Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (9): 2847-2865.doi: 10.19799/j.cnki.2095-4239.2022.0097
• Special Issue for the 10th Anniversary • Previous Articles Next Articles
Pengbo ZHAI1( ), Dongmei CHANG2, Zhijie BI1, Ning ZHAO1, Xiangxin GUO1(
), Dongmei CHANG2, Zhijie BI1, Ning ZHAO1, Xiangxin GUO1( )
)
Received:2022-02-24
Revised:2022-04-12
Online:2022-09-05
Published:2022-08-30
Contact:
Xiangxin GUO
E-mail:woshizpb@qdu.edu.cn;xxguo@qdu.edu.cn
CLC Number:
Pengbo ZHAI, Dongmei CHANG, Zhijie BI, Ning ZHAO, Xiangxin GUO. Research progress on key interfacial issues in lithium lanthanum zirconium oxide-based solid-state[J]. Energy Storage Science and Technology, 2022, 11(9): 2847-2865.
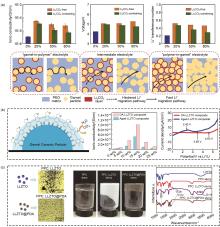
Fig. 2
(a) The influence of Li2CO3 coating on lithium-ion transport path of the composite solid electrolyte with different LLZTO additions[32]; (b) Electrochemical performances of composite electrolyte prepared using polydopamine-coated LLZTO (DA-LLZTO) as inorganic filler[34]; (c) Polydopamine coated LLZTO (DA-LLZTO) can be uniformly dispersed in PPC"

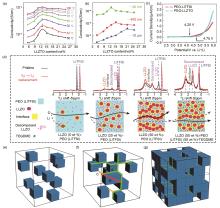
Fig. 3
(a) Lithium-ion conductivity of PEO/LLZTO composite solid electrolyte membranes with different LLZTO volume fractions at different temperatures[44]; (b) The Li-ion conductivity as a function of LLZTO volume fraction for the LLZTO particles with different sizes; (c) LSV curves of PEO:LiTFSI: LLZTO and PEO: LLZTO composite electrolyte; (d) Exploring the lithium-ion transfer pathway in PEO/LLZTO composite solid electrolyte using solid-state nuclear magnetic technology[45]; Schematic diagrams of the lithium-ion transfer pathways when the fractions of LLZO is (e) less than the percolation threshold; (f) in the interval of percolation threshold (g) larger than the percolation threshold in the PEO/LLZO composite electrolyte[46]"

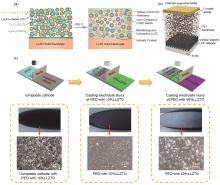
Fig. 4
(a) Li2.3-x C0.7+x B0.3-x O3 solid electrolyte interphase coated on both LLZO and LiCoO2 through the reaction between the Li2.3C0.7B0.3O3 solder and the Li2CO3 layers[51]. (b) Schematic illustration of solid-state battery based on Li2CO3 free LLZO/PEO composite electrolyte[36]. (c) Schematic illustration and corresponding photographs and SEM images of cathode supported composite electrolyte[52]"

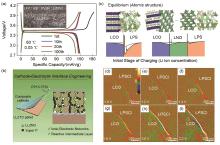
Fig. 5
(a) Solid composite cathode synthesized by spreading method and corresponding charge/discharge curve[54]; (b) Lithium ion/electron co-conducting network constructed by in-situ coating/external adding method[55]; (c) Schematic illustration of space-charge layer effect at the interface between sulfide electrolyte and oxide cathode[56]; (d)~(i) space-charge layer observed by in-situ DPC-STEM[57]"
