Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (7): 2270-2285.doi: 10.19799/j.cnki.2095-4239.2024.0294
• Special Issue on Low Temperature Batteries • Previous Articles Next Articles
Sen JIANG1,2( ), Long CHEN1, Chuangchao SUN1, Jinze WANG1, Ruhong LI1,2(
), Long CHEN1, Chuangchao SUN1, Jinze WANG1, Ruhong LI1,2( ), Xiulin FAN1(
), Xiulin FAN1( )
)
Received:2024-04-03
Revised:2024-04-17
Online:2024-07-28
Published:2024-07-23
Contact:
Ruhong LI, Xiulin FAN
E-mail:jiangsen@zju.edu.cn;ruhong@zju.edu.cn;xlfan@zju.edu.cn
CLC Number:
Sen JIANG, Long CHEN, Chuangchao SUN, Jinze WANG, Ruhong LI, Xiulin FAN. Low-temperature lithium battery electrolytes: Progress and perspectives[J]. Energy Storage Science and Technology, 2024, 13(7): 2270-2285.
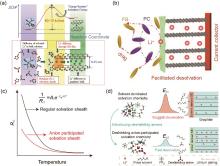
Fig. 3
(a) Schematic description of energetic coordinates for Li+ transfer at graphite/electrolyte junction[27]; (b) Schematic diagram of the interfacial behaviors and facilitated desolvation in the electrolyte with loose solvation structure[29]; The effect of solvation sheath design on (c) the change of Rct and (d) the intercalation process for graphite anode at low temperature[30]"
