. Energy Storage Materials, 2019, 23: 144-153. |
| 10 |
曹余良. 钠离子电池机遇与挑战[J]. 储能科学与技术, 2020, doi:10.19799/j.cnki.2095-4239.2020.0026:
doi: 10.19799/j.cnki.2095-4239.2020.0026: |
|
CAO Y L. The opportunities and challenges of sodium ion battery[J]. Energy Storage Science and Technology, 2020, doi:10.19799/j.cnki.2095-4239.2020.0026:
doi: 10.19799/j.cnki.2095-4239.2020.0026: |
|
| 11 | HU Y S, LU Y. 2019 Nobel Prize for the Li-ion batteries and new opportunities and challenges in Na-ion batteries[J]. ACS Energy Letters, 2019, 4(11): 2689-2690. |
| 12 | 陆雅翔, 赵成龙, 容晓晖, 等. 室温钠离子电池材料及器件研究进展[J]. 物理学报, 2018, 067(012): 37-48. |
| LU Y X, ZHAO C L, RONG X H, et al. Research progress of materials and devices for room-temperature Na-ion batteries[J]. Acta Phys. –Chim. Sin. 2018, 67(12): 120601. | |
| 13 | FANG Y, XIAO L, AI X, et al. Hierarchical carbon framework wrapped Na3V2(PO4)3 as a superior high-rate and extended lifespan cathode for sodium-ion batteries[J]. Advanced Materials, 2015, 27(39): 5895-5900. |
| 14 | ADELHELM P, HARTMANN P, BENDER C L, et al. From lithium to sodium: cell chemistry of room temperature sodium-air and sodium-sulfur batteries[J]. Beilstein J Nanotechnol, 2015, 6: 1016-1055. |
| 15 | HWANG J Y, MYUNG S T, SUN Y K. Sodium-ion batteries: present and future[J]. Chemical Society Reviews, 2017, 46(12): 3529-3614. |
| 16 | DENG J, LUO W-B, S-L CHOU, et al. Sodium-ion batteries: From academic research to practical commercialization[J]. Advanced Energy Materials, 2018, 8(4): 1701428. |
| 17 | YU F, DU L, ZHANG G, et al. Electrode engineering by atomic layer deposition for sodium‐ion batteries: From traditional to advanced batteries[J]. Advanced Functional Materials, 2019, 10.1002/adfm.201906890: 1906890. |
| 18 | NAYAK P K, YANG L, BREHM W, et al. From lithium-ion to sodium-ion batteries: advantages, challenges, and surprises[J]. Angew Chem Int Ed Engl, 2018, 57(1): 102-120. |
| 19 | 陈光海, 白莹, 高永晟, 等. 全固态钠离子电池硫系化合物电解质[J]. 物理化学学报, 2020, 36(5): 1905009. |
| CHEN G H, BAI Y, GAO Y S, et al. Chalcogenide electrolytes for all-solid-state sodium ion batteries[J]. Acta Phys. –Chim. Sin. 2020, 36(5), 1905009. | |
| 20 | LEWIS J A, TIPPENS J, CORTES F J Q, et al. Chemo-mechanical challenges in solid-state batteries[J]. Trends in Chemistry, 2019, 1(9): 845-857. |
| 21 | 张隆, 杨坤, 董建英, 等. 硫代快离子导体电解质材料的研究进展[J]. 燕大学报, 2015, 39(2): 95-106. |
| ZHANG L,YANG K, DONG J Y, et al. Recent developments in thio-LISICON solid electrolytes[J]. Journal of Yanshan University, 2015, 39(2): 95-106. | |
| 22 | 刘丽露, 戚兴国, 邵元骏, 等. 全固态锂电池界面的研究进展[J]. 储能科学与技术, 2017, 6(5): 962-980. |
| LIU L L, QI X G, SHAO Y J, et al. Research progress on sodium ion solid-state electrolytes[J]. Energy Storage Science and Technology, 2017, 6(5): 962-980. | |
| 23 | FAMPRIKIS T, CANEPA P, DAWSON J A, et al. Fundamentals of inorganic solid-state electrolytes for batteries[J]. Nature Materials, 2019, 18(12): 1278-1291. |
| 24 | KIM J G, SON B, MUKHERJEE S, et al. A review of lithium and non-lithium based solid state batteries[J]. Journal of Power Sources, 2015, 282: 299-322. |
| 25 | FAN L, WEI S, LI S, et al. Recent progress of the solid-state electrolytes for high-energy metal-based batteries[J]. Advanced Energy Materials, 2018, 8(11): 1702657. |