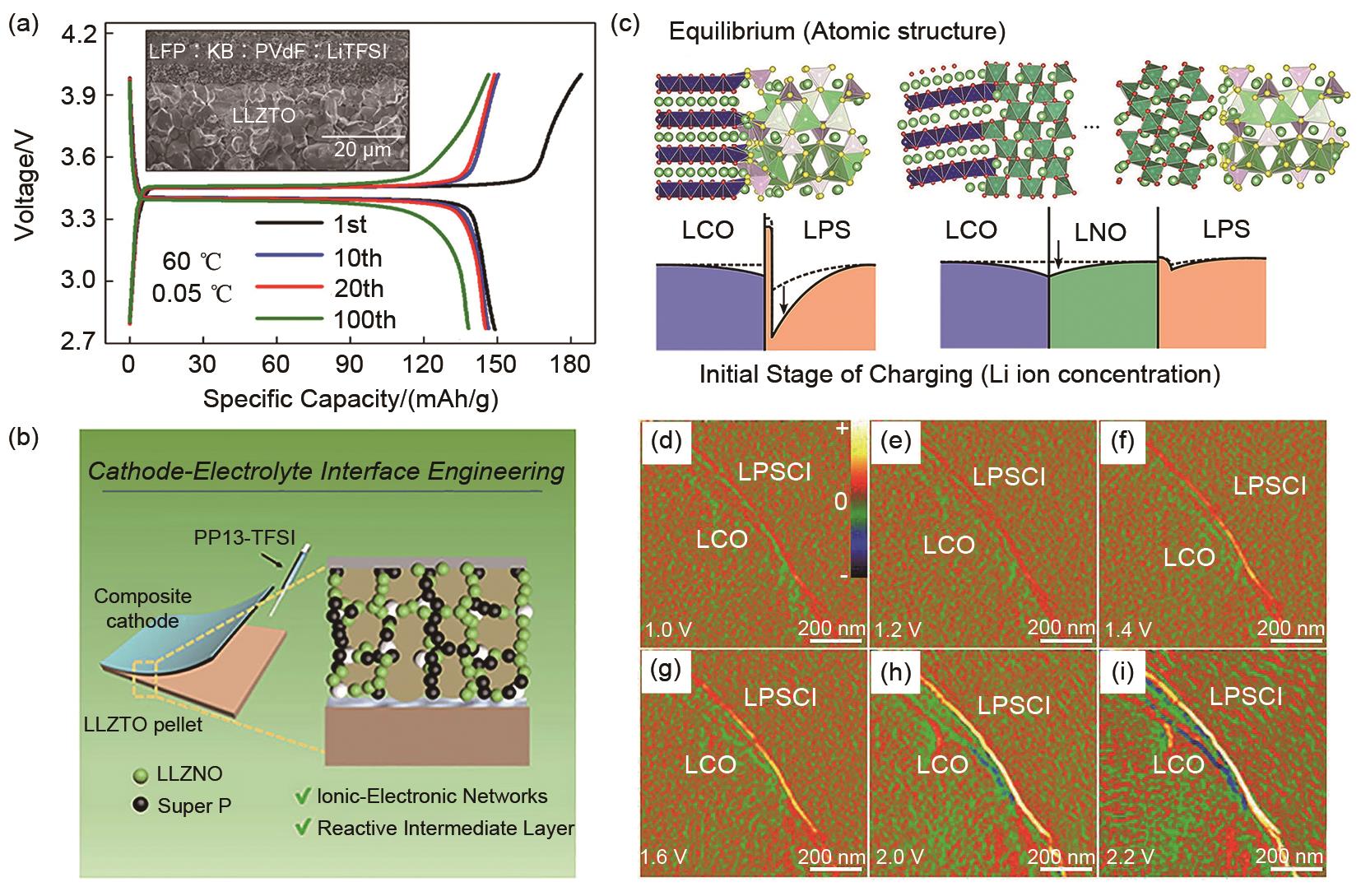

Fig. 7
(a) Schematic diagram of Li deposition behavior on the surface of LLZO solid electrolyte with defects[70]; (b) Effect of Li2CO3 Layer on the lithiophilicity of LLZO Solid Electrolyte[71]; (c) Using Au-Li alloy layer to improve the wettability of lithium metal on the surface of LLZO solid electrolyte[72]; (d) In-situ characterization of the electrochemical process of lithium deposition in solid electrolytes using neutron radiation technology[73]; (e) Mechanism diagram of lithium metal penetration behavior in solid electrolyte[74]"
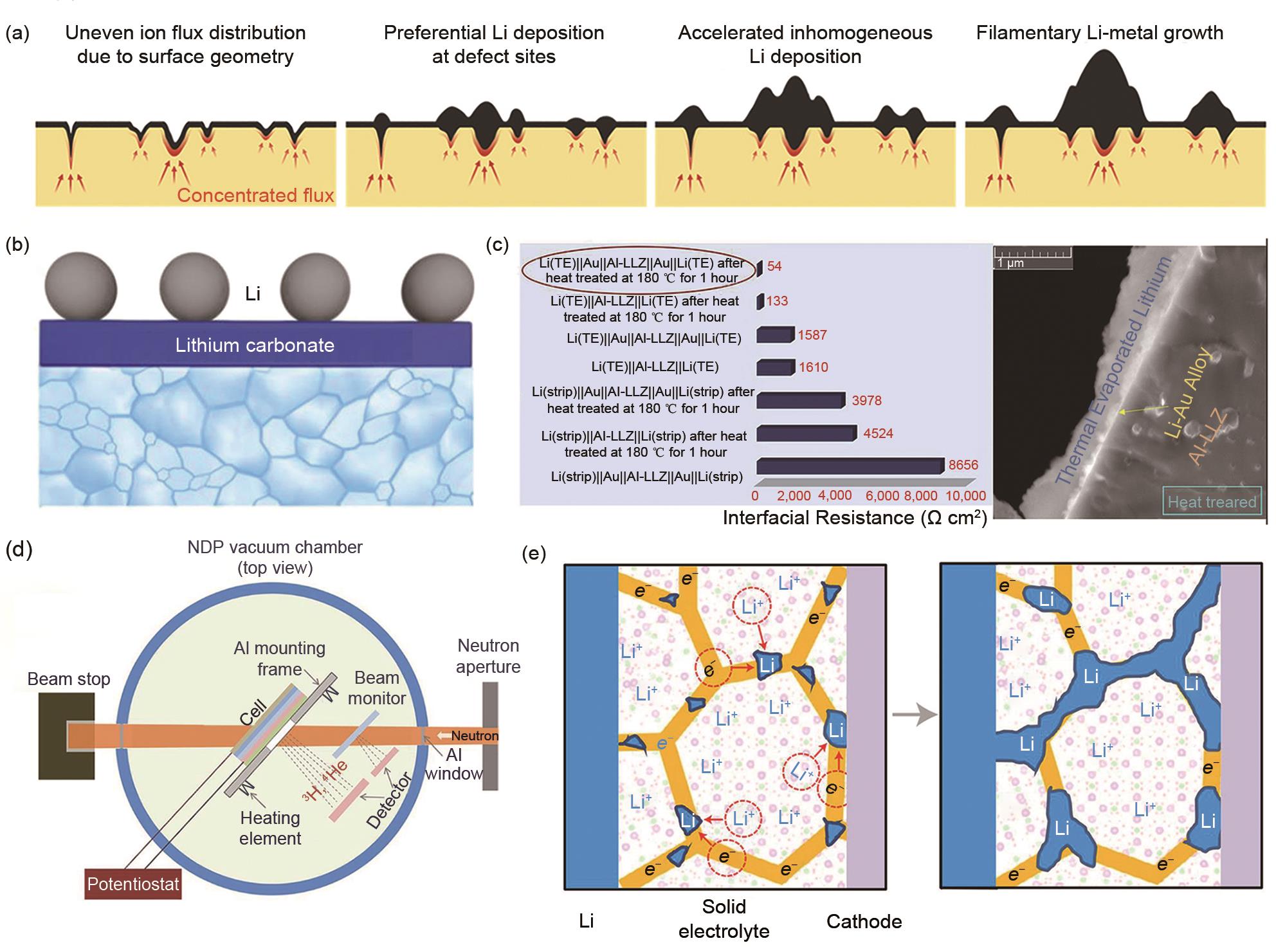
Fig. 10
(a) Electrochemical mechanical model of lithium metal deposition at the Li/LLZO interface[78]; (b) The relationship between the overpotential/crack growth stress of lithium metal deposition and the size of defects in solid electrolyte; (c) Direct imaging of lithium metal deposition behavior in solid electrolyte by In-situ X-ray tomography (X-CT)[79]; (d) Utilizing multilayer composite solid electrolyte to achieve rational mechanical properties control and solve the problems of interfacial stress/strain[80]"

| 1 | DUNN B, KAMATH H, TARASCON J M. Electrical energy storage for the grid: A battery of choices[J]. Science, 2011, 334(6058): 928-935. |
| 2 | ARMAND M, TARASCON J M. Building better batteries[J]. Nature, 2008, 451(7179): 652-657. |
| 3 | GOODENOUGH J B. Rechargeable batteries: Challenges old and new[J]. Journal of Solid State Electrochemistry, 2012, 16(6): 2019-2029. |
| 4 | LIN D C, LIU Y Y, CUI Y. Reviving the lithium metal anode for high-energy batteries[J]. Nature Nanotechnology, 2017, 12(3): 194-206. |
| 5 | LIU J, BAO Z N, CUI Y, et al. Pathways for practical high-energy long-cycling lithium metal batteries[J]. Nature Energy, 2019, 4(3): 180-186. |
| 6 | LIN D C, LIU Y Y, PEI A, et al. Nanoscale perspective: Materials designs and understandings in lithium metal anodes[J]. Nano Research, 2017, 10(12): 4003-4026. |
| 7 | 李泓. 全固态锂电池: 梦想照进现实[J]. 储能科学与技术, 2018, 7(2): 188-193. |
| 8 | WU F X, MAIER J, YU Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries[J]. Chemical Society Reviews, 2020, 49(5): 1569-1614. |
| 9 | YANG C P, FU K, ZHANG Y, et al. Protected lithium-metal anodes in batteries: From liquid to solid[J]. Advanced Materials, 2017, 29(36): 1701169. |
| 10 | XIN S, YOU Y, WANG S F, et al. Solid-state lithium metal batteries promoted by nanotechnology: Progress and prospects[J]. ACS Energy Letters, 2017, 2(6): 1385-1394. |
| 11 | JIA M Y, ZHAO N, HUO H Y, et al. Comprehensive investigation into garnet electrolytes toward application-oriented solid lithium batteries[J]. Electrochemical Energy Reviews, 2020, 3(4): 656-689. |