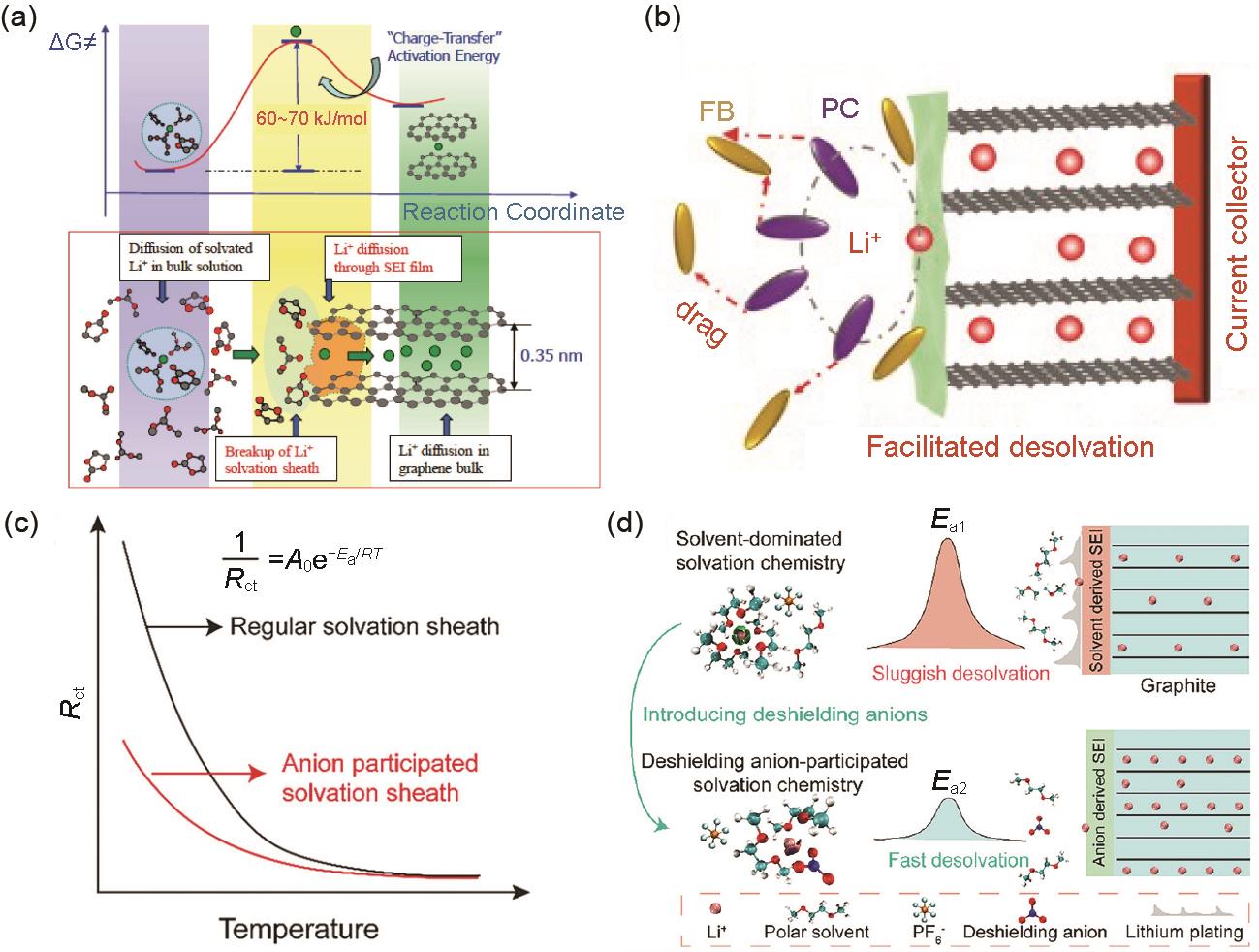

Fig. 4
(a) Model of the Li deposition on the surface of graphite anode at low temperature; (b) The relationship between cell voltage and anode potential at various current densities[31]; (c) Schematic diagram of the effects of Li+ transfer and charge transfer on Li deposition behavior at room temperature and low temperature[32]"
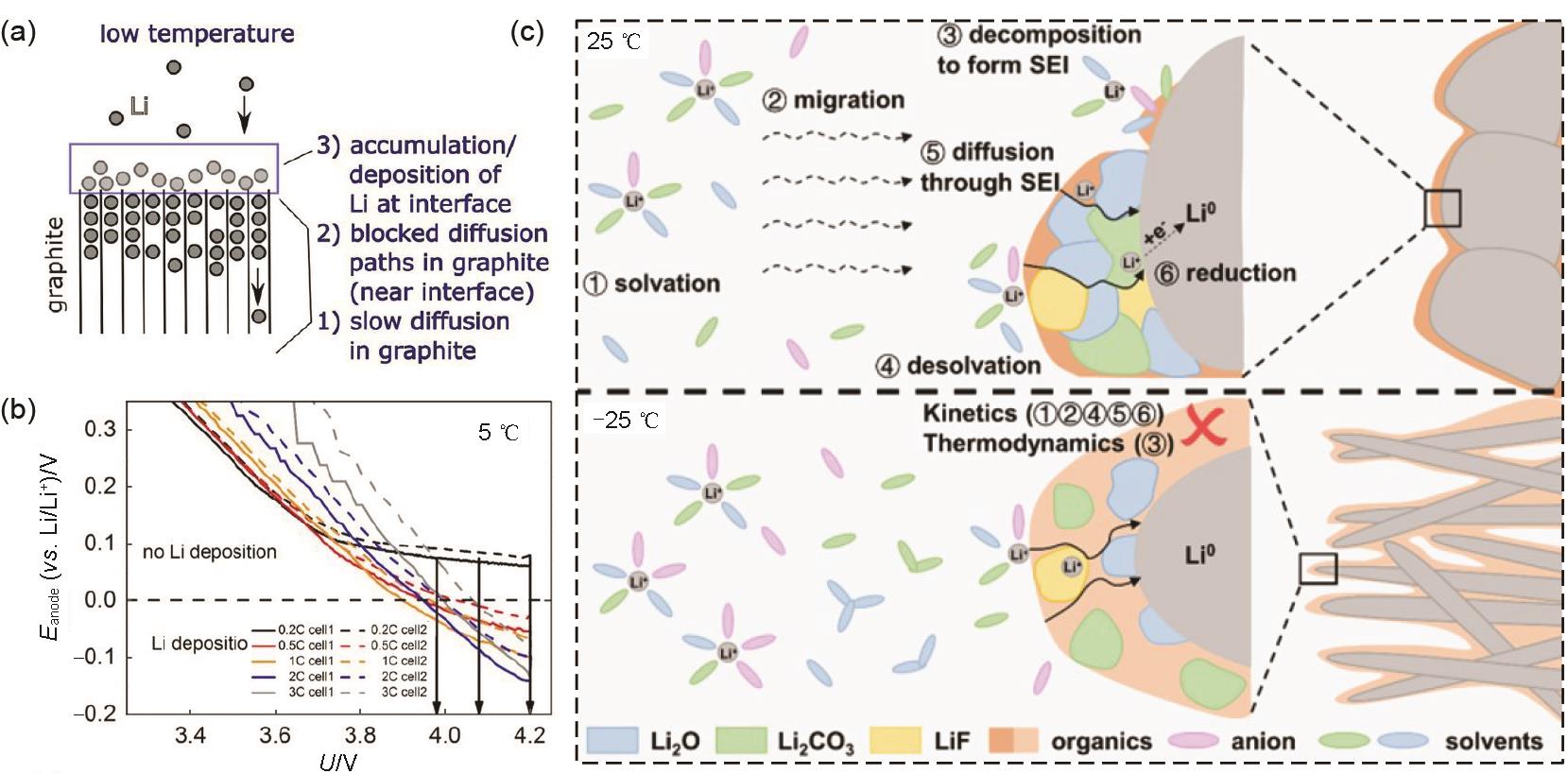
Table 1
Characteristics of commonly used lithium salts in LIBs"
| 锂盐 | 优点 | 缺点 |
|---|---|---|
| 高氯酸锂(LiClO4) | 高离子电导率和氧化电位,对湿度不敏感 | 高氯酸根极易与有机电解液反应,尤其在高温和大电流密度条件下 |
| 六氟砷酸锂(LiAsF6) | 电导率高,电化学稳定好 | As的毒性限制了该锂盐的应用 |
| 六氟磷酸锂(LiPF6) | 综合性能优异,高的溶解性和离子电导率 | 热稳定性差,对水非常敏感,反应生成HF等腐蚀电极材料 |
| 四氟硼酸锂(LiBF4) | 对湿度不敏感,热稳定性好,低温性能较好 | 离子电导率低,常用作电解液添加剂 |
| 二草酸硼酸锂[LiB(C2O4)2或LiBOB] | 热稳定性好,形成致密的SEI膜 | 溶解度较低,导致电解液体系的离子电导率低 |
| 二氟二草酸硼酸锂[LiBF2(C2O4)2] | 热稳定性好,负极成膜性能优异,形成的SEI膜阻抗小 | 溶解度较低,常用作电解液添加剂 |
| 三氟甲磺酸锂(LiCF3SO3) | 高抗氧化能力和热稳定性 | 成本较高,严重腐蚀Al集流体 |