| 26 | ZHANG Z, ZHANG L, LIU Y, et al. Synthesis and characterization of argyrodite solid electrolytes for all-solid-state Li-ion batteries[J]. Journal of Alloys and Compounds, 2018, 747: 227-235. |
| 27 | YANG K, DONG J, ZHANG L, et al. Dual doping: An effective method to enhance the electrochemical properties of Li10GeP2S12-based solid electrolytes[J]. Journal of the American Ceramic Society, 2015, 98(12): 3831-3835. |
| 28 | NAKAMURA T, AMEZAWA K, KULISCH J, et al. Guidelines for all-solid-state battery design and electrode buffer layers based on chemical potential profile calculation[J]. ACS Applied Materials Interfaces, 2019, 11(22): 19968-19976. |
| 29 | LEE B, PAEK E, MITLIN D, et al. Sodium metal anodes: Emerging solutions to dendrite growth[J]. Chemical Reviews, 2019, 119(8): 5416-5460. |
| 30 | TATSUMISAGO M, HAYASHI A. Sulfide glass-ceramic electrolytes for all-solid-state lithium and sodium batteries[J]. International Journal of Applied Glass Science, 2014, 5(3): 226-235. |
| 31 | CHEN T, ZHANG L, ZHANG Z, et al. Argyrodite solid electrolyte with a stable interface and superior dendrite suppression capability realized by ZnO co-doping[J]. ACS Applied Material Interfaces, 2019, 11(43): 40808-40816. |
| 32 | ZHANG Z, SHAO Y, LOTSCH B, et al. New horizons for inorganic solid state ion conductors[J]. Energy & Environmental Science, 2018, 11(8): 1945-1976. |
| 33 |
PARK K H. Design strategies, practical considerations, and new solution processes of sulfide solid electrolytes for all‐solid‐state batteries[J]. Advanced Energy Materials, 2018, doi:10.1002/aenm.201800035:
doi: 10.1002/aenm.201800035: |
| 34 | HAYASHI A, NOI K, SAKUDA A, et al. Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries[J]. Nature Communications, 2012, 3: 856. |
| 35 | TANIBATA N, NOI K, HAYASHI A, et al. Preparation and characterization of highly sodium ion conducting Na3PS4–Na4SiS4 solid electrolytes[J]. RSC Advances, 2014, 4(33): 17120-17123. |
| 36 | YU Z, SHANG S L, SEO J H, et al. Exceptionally high ionic conductivity in Na3P0.62As0.38S4 with improved moisture stability for solid-state sodium-ion batteries[J]. Advanced Materials, 2017, 29(16): 1605561. |
| 37 | RAO R P, CHEN H, WONG L L, et al. Na3+xMxP1-xS4(M = Ge4+, Ti4+, Sn4+) enables high rate all-solid-state Na-ion batteries Na2+2δFe2-δ(SO4)3|Na3+xMxP1-xS4|Na2Ti3O7[J]. Journal of Materials Chemistry A, 2017, 5(7): 3377-3388. |
| 38 | ZHANG D, CAO X, XU D, et al. Synthesis of cubic Na3SbS4 solid electrolyte with enhanced ion transport for all-solid-state sodium-ion batteries[J]. Electrochimica Acta, 2018, 259: 100-109. |
| 39 | WANG H, YU M, WANG Y, et al. In-situ investigation of pressure effect on structural evolution and conductivity of Na3SbS4 superionic conductor[J]. Journal of Power Sources, 2018, 401: 111-116. |
| 40 | ZHANG L, YANG K, MI J, et al. Na3PSe4: A novel chalcogenide solid electrolyte with high ionic conductivity[J]. Advanced Energy Materials, 2015, 5(24): 1501294. |
| 41 | ZHANG L, ZHANG D, YANG K, et al. Vacancy-contained tetragonal Na3SbS4 superionic conductor[J]. Advanced Science, 2016, 3(10): 1600089. |