| 12 | HUANG W L, BI Z J, ZHAO N, et al. Chemical interface engineering of solid garnet batteries for long-life and high-rate performance[J]. Chemical Engineering Journal, 2021, 424: 130423. |
| 13 | MURUGAN R, THANGADURAI V, WEPPNER W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12[J]. Angewandte Chemie International Edition, 2007, 46(41): 7778-7781. |
| 14 | KOBAYASHI T, IMADE Y, SHISHIHARA D, et al. All solid-state battery with sulfur electrode and thio-LISICON electrolyte[J]. Journal of Power Sources, 2008, 182(2): 621-625. |
| 15 | ZHANG Z X, ZHANG L, LIU Y Y, et al. Synthesis and characterization of argyrodite solid electrolytes for all-solid-state Li-ion batteries[J]. Journal of Alloys and Compounds, 2018, 747: 227-235. |
| 16 | ANANTHARAMULU N, KOTESWARA RAO K, RAMBABU G, et al. A wide-ranging review on Nasicon type materials[J]. Journal of Materials Science, 2011, 46(9): 2821-2837. |
| 17 | MIZUNO F, HAYASHI A, TADANAGA K, et al. New, highly ion-conductive crystals precipitated from Li2S-P2S5 glasses[J]. Advanced Materials, 2005, 17(7): 918-921. |
| 18 | SENEVIRATHNE K, DAY C S, GROSS M D, et al. A new crystalline LiPON electrolyte: Synthesis, properties, and electronic structure[J]. Solid State Ionics, 2013, 233: 95-101. |
| 19 | ZHAO N, KHOKHAR W, BI Z J, et al. Solid garnet batteries[J]. Joule, 2019, 3(5): 1190-1199. |
| 20 | WANG C W, FU K, KAMMAMPATA S P, et al. Garnet-type solid-state electrolytes: Materials, interfaces, and batteries[J]. Chemical Reviews, 2020, 120(10): 4257-4300. |
| 21 | GUO S J, SUN Y G, CAO A M. Garnet-type solid-state electrolyte Li7La3Zr2O12: Crystal structure, element doping and interface strategies for solid-state lithium batteries[J]. Chemical Research in Chinese Universities, 2020, 36(3): 329-342. |
| 22 | FERRARESI G, EL KAZZI M, CZORNOMAZ L, et al. Electrochemical performance of all-solid-state Li-ion batteries based on garnet electrolyte using silicon as a model electrode[J]. ACS Energy Letters, 2018, 3(4): 1006-1012. |
| 23 | PARK K, YU B C, JUNG J W, et al. Electrochemical nature of the cathode interface for a solid-state lithium-ion battery: Interface between LiCoO2 and Garnet-Li7La3Zr2O12[J]. Chemistry of Materials, 2016, 28(21): 8051-8059. |
| 24 | CHENG L, CRUMLIN E J, CHEN W, et al. The origin of high electrolyte-electrode interfacial resistances in lithium cells containing garnet type solid electrolytes[J]. Physical Chemistry Chemical Physics: PCCP, 2014, 16(34): 18294-18300. |
| 25 | MA C, RANGASAMY E, LIANG C D, et al. Excellent stability of a lithium-ion-conducting solid electrolyte upon reversible Li+/H+ exchange in aqueous solutions[J]. Angewandte Chemie International Edition, 2015, 54(1): 129-133. |