| 双氟磺酰亚胺锂[LiN(FSO2)2或LiFSI] | 电导率高,易生成富LiF的SEI膜,低温性能优异 | 成本较高,腐蚀Al集流体 |
| 双三氟甲烷磺酰亚胺锂[LiN(CF3SO2)2或LiTFSI] | 电导率较高,热稳定性好,生成的SEI膜致密 | 成本较高,腐蚀Al集流体 |

Fig. 5
(a) Ionic conductivities of electrolytes with various Li salts in different carbonate-based solvents at -40—60 ℃[35]; (b) Nyquist plot of Li/Li cells with 0.9 mol/L LiPF6 and LiN(CF3SO2)2 respectively[37]; (c) Charge-discharge curves at the 10th cycle of LiNi0.7Co0.1Mn0.2O2/Li cells at -25 ℃[42]; (d) Schematic diagram illustrating the ion transport between electrode and electrolyte in HE electrolyte[43]"
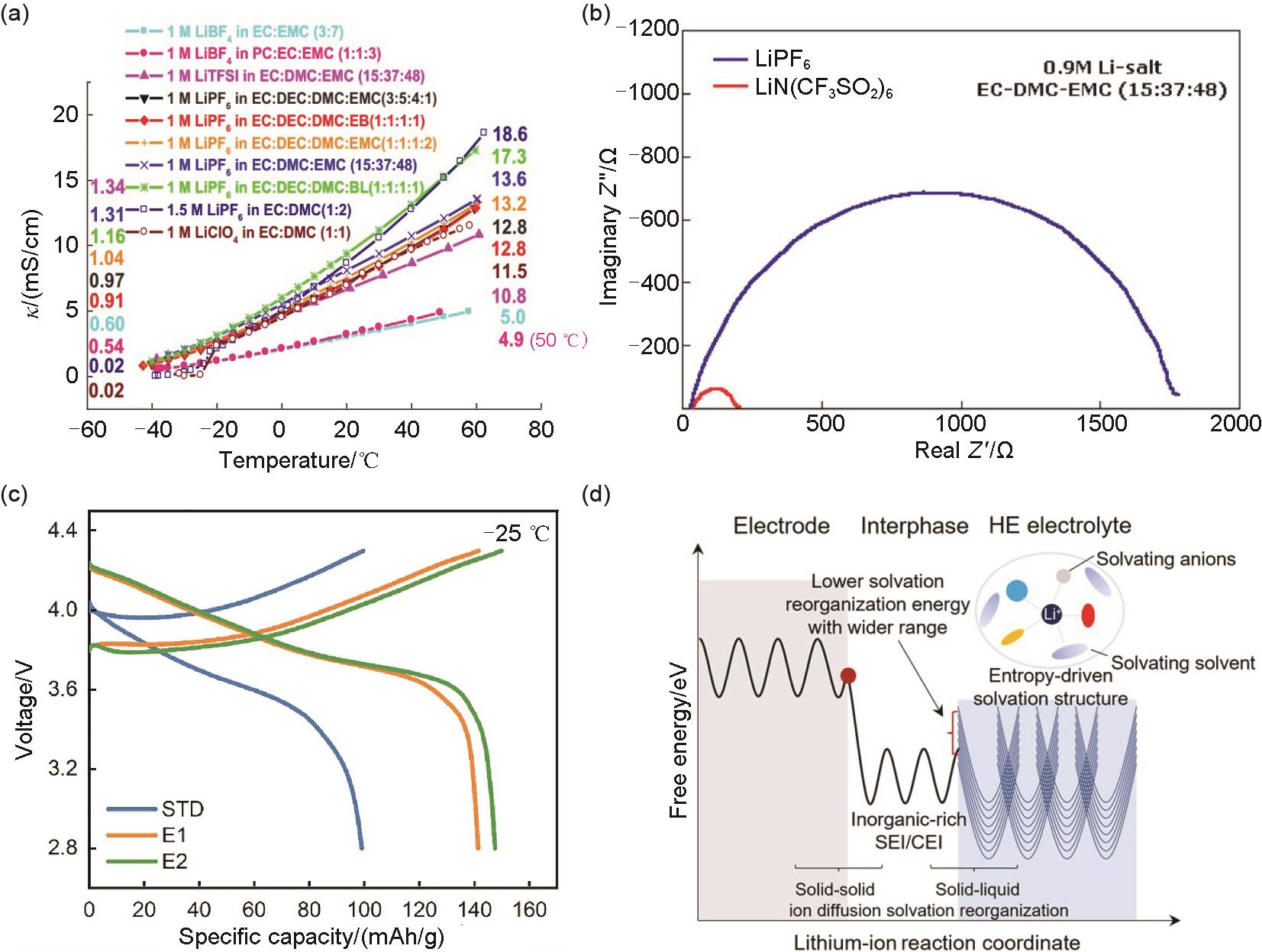

Fig. 7
(a) Cyclability of NCM622/Gr cells with different electrolytes at -20 ℃ and C/3 rate[47]; (b) Charge/discharge curves at 0.2C and different temperatures and (c) schematic illustration of the NCM811/Gr cell in the EDFA-FEC electrolyte[48]; (d) Electrostatic potential and ionic conductivities of various electrolytes at 30 ℃, and (e) solvating energies including DEE, BTFE, BDE, TFFE, DFE, and BFE[49]"
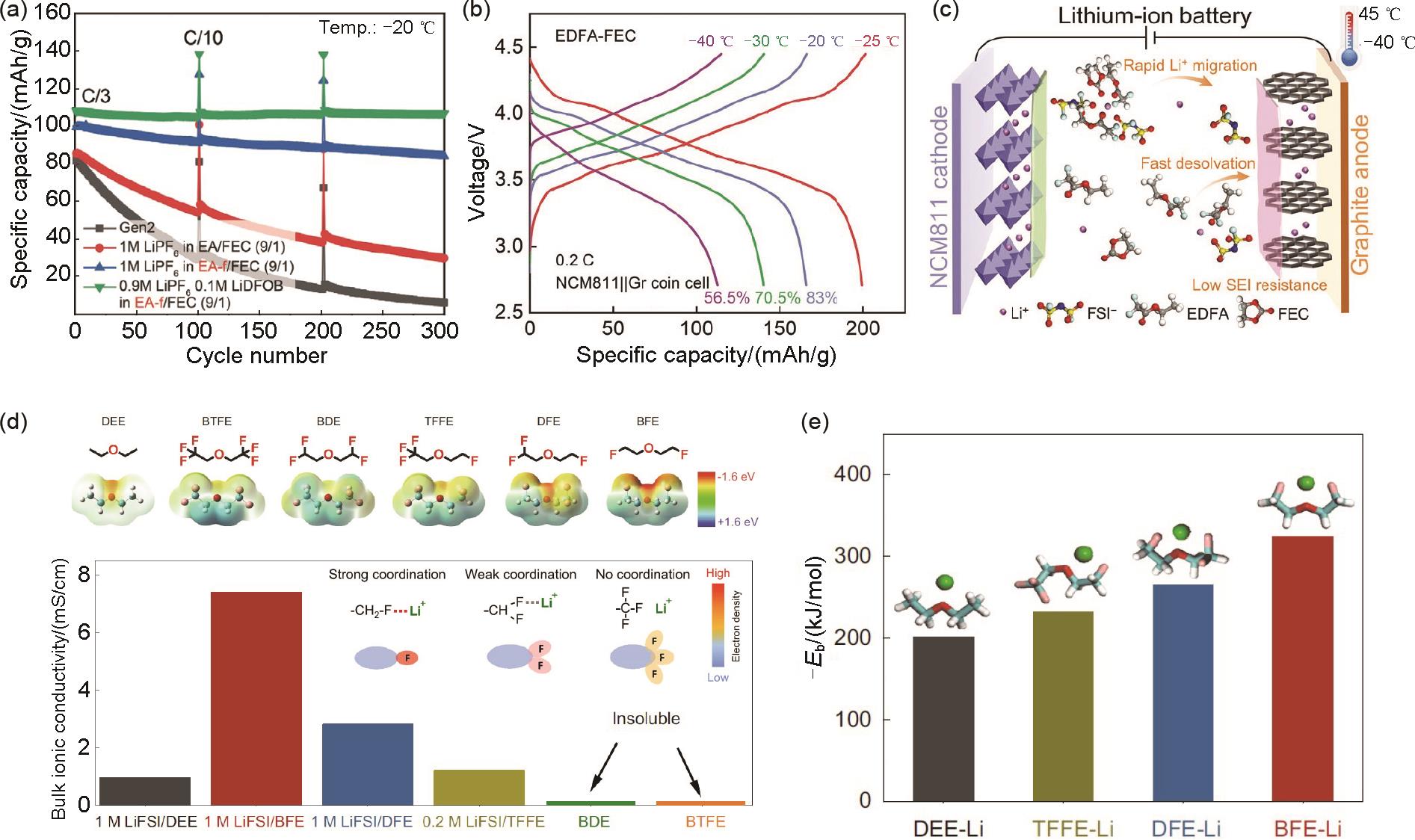