| 42 | CHU I H, KOMPELLA C S, NGUYEN H, et al. Room-temperature all-solid-state rechargeable sodium-ion batteries with a Cl-doped Na3PS4 superionic conductor[J]. Scientific Reports, 2016, 6: 33733. |
| 43 | YUBUCHI S, HAYASHI A, TATSUMISAGO M. Sodium-ion conducting Na3PS4 electrolyte synthesized via a liquid-phase process using N-methylformamide[J]. Chemistry Letters, 2015, 44(7): 884-886. |
| 44 | WAN H, MWIZERWA J P, QI X, et al. Core-shell Fe1- xS@Na2.9PS3.95Se0.05 nanorods for room temperature all-solid-state sodium batteries with high energy density[J]. ACS Nano, 2018, 12(3): 2809-2817. |
| 45 | KIM T W, PARK K H, CHOI Y E, et al. Aqueous-solution synthesis of Na3SbS4 solid electrolytes for all-solid-state Na-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(3): 840-844. |
| 46 | JANSEN M, HENSELER U. Synthesis, structure determination, and ionic conductivity of sodium tetrathiophosphate[J]. Journal of Solid State Chemistry, 1992, 99: 110-119. |
| 47 | HAYASHI A, NOI K, TANIBATA N, et al. High sodium ion conductivity of glass–ceramic electrolytes with cubic Na3PS4[J]. Journal of Power Sources, 2014, 258: 420-423. |
| 48 | DE KLERK N J J, WAGEMAKER M. Diffusion mechanism of the sodium-ion solid electrolyte Na3PS4 and potential improvements of halogen doping[J]. Chemistry of Materials, 2016, 28(9): 3122-3130. |
| 49 | YU C, GANAPATHY S, DE KLERK N J J, et al. Na-ion dynamics in tetragonal and cubic Na3PS4, a Na-ion conductor for solid state Na-ion batteries[J]. Journal of Materials Chemistry A, 2016, 4(39): 15095-15105. |
| 50 | TAKEUCHI S, SUZUKI K, HIRAYAMA M, et al. Sodium superionic conduction in tetragonal Na3PS4[J]. Journal of Solid State Chemistry, 2018, 265: 353-358. |
| 51 | KRAUSKOPF T, CULVER S P, ZEIER W G. Local tetragonal structure of the cubic superionic conductor Na3PS4[J]. Inorganic Chemistry, 2018, 57(8): 4739-4744. |
| 52 | ÅVALL G, MINDEMARK J, BRANDELL D, et al. Sodium-ion battery electrolytes: Modeling and simulations[J]. Advanced Energy Materials, 2018, 8(17): 1703036. |
| 53 | WANG N, YANG K, ZHANG L, et al. Improvement in ion transport in Na3PSe4–Na3SbSe4 by Sb substitution[J]. Journal of Materials Science, 2018, 53(3): 1987-1994. |
| 54 | BO S H, WANG Y, KIM J C, et al. Computational and experimental investigations of Na-ion conduction in cubic Na3PSe4[J]. Chemistry of Materials, 2015, 28(1): 252-258. |
| 55 | BO S H, WANG Y, CEDER G. Structural and Na-ion conduction characteristics of Na3PSxSe4-x[J]. Journal of Materials Chemistry A, 2016, 4(23): 9044-9053. |
| 56 | KRAUSKOPF T, POMPE C, KRAFT M A, et al. Influence of lattice dynamics on Na+ transport in the solid electrolyte Na3PS4–xSex[J]. Chemistry of Materials, 2017, 29(20): 8859-8869. |
| 57 | UEMATSU M, YUBUCHI S, TSUJI F, et al. Suspension synthesis of Na3-xPS4-xClx solid electrolytes[J]. Journal of Power Sources, 2019, 428: 131-135. |
| 58 | TANIBATA N, NOI K, HAYASHI A, et al. X-ray crystal structure analysis of sodium-ion conductivity in 94 Na3PS4⋅6 Na4SiS4 glass-ceramic electrolytes[J]. ChemElectroChem, 2014, 1(7): 1130-1132. |
| 59 | ZHU Z, CHU I H, DENG Z, et al. Role of Na+ interstitials and dopants in enhancing the Na+ conductivity of the cubic Na3PS4 superionic conductor[J]. Chemistry of Materials, 2015, 27(24): 8318-8325. |