| 26 | HUO H Y, CHEN Y, ZHAO N, et al. In-situ formed Li2CO3-free garnet/Li interface by rapid acid treatment for dendrite-free solid-state batteries[J]. Nano Energy, 2019, 61: 119-125. |
| 27 | HU Y S. Batteries: Getting solid[J]. Nature Energy, 2016, 1: 16042. |
| 28 | VARDAR G, BOWMAN W J, LU Q, et al. Structure, chemistry, and charge transfer resistance of the interface between Li7La3Zr2O12 electrolyte and LiCoO2 cathode [J]. Chemistry of Materials, 2018, 30(18): 6259-6276. |
| 29 | HAN X G, GONG Y H, FU K, et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries[J]. Nature Materials, 2017, 16(5): 572-579. |
| 30 | LIU Q, GENG Z, HAN C P, et al. Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries[J]. Journal of Power Sources, 2018, 389: 120-134. |
| 31 | NOLAN A M, WACHSMAN E D, MO Y F. Computation-guided discovery of coating materials to stabilize the interface between lithium garnet solid electrolyte and high-energy cathodes for all-solid-state lithium batteries[J]. Energy Storage Materials, 2021, 41: 571-580. |
| 32 | KRAUSKOPF T, DIPPEL R, HARTMANN H, et al. Lithium-metal growth kinetics on LLZO garnet-type solid electrolytes[J]. Joule, 2019, 3(8): 2030-2049. |
| 33 | MU S, HUANG W L, SUN W H, et al. Heterogeneous electrolyte membranes enabling double-side stable interfaces for solid lithium batteries[J]. Journal of Energy Chemistry, 2021, 60: 162-168. |
| 34 | CAO Y, LI Y Q, GUO X X. Densification and lithium ion conductivity of garnet-type Li7– xLa3Zr2– xTaxO12(x= 0.25) solid electrolytes[J]. Chinese Physics B, 2013, 22(7): 078201. |
| 35 | LI Y Q, CAO Y, GUO X X. Influence of lithium oxide additives on densification and ionic conductivity of garnet-type Li6.75La3Zr1.75Ta0.25O12 solid electrolytes[J]. Solid State Ionics, 2013, 253: 76-80. |
| 36 | HUO H Y, LI X N, SUN Y P, et al. Li2CO3 effects: New insights into polymer/garnet electrolytes for dendrite-free solid lithium batteries[J]. Nano Energy, 2020, 73: 104836. |
| 37 | HUO H Y, LUO J, THANGADURAI V, et al. Li2CO3: A critical issue for developing solid garnet batteries[J]. ACS Energy Letters, 2020, 5(1): 252-262. |
| 38 | JIA M Y, ZHAO N, BI Z J, et al. Polydopamine-coated garnet particles homogeneously distributed in poly(propylene carbonate) for the conductive and stable membrane electrolytes of solid lithium batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(41): 46162-46169. |
| 39 | FAN L Z, HE H C, NAN C W. Tailoring inorganic-polymer composites for the mass production of solid-state batteries[J]. Nature Reviews Materials, 2021, 6(11): 1003-1019. |
| 40 | KELLER M, APPETECCHI G B, KIM G T, et al. Electrochemical performance of a solvent-free hybrid ceramic-polymer electrolyte based on Li7La3Zr2O12 in P(EO)15LiTFSI[J]. Journal of Power Sources, 2017, 353: 287-297. |