Fig. 8
The effects of FEC additive on (a) the first discharge capacity and (b) Tafel polarization[57]; (c) the proposed possible decomposition mechanism of LiDFBOP; The impedance of the Li/Gr cells in the electrolyte (d) without and (e) with LiDFBOP[61]; (f) The working mechanism of TMSP and PCS functional additives and (g) the corresponding cycling performance of LNMO/MCMB full cells at 0.3C and -5 ℃[73]"
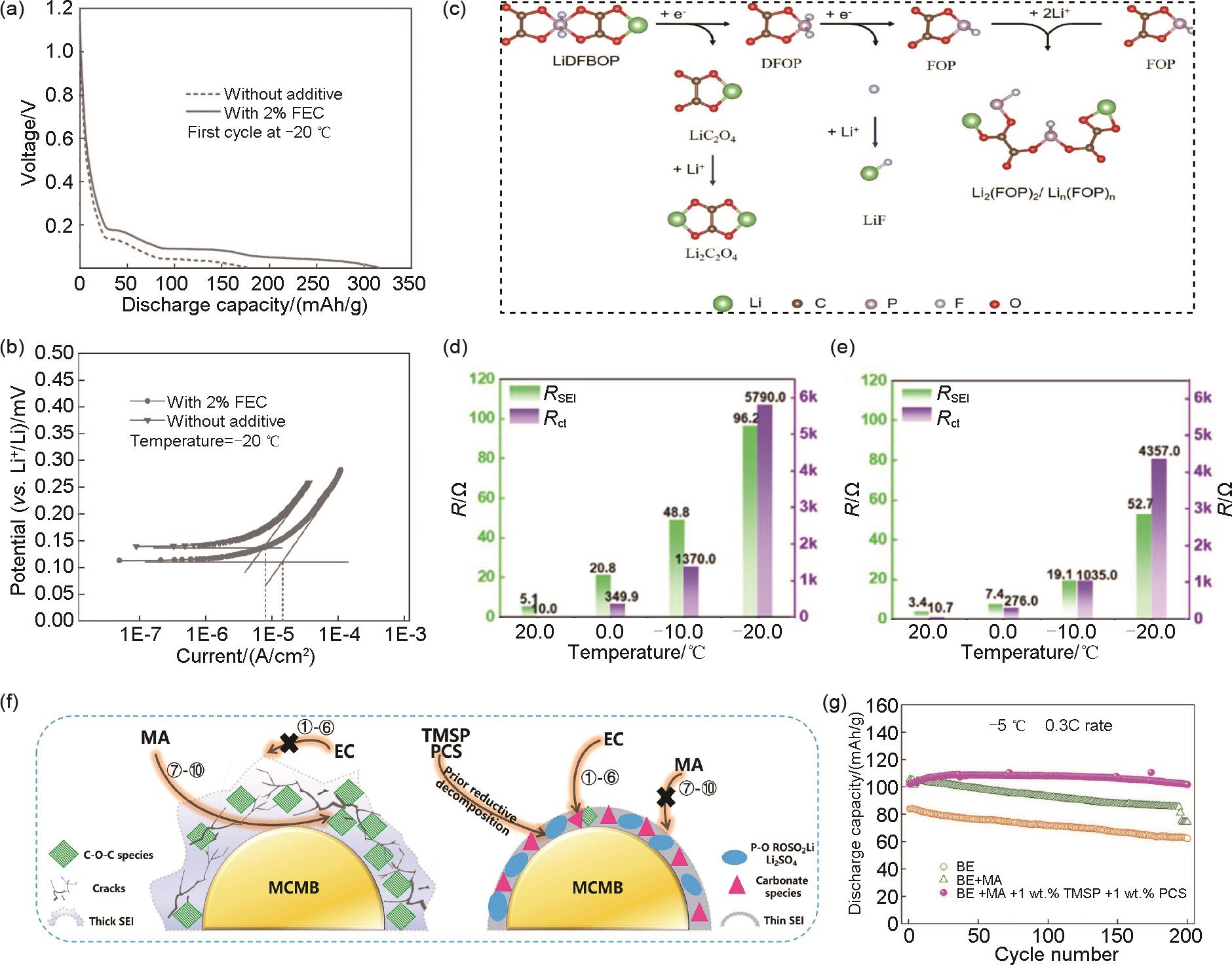
Fig. 9
(a) Cycling performance of NCM811/Gr cells with 3 mol/L LiPF6 EA-FEC electrolyte at -20 ℃ and 0.5C, (b) schematic diagram of the interfacial evolution of graphite anodes[12]; (c) Schematics of the solvation structures and (d) ionic conductivities in commercial dilute electrolyte and HCE at a temperature range of -80—100 ℃[74]; (e) Discharge profiles at different temperatures and (f) cycling performance at -20 ℃ of NCA/Li cells using 1.28 mol/L LiFSI-FEC/FEMC-D2 electrolyte[11]"
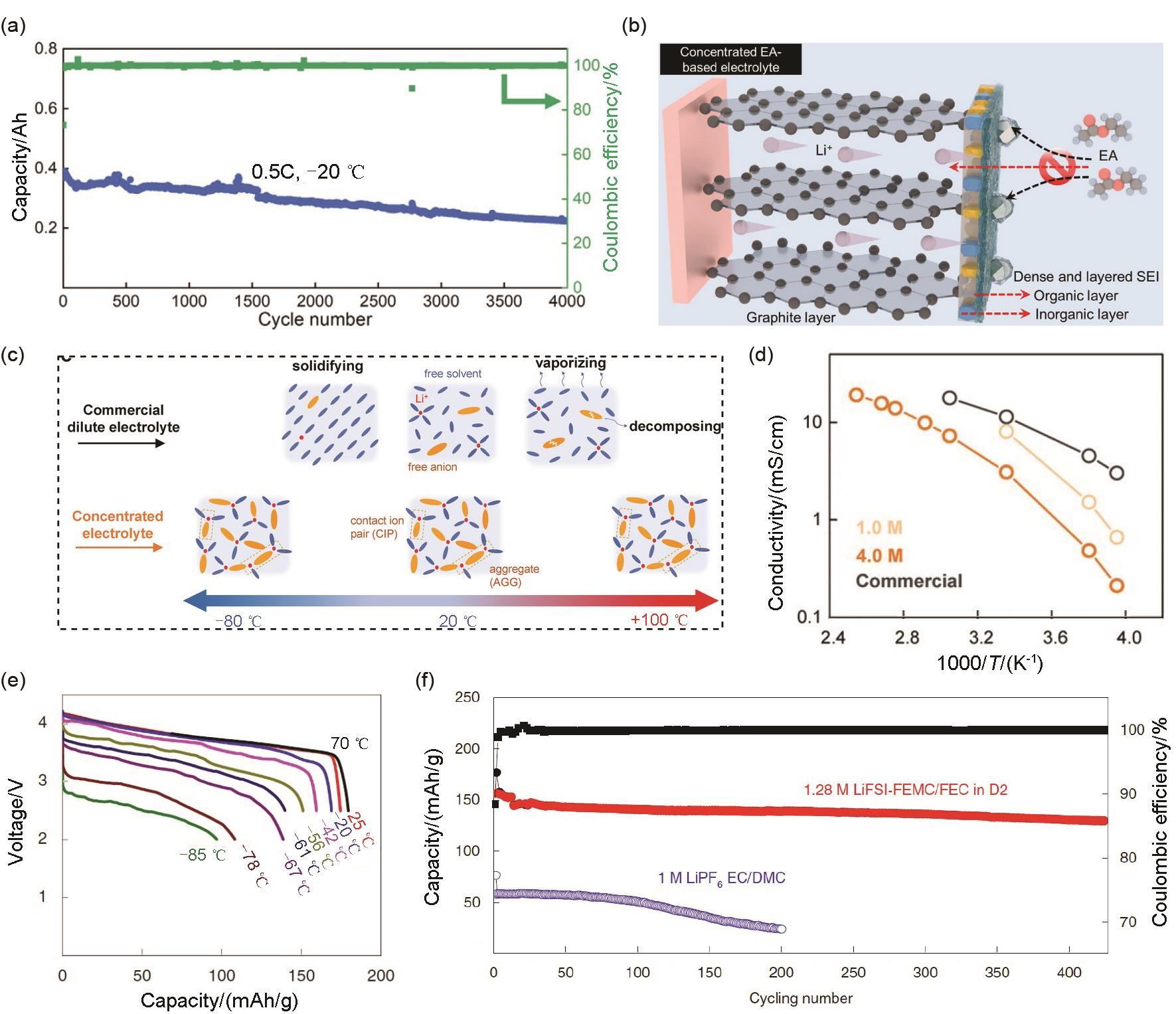
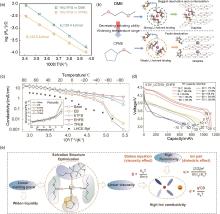
Fig. 10