| 60 | SHANG S L, YU Z, WANG Y, et al. Origin of outstanding phase and moisture stability in a Na3P1-xAsxS4 superionic conductor[J]. ACS Applied Material Interfaces, 2017, 9(19): 16261-16269. |
| 61 | MOON C K, H-J LEE, PARK K H, et al. Vacancy-driven Na+ superionic conduction in new Ca-doped Na3PS4 for all-solid-state Na-ion batteries[J]. ACS Energy Letters, 2018, 3(10): 2504-2512. |
| 62 | HOU W, GUO X, SHEN X, et al. Solid electrolytes and interfaces in all-solid-state sodium batteries: Progress and perspective[J]. Nano Energy, 2018, 52: 279-291. |
| 63 | BANERJEE A, PARK K H, HEO J W, et al. Na3SbS4: A solution processable sodium superionic conductor for all-solid-state sodium-ion batteries[J]. Angewandte Chemie International Edition, 2016, 55(33): 9634-9638. |
| 64 | WANG H, CHEN Y, HOOD Z D, et al. An air-stable Na3SbS4 superionic conductor prepared by a rapid and economic synthetic procedure[J]. Angewandte Chemie International Edition, 2016, 55(30): 8551-8555. |
| 65 | XIONG S, LIU Z, RONG H, et al. Na3SbSe4-xSx as Sodium Superionic Conductors[J]. Scientific Reports, 2018, 8(1): 9146. |
| 66 | WAN H, MWIZERWA J P, HANC F, et al. Grain-boundary-resistance-less Na3SbS4-xSex solid electrolytes for all-solid-state sodium batteries[J]. Nano Energy, 2019, 66: 104109. |
| 67 | HEO J W, BANERJEE A, PARK K H, et al. New Na-ion solid electrolytes Na4-xSn1-xSbxS4(0.02 ≤ x≤ 0.33) for all-solid-state Na-ion batteries[J]. Advanced Energy Materials, 2018, 8(11): 1702716. |
| 68 | 张德超, 张隆. 掺杂型 Na3SbS4 钠离子固体电解质的制备与离子输运性能研究[J]. 燕大学报, 2018, 43(2): 118-138. |
| ZHANG D C, ZHANG L. Synthesis and conductivity of Na3SbS4–based sodium solid electrolytes[J]. Journal of Yanshan University, 2018, 43(2): 118-138. | |
| 69 | HAYASHI A, MASUZAWA N, YUBUCHI S, et al. A sodium-ion sulfide solid electrolyte with unprecedented conductivity at room temperature[J]. Nature Communications, 2019, 10(1): 5266. |
| 70 | FUCHS T, CULVER S P, TILL P, et al. Defect-mediated conductivity enhancements in Na3–xPn1–xWxS4 (Pn = P, Sb) using aliovalent substitutions[J]. ACS Energy Letters, 2019, 5(1): 146-151. |
| 71 |
YUBUCHI S, ITO A, MASUZAWA N, et al. Aqueous solution synthesis of Na3SbS4–Na2WS4 superionic conductors[J]. Journal of Materials Chemistry A, 2020, doi:10.1039/c9ta02246e:
doi: 10.1039/c9ta02246e: |
| 72 | KAMAYA N, HOMMA K, YAMAKAWA Y, et al. A lithium superionic conductor[J]. Nature Materials, 2011, 10(9): 682-686. |
| 73 | KANDAGAL V S, BHARADWAJ M D, WAGHMARE U V. Theoretical prediction of a highly conducting solid electrolyte for sodium batteries: Na10GeP2S12[J]. Journal of Materials Chemistry A, 2015, 3(24): 12992-12999. |
| 74 | TSUJI F, TANIBATA N, SAKUDA A, et al. Preparation of sodium ion conductive Na10GeP2S12 glass-ceramic electrolytes[J]. Chemistry Letters, 2018, 47(1): 13-15. |
| 75 | RICHARDS W D, TSUJIMURA T, MIARA L J, et al. Design and synthesis of the superionic conductor Na10SnP2S12[J]. Nature Communications, 2016, 7: 11009. |
| 76 | ZHANG Z, RAMOS E, LALèRE F, et al. Na11Sn2PS12: a new solid state sodium superionic conductor[J]. Energy & Environmental Science, 2018, 11(1): 87-93. |