| 41 | LU W Z, XUE M Z, ZHANG C M. Modified Li7La3Zr2O12 (LLZO) and LLZO-polymer composites for solid-state lithium batteries[J]. Energy Storage Materials, 2021, 39: 108-129. |
| 42 | LI L S, DENG Y F, CHEN G H. Status and prospect of garnet/polymer solid composite electrolytes for all-solid-state lithium batteries[J]. Journal of Energy Chemistry, 2020, 50: 154-177. |
| 43 | YANG T, ZHENG J, CHENG Q, et al. Composite polymer electrolytes with Li7La3Zr2O12 garnet-type nanowires as ceramic fillers: Mechanism of conductivity enhancement and role of doping and morphology[J]. ACS Applied Materials & Interfaces, 2017, 9(26): 21773-21780. |
| 44 | ZHANG J X, ZHAO N, ZHANG M, et al. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide[J]. Nano Energy, 2016, 28: 447-454. |
| 45 | ZHENG J, HU Y Y. New insights into the compositional dependence of Li-ion transport in polymer-ceramic composite electrolytes[J]. ACS Applied Materials & Interfaces, 2018, 10(4): 4113-4120. |
| 46 | CHOUDHURY S, STALIN S, VU D, et al. Solid-state polymer electrolytes for high-performance lithium metal batteries[J]. Nature Communications, 2019, 10: 4398. |
| 47 | 梁宇皓, 范丽珍. 固态锂电池中的机械力学失效及解决策略[J]. 物理学报, 2020, 69(22): 226201. |
| LIANG Y H, FAN L Z. Mechanical failures in solid-state lithium batteries and their solution[J]. Acta Physica Sinica, 2020, 69(22): 226201. | |
| 48 | BUCCI G, SWAMY T, CHIANG Y M, et al. Modeling of internal mechanical failure of all-solid-state batteries during electrochemical cycling, and implications for battery design[EB/OL]. 2017: arXiv: 1703.00113[cond-mat.mtrl-sci]. https://arxiv.org/abs/1703.00113 |
| 49 | YAN X F, LI Z B, WEN Z Y, et al. Li/Li7La3Zr2O12/LiFePO4 all-solid-state battery with ultrathin nanoscale solid electrolyte[J]. The Journal of Physical Chemistry C, 2017, 121(3): 1431-1435. |
| 50 | OHTA S, KOMAGATA S, SEKI J, et al. All-solid-state lithium ion battery using garnet-type oxide and Li3BO3 solid electrolytes fabricated by screen-printing[J]. Journal of Power Sources, 2013, 238: 53-56. |
| 51 | HAN F D, YUE J, CHEN C, et al. Interphase engineering enabled all-ceramic lithium battery[J]. Joule, 2018, 2(3): 497-508. |
| 52 | BI Z J, MU S, ZHAO N, et al. Cathode supported solid lithium batteries enabling high energy density and stable cyclability[J]. Energy Storage Materials, 2021, 35: 512-519. |
| 53 | BI Z J, HUANG W L, MU S, et al. Dual-interface reinforced flexible solid garnet batteries enabled by in situ solidified gel polymer electrolytes[J]. Nano Energy, 2021, 90: 106498. |
| 54 | DU F M, ZHAO N, LI Y Q, et al. All solid state lithium batteries based on lamellar garnet-type ceramic electrolytes[J]. Journal of Power Sources, 2015, 300: 24-28. |