(a) Li+ desolvation activation energy and (b) schematic diagram of the desolvation process in the DME and CPME electrolyte[80]; (c) Ionic conductivity of the investigated electrolytes; (d) Discharge profiles of LCO/Gr cells at different temperature; (e) Relationship of solvation structure and physical properties of electrolyte[15]"
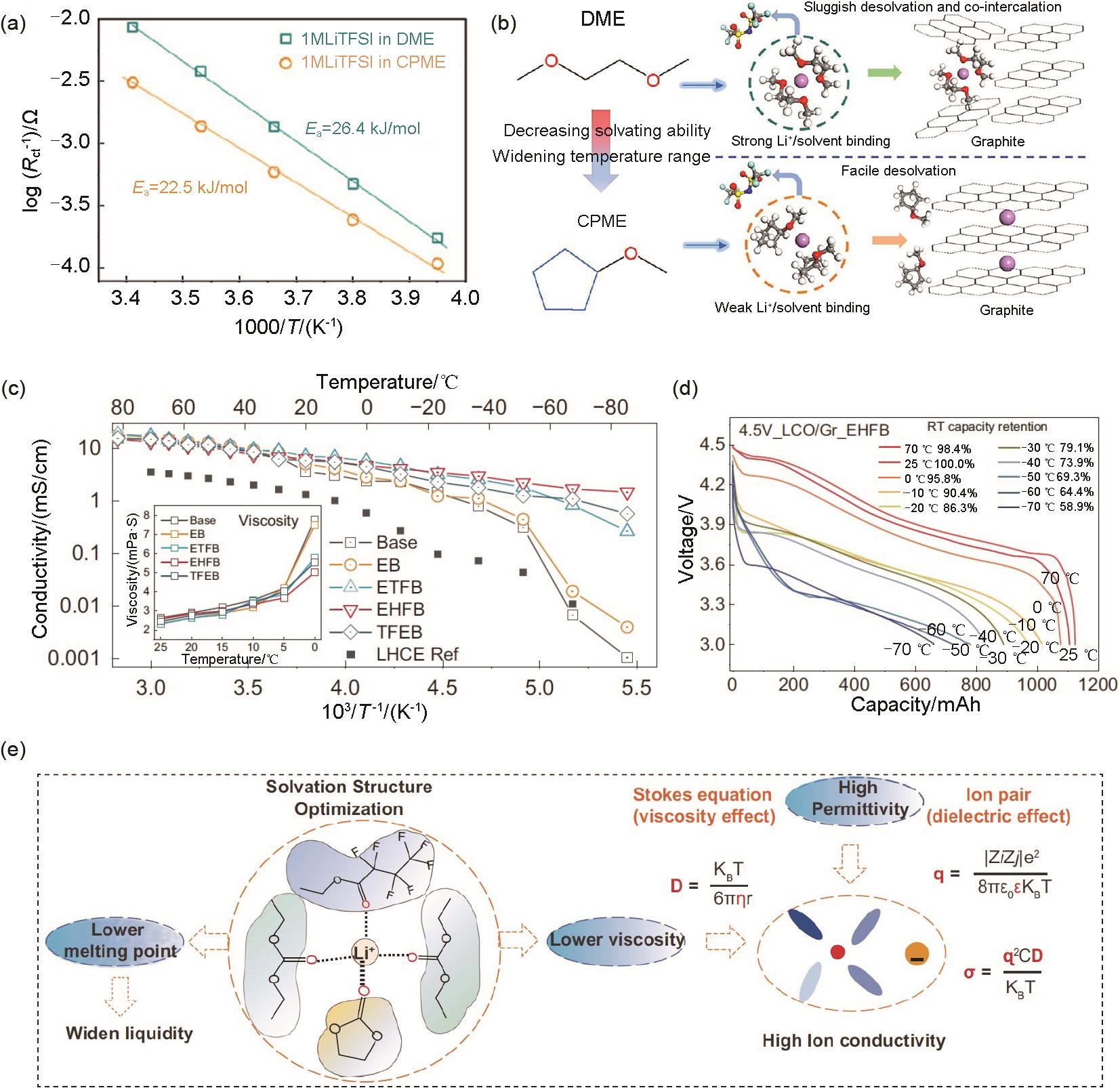
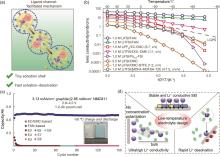
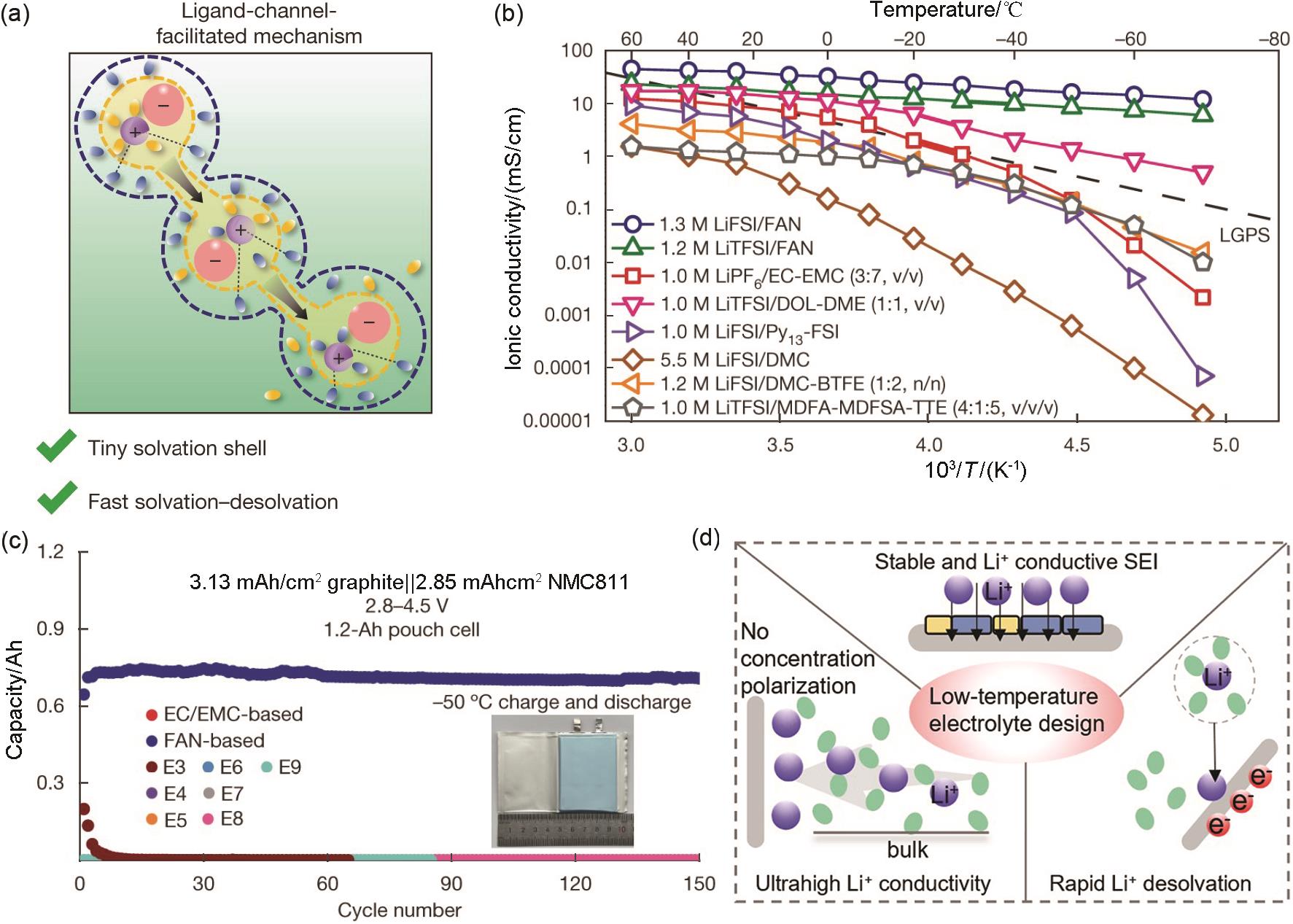
Fig. 11
(a) Ligand-channel-facilitated mechanism of Li+ transport behaviors in the FAN electrolyte; (b) Ionic conductivities of liquid and solid-state electrolytes; (c) Cycling performance of 1.2 Ah NMC811/Gr pouch cells with different electrolytes at -50 ℃[14]; (d) The design principle of low-temperature electrolyte"
| 1 | ARMAND M, TARASCON J M. Building better batteries[J]. Nature, 2008, 451: 652-657. |
| 2 | XU K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries[J]. Chemical Reviews, 2004, 104(10): 4303-4417. |
| 3 | TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414: 359-367. |