| 77 | OH K, CHANG D, PARK I, et al. First-principles investigations on sodium superionic conductor Na11Sn2PS12[J]. Chemistry of Materials, 2019, 31(16): 6066-6075. |
| 78 | DUCHARDT M, RUSCHEWITZ U, ADAMS S, et al. Vacancy-controlled Na+ superion conduction in Na11Sn2PS12[J]. Angew Chemie International Edition, 2018, 57: 1351-1355. |
| 79 | YU Z, SHANG S-L, GAO Y, et al. A quaternary sodium superionic conductor - Na10.8Sn1.9PS11.8[J]. Nano Energy, 2018, 47: 325-330. |
| 80 | RAO R P, ZHANG X, PHUAH K C, et al. Mechanochemical synthesis of fast sodium ion conductor Na11Sn2PSe12 enables first sodium–selenium all-solid-state battery[J]. Journal of Materials Chemistry A, 2019, 7(36): 20790-20798. |
| 81 | RAMOS E P, ZHANG Z, ASSOUD A, et al. Correlating Ion mobility and single crystal structure in sodium-ion chalcogenide-based solid state fast ion conductors: Na11Sn2PnS12 (Pn = Sb, P)[J]. Chemistry of Materials, 2018, 30(21): 7413-7417. |
| 82 | LIU J, LU Z, EFFAT M B, et al. A theoretical study on the stability and ionic conductivity of the Na11M2PS12 (M = Sn, Ge) superionic conductors[J]. Journal of Power Sources, 2019, 409: 94-101. |
| 83 | WAN H, CAI L, WENG W, et al. Cobalt-doped pyrite for Na11Sn2SbS11.5Se0.5 electrolyte based all-solid-state sodium battery with enhanced capacity[J]. Journal of Power Sources, 2020, 449: |
| 84 | TIAN Y, SHI T, RICHARDS W D, et al. Compatibility issues between electrodes and electrolytes in solid-state batteries[J]. Energy & Environmental Science, 2017, 10: 1150-1166. |
| 85 |
ZHANG L, YANG T, DU C, et al. Lithium whisker growth and stress generation in an in situ atomic force microscope-environmental transmission electron microscope set-up[J]. Nature Nanotechnology, 2020, doi:10.1038/s41565-019-0604-x: 1-5.
doi: 10.1038/s41565-019-0604-x: 1-5 |
| 86 | HAN F, WESTOVER A S, YUE J, et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes[J]. Nature Energy, 2019, 4(3): 187-196. |
| 87 | ALBERTUS P, BABINEC S, LITZELMAN S, et al. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries[J]. Nature Energy, 2017, 3(1): 16-21. |
| 88 | LACIVITA V, WANG Y, BO S-H, et al. Ab initio investigation of the stability of electrolyte/electrode interfaces in all-solid-state Na batteries[J]. Journal of Materials Chemistry A, 2019, 7(14): 8144-8155. |
| 89 | WANG Y, WANG Y, WANG Y X, et al. Developments and perspectives on emerging high-energy-density sodium-metal batteries[J]. Chem, 2019, 5(10): 2547-2570. |
| 90 | MATIOS E, WANG H, WANG C, et al. Enabling safe sodium metal batteries by solid electrolyte interphase engineering: A Review[J]. Industrial & Engineering Chemistry Research, 2019, 58(23): 9758-9780. |
| 91 | ZHANG Z, CAO H, YANG M, et al. High performance room temperature all-solid-state Na-SexS battery with Na3SbS4–coated cathode via aqueous solution[J]. Journal of Energy Chemistry, 2020, 48: 250-258. |
| 92 | WAN H, MWIZERWA J P, QI X, et al. Nanoscaled Na3PS4 solid electrolyte for all-solid-state FeS2/Na batteries with ultrahigh initial coulombic efficiency of 95% and excellent cyclic performances[J]. ACS Appl Mater Interfaces, 2018, 10(15): 12300-12304. |