| 55 | BI Z J, ZHAO N, MA L N, et al. Interface engineering on cathode side for solid garnet batteries[J]. Chemical Engineering Journal, 2020, 387: 124089. |
| 56 | HARUYAMA J, SODEYAMA K, HAN L Y, et al. Space-charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery[J]. Chemistry of Materials, 2014, 26(14): 4248-4255. |
| 57 | WANG L L, XIE R C, CHEN B B, et al. In-situ visualization of the space-charge-layer effect on interfacial lithium-ion transport in all-solid-state batteries[J]. Nature Communications, 2020, 11: 5889. |
| 58 | DE KLERK N J J, WAGEMAKER M. Space-charge layers in all-solid-state batteries; Important or negligible?[J]. ACS Applied Energy Materials, 2018, 1(10): 5609-5618. |
| 59 | OHTA S, KOBAYASHI T, ASAOKA T. High lithium ionic conductivity in the garnet-type oxide Li7-X La3(Zr2-X, NbX)O12 (X = 0-2)[J]. Journal of Power Sources, 2011, 196(6): 3342-3345. |
| 60 | HAN F D, ZHU Y Z, HE X F, et al. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes[J]. Advanced Energy Materials, 2016, 6(8): 1501590. |
| 61 | ZHUANG Y, ZOU Z Y, LU B, et al. Understanding the Li diffusion mechanism and positive effect of current collector volume expansion in anode free batteries[J]. Chinese Physics B, 2020, 29(6): 068202. |
| 62 | XIONG Z H, SHI S Q, OUYANG C Y, et al. Ab initio investigation of the surface properties of Cu(111) and Li diffusion in Cu thin film[J]. Physics Letters A, 2005, 337(3): 247-255. |
| 63 | 冯吴亮, 王飞, 周星, 等. 固态电解质与电极界面的稳定性[J]. 物理学报, 2020, 69(22): 137-149. |
| FENG W L, WANG F, ZHOU X, et al. Stability of interphase between solid state electrolyte and electrode[J]. Acta Physica Sinica, 2020, 69(22): 137-149. | |
| 64 | MONROE C, NEWMAN J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces[J]. Journal of the Electrochemical Society, 2005, 152(2): A396. |
| 65 | HUO H Y, CHEN Y, LI R Y, et al. Design of a mixed conductive garnet/Li interface for dendrite-free solid lithium metal batteries[J]. Energy & Environmental Science, 2020, 13(1): 127-134. |
| 66 | HUO H Y, LIANG J N, ZHAO N, et al. Dynamics of the garnet/Li interface for dendrite-free solid-state batteries[J]. ACS Energy Letters, 2020, 5(7): 2156-2164. |
| 67 | KHOKHAR W A, ZHAO N, HUANG W L, et al. Different behaviors of metal penetration in Na and Li solid electrolytes[J]. ACS Applied Materials & Interfaces, 2020, 12(48): 53781-53787. |
| 68 | ZHAO N, FANG R, HE M H, et al. Cycle stability of lithium/garnet/lithium cells with different intermediate layers[J]. Rare Metals, 2018, 37(6): 473-479. |
| 69 | SHARAFI A, KAZYAK E, DAVIS A L, et al. Surface chemistry mechanism of ultra-low interfacial resistance in the solid-state electrolyte Li7La3Zr2O12[J]. Chemistry of Materials, 2017, 29(18): 7961-7968. |