| 4 | XU K. Electrolytes and interphases in Li-ion batteries and beyond[J]. Chemical Reviews, 2014, 114(23): 11503-11618. |
| 5 | SMART M C, RATNAKUMAR B V, SURAMPUDI S. Electrolytes for low-temperature lithium batteries based on ternary mixtures of aliphatic carbonates[J]. Journal of the Electrochemical Society, 2019, 146(2): 486-492. |
| 6 | ZHANG S S, XU K, JOW T R. A new approach toward improved low temperature performance of Li-ion battery[J]. Electrochemistry Communications, 2002, 4(11): 928-932. |
| 7 | MIKHAYLIK Y V, AKRIDGE J R. Low temperature performance of Li/S batteries[J]. Journal of the Electrochemical Society, 2003, 150(3): A306. |
| 8 | REN Y H, YANG C W, WU B R, et al. Novel low-temperature electrolyte for Li-ion battery[J]. Advanced Materials Research, 2011, 287/288/289/290: 1283-1289. |
| 9 | RUSTOMJI C S, YANG Y, KIM T K, et al. Liquefied gas electrolytes for electrochemical energy storage devices[J]. Science, 2017, 356(6345): eaal4263. |
| 10 | DONG X L, GUO Z W, GUO Z Y, et al. Organic batteries operated at –70℃[J]. Joule, 2018, 2(5): 902-913. |