| 93 | PARK K H, KIM D H, KWAK H, et al. Solution-derived glass-ceramic NaI center dot Na3SbS4 superionic conductors for all-solid-state Na-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6: 17192-17200. |
| 94 | FAN L, LI X. Recent advances in effective protection of sodium metal anode[J]. Nano Energy, 2018, 53: 630-642. |
| 95 | WU E A, KOMPELLA C S, ZHU Z, et al. New insights into the interphase between the Na metal anode and sulfide solid-state electrolytes: A joint experimental and computational study[J]. ACS Applied Material Interfaces, 2018, 10(12): 10076-10086. |
| 96 | WENZEL S, LEICHTWEISS T, WEBER D A, et al. Interfacial reactivity renchmarking of the sodium ion conductors Na3PS4 and sodium beta-alumina for protected sodium metal anodes and sodium all-solid-state batteries[J]. ACS Applied Material Interfaces, 2016, 8(41): 28216-28224. |
| 97 | XU X, LI Y, CHENG J, et al. Composite solid electrolyte of Na3PS4-PEO for all-solid-state SnS2/Na batteries with excellent interfacial compatibility between electrolyte and Na metal[J]. Journal of Energy Chemistry, 2020, 41: 73-78. |
| 98 | CHOUDHURY S, WEI S, OZHABES Y, et al. Designing solid-liquid interphases for sodium batteries[J]. Nature Communications, 2017, 8(1): 1-10. |
| 99 | TIAN Y, SUN Y, HANNAH D C, et al. Reactivity-guided interface design in Na metal solid-state batteries[J]. Joule, 2019, 3(4): 1037-1050. |
| 100 | HU P, ZHANG Y, CHI X, et al. Stabilizing the Interface between sodium metal anode and sulfide-based solid-state electrolyte with an electron-blocking interlayer[J]. ACS Applied Material Interfaces, 2019, 11(10): 9672-9678. |
| 101 | 创 刘, 卢海燕, 曹余良. 钠离子电池合金类负极材料的研究进展[J]. 中国材料进展, 2017, 36(10): 718-727. |
| LIU C, LU H Y, CAO Y L. Research progress on alloy anode materials for sodium ion batteries[J]. MATERIALS CHINA, 2017, 36(10): 718-727. | |
| 102 | NAM D H, HONG K S, LIM S J, et al. Electrochemical properties of electrodeposited Sn anodes for Na-ion batteries[J]. The Journal of Physical Chemistry C, 2014, 118(35): 20086-20093. |
| 103 | DATTA M K, EPUR R, SAHA P, et al. Tin and graphite based nanocomposites: Potential anode for sodium ion batteries[J]. Journal of Power Sources, 2013, 225: 316-322. |
| 104 | TANIBATA N, HAYASHI A, TATSUMISAGO M. Improvement of rate performance for all-solid-state Na15Sn4/Amorphous TiS3 cells using 94Na3PS4·6Na4SiS4 glass-ceramic electrolytes[J]. Journal of The Electrochemical Society, 2015, 162(6): A793-A795. |
| 105 | TANIBATA N, MATSUYAMA T, HAYASHI A, et al. All-solid-state sodium batteries using amorphous TiS3 electrode with high capacity[J]. Journal of Power Sources, 2015, 275: 284-287. |
| 106 | LIU Y, LIU X, WANG T, et al. Research and application progress on key materials for sodium-ion batteries[J]. Sustainable Energy & Fuels, 2017, 1(5): 986-1006. |
| 107 | HUANG Y, ZHAO L, LI L, et al. Electrolytes and electrolyte/electrode interfaces in sodium-ion batteries: From scientific research to practical application[J]. Advanced Materials, 2019, 31(21): 1808393. |
| [1] | LI Yitao, SHEN Kaier, PANG Quanquan. Advance in organics enhanced sulfide-based solid-state batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1902-1918. |