| 70 | KIM S, JUNG C, KIM H, et al. The role of interlayer chemistry in Li-metal growth through a garnet-type solid electrolyte[J]. Advanced Energy Materials, 2020, 10(12): 1903993. |
| 71 | HUANG W L, ZHAO N, BI Z J, et al. Can we find solution to eliminate Li penetration through solid garnet electrolytes? [J]. Materials Today Nano, 2020, 10: 100075. |
| 72 | ALEXANDER G V, PATRA S, SOBHAN RAJ S V, et al. Electrodes-electrolyte interfacial engineering for realizing room temperature lithium metal battery based on garnet structured solid fast Li+ conductors[J]. Journal of Power Sources, 2018, 396: 764-773. |
| 73 | HAN F D, WESTOVER A S, YUE J, et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes[J]. Nature Energy, 2019, 4(3): 187-196. |
| 74 | LIU X M, GARCIA-MENDEZ R, LUPINI A R, et al. Local electronic structure variation resulting in Li 'filament' formation within solid electrolytes[J]. Nature Materials, 2021, 20(11): 1485-1490. |
| 75 | HUO H Y, GAO J, ZHAO N, et al. A flexible electron-blocking interfacial shield for dendrite-free solid lithium metal batteries[J]. Nature Communications, 2021, 12: 176. |
| 76 | MA C, CHENG Y Q, YIN K B, et al. Interfacial stability of Li metal-solid electrolyte elucidated via in situ electron microscopy[J]. Nano Letters, 2016, 16(11): 7030-7036. |
| 77 | ZHU Y S, CONNELL J G, TEPAVCEVIC S, et al. Dopant-dependent stability of garnet solid electrolyte interfaces with lithium metal[J]. Advanced Energy Materials, 2019, 9(12): 1803440. |
| 78 | PORZ L, SWAMY T, SHELDON B W, et al. Mechanism of lithium metal penetration through inorganic solid electrolytes[J]. Advanced Energy Materials, 2017, 7(20): 1701003. |
| 79 | NING Z Y, JOLLY D S, LI G C, et al. Visualizing plating-induced cracking in lithium-anode solid-electrolyte cells[J]. Nature Materials, 2021, 20(8): 1121-1129. |
| 80 | HUO H Y, CHEN Y, LUO J, et al. Rational design of hierarchical "ceramic-in-polymer" and "polymer-in-ceramic" electrolytes for dendrite-free solid-state batteries[J]. Advanced Energy Materials, 2019, 9(17): 1804004. |
| 81 | TIAN H K, CHAKRABORTY A, TALIN A A, et al. Evaluation of the electrochemo-mechanically induced stress in all-solid-state Li-ion batteries[J]. Journal of the Electrochemical Society, 2020, 167(9): 090541. |
| 82 | HUO H Y, SUN J Y, CHEN C, et al. Flexible interfaces between Si anodes and composite electrolytes consisting of poly(propylene carbonates) and garnets for solid-state batteries[J]. Journal of Power Sources, 2018, 383: 150-156. |
| 83 | DOUX J M, NGUYEN H, TAN D H S, et al. Stack pressure considerations for room-temperature all-solid-state lithium metal batteries[J]. Advanced Energy Materials, 2020, 10(1): 1903253. |