| [2] | Zhiwei ZHAO, Zhi YANG, Zhangquan PENG. Application of time-of-flight secondary ion mass spectrometry in lithium-based rechargeable batteries [J]. Energy Storage Science and Technology, 2022, 11(3): 781-794. |
| [3] | Zhao DU, Kang YANG, Gao SHU, Pan WEI, Xiaohu YANG. Experimental Study on the Heat Storage and Release of the Solid-Liquid Phase Change in Metal-Foam-Filled Tube [J]. Energy Storage Science and Technology, 2022, 11(2): 531-537. |
| [4] | Zhuo XU, Lili ZHENG, Bing CHEN, Tao ZHANG, Xiuling CHANG, Shouli WEI, Zuoqiang DAI. Overview of research on composite electrolytes for solid-state batteries [J]. Energy Storage Science and Technology, 2021, 10(6): 2117-2126. |
| [5] | Shangsen CHI, Yidong JIANG, Qingrong WANG, Ziwei YE, Kai YU, Jun MA, Jun JIN, Jun WANG, Chaoyang WANG, Zhaoyin WEN, Yonghong DENG. The liquid electrolyte modified interface between garnet-type solid-state electrolyte and lithium anode [J]. Energy Storage Science and Technology, 2021, 10(3): 914-924. |
| [6] | Saisai ZHANG, Hailei ZHAO. Electrode/electrolyte interfaces in Li7La3Zr2O12 garnet-based solid-state lithium metal battery: Challenges and progress [J]. Energy Storage Science and Technology, 2021, 10(3): 863-871. |
| [7] | Xinxin ZHU, Wei JIANG, Zhengwei WAN, Shu ZHAO, Zeheng LI, Liguang WANG, Wenbin NI, Min LING, Chengdu LIANG. Research progress in electrolyte and interfacial issues of solid lithium sulfur batteries [J]. Energy Storage Science and Technology, 2021, 10(3): 848-862. |
| [8] | Jingjing ZHANG, Xiaoling CUI, Dongni ZHAO, Li YANG, Jie WANG. Effects of concentrated electrolytes on the electrode /electrolyte interface [J]. Energy Storage Science and Technology, 2021, 10(1): 143-149. |
| [9] | Xie WU, Li ZHOU, Zhaoming XUE. Synthesis and performance of solid polymer electrolytes based on chelated boron lithium salts [J]. Energy Storage Science and Technology, 2021, 10(1): 96-103. |
| [10] | Yue MU, Yun DU, Hai MING, Songtong ZHANG, Jingyi QIU. Methods of investigating structural evolution and interface behavior in cathode materials for Li-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(1): 7-26. |
| [11] | Jing YANG, Gaozhan LIU, Lin SHEN, Xiayin YAO. Research progress on NASICON-structured sodium solid electrolytes and their derived solid state sodium batteries [J]. Energy Storage Science and Technology, 2020, 9(5): 1284-1299. |
| [12] | Linfeng PENG, Huanhuan JIA, Qing DING, Yuming ZHAO, Jia XIE, Shijie CHENG. Research progress of solid-state sodium batteries using inorganic sodium ion conductors [J]. Energy Storage Science and Technology, 2020, 9(5): 1370-1382. |
| [13] | Ge SUN, Zhixuan WEI, Xinyuan ZHANG, Nan CHEN, Gang CHEN, Fei DU. Recent progress of sodium-based inorganic solid electrolytes [J]. Energy Storage Science and Technology, 2020, 9(5): 1251-1265. |
| [14] | Jie WU, Xiaobiao JIANG, Yang YANG, Yongmin WU, Lei ZHU, Weiping TANG. Progress of NASICON-structured Li1+xAlxTi2-x(PO4)3 (0 ≤x≤ 0.5) solid electrolyte [J]. Energy Storage Science and Technology, 2020, 9(5): 1472-1488. |
| [15] | Manman JIA, Long ZHANG. Recent development on sulfide solid electrolytes for solid-state sodium batteries [J]. Energy Storage Science and Technology, 2020, 9(5): 1266-1283. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