| 84 | TAN D H S, CHEN Y T, YANG H D, et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes[J]. Science, 2021, 373(6562): 1494-1499. |
| [1] | Jinghua WU, Jing YANG, Gaozhan LIU, Zhiyan WANG, Zhihua ZHANG, Hailong YU, Xiayin YAO, Xuejie HUANG. Review and prospective of solid-state lithium batteries in the past decade (2011—2021) [J]. Energy Storage Science and Technology, 2022, 11(9): 2713-2745. |
| [2] | ZHOU Weidong, HUANG Qiu, XIE Xiaoxin, CHEN Kejun, LI Wei, QIU Jieshan. Research progress of polymer electrolyte for solid state lithium batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1788-1805. |
| [3] | Yun TANG, Fang YUE, Kaimo GUO, Lanchun LI, Wangsong KE, Wei CHEN. Analysis of the development trend and the innovation ability of an all-solid-state lithium battery technology [J]. Energy Storage Science and Technology, 2022, 11(1): 359-369. |
| [4] | Yanming CUI, Zhihua ZHANG, Yuanqiao HUANG, Jiu LIN, Xiayin YAO, Xiaoxiong XU. Prototype all-solid-state battery electrodes preparation and assembly technology [J]. Energy Storage Science and Technology, 2021, 10(3): 836-847. |
| [5] | Hongming YI, Zhiqiang LYU, Huamin ZHANG, Mingming SONG, Qiong ZHENG, Xianfeng LI. Recent progress and application challenges in V-based polyanionic compounds for cathodes of sodium-ion batteries [J]. Energy Storage Science and Technology, 2020, 9(5): 1350-1369. |
| [6] | Linfeng PENG, Huanhuan JIA, Qing DING, Yuming ZHAO, Jia XIE, Shijie CHENG. Research progress of solid-state sodium batteries using inorganic sodium ion conductors [J]. Energy Storage Science and Technology, 2020, 9(5): 1370-1382. |
| [7] | WU Jinghua, YAO Xiayin. Recent progress in interfaces of all-solid-state lithium batteries based on sulfide electrolytes [J]. Energy Storage Science and Technology, 2020, 9(2): 501-514. |
| [8] | XIA Qiuying, SUN Shuo, XU Jing, ZAN Feng, YUE Jili, XIA Hui. All-solid-state thin film lithium batteries [J]. Energy Storage Science and Technology, 2018, 7(4): 565-574. |
| [9] | GUO Yuguo . Project “High-energy solid-state lithium metal batteries based on nanostructured materials” [J]. Energy Storage Science and Technology, 2016, 5(6): 919-921. |
| [10] | MA Hongyun, FAN Yongsheng, HONG Weichen, WANG Baoguo. Principle and application of electrochemical impedance spectroscopy method [J]. Energy Storage Science and Technology, 2014, 3(5): 544-549. |
| [11] | JIA Zhijun, MA Hongyun, WU Xuran, LIAO Sida, WANG Baoguo. Fundamentals of electrochemistry(Ⅴ)--Electrochemical kinetic and charge-transfer process for electrochemical reaction [J]. Energy Storage Science and Technology, 2013, 2(4): 402-409. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