| 11 | FAN X L, JI X, CHEN L, et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents[J]. Nature Energy, 2019, 4: 882-890. |
| 12 | LI Z H, YAO N, YU L G, et al. Inhibiting gas generation to achieve ultralong-lifespan lithium-ion batteries at low temperatures[J]. Matter, 2023, 6(7): 2274-2292. |
| 13 | LUO L B, CHEN K A, CHEN H, et al. Enabling ultralow-temperature (-70 ℃) lithium-ion batteries: Advanced electrolytes utilizing weak-solvation and low-viscosity nitrile cosolvent[J]. Advanced Materials, 2024, 36(5): 2308881. |
| 14 | LU D, LI R H, RAHMAN M M, et al. Ligand-channel-enabled ultrafast Li-ion conduction[J]. Nature, 2024, 627: 101-107. |
| 15 | CHEN Y Q, HE Q, ZHAO Y, et al. Breaking solvation dominance of ethylene carbonate via molecular charge engineering enables lower temperature battery[J]. Nature Communications, 2023, 14: 8326. |
| 16 | MENG Y S, SRINIVASAN V, XU K. Designing better electrolytes[J]. Science, 2022, 378(6624): eabq3750. |
| 17 | XU K. Li-ion battery electrolytes[J]. Nature Energy, 2021, 6: 763-763. |
| 18 | ZHANG S B, XU K, JOW T. EIS study on the formation of solid electrolyte interface in Li-ion battery[J]. Electrochimica Acta, 2006, 51: 1636-1640. |
| 19 | GOODENOUGH J B, KIM Y. Challenges for rechargeable Li batteries[J]. Chemistry of Materials, 2010, 22(3): 587-603. |
| 20 | YAO Y X, WAN J, LIANG N Y, et al. Nucleation and growth mode of solid electrolyte interphase in Li-ion batteries[J]. Journal of the American Chemical Society, 2023, 145(14): 8001-8006. |
| 21 | XU K. Whether EC and PC differ in interphasial chemistry on graphitic anode and how[J]. Journal of the Electrochemical Society, 2009, 156(9): A751-755. |
| 22 | XING L D, ZHENG X W, SCHROEDER M, et al. Deciphering the ethylene carbonate-propylene carbonate mystery in Li-ion batteries[J]. Accounts of Chemical Research, 2018, 51(2): 282-289. |
| 23 | SMART M C, RATNAKUMAR B V, CHIN K B, et al. Lithium-ion electrolytes containing ester cosolvents for improved low temperature performance[J]. Journal of the Electrochemical Society, 2010, 157(12): A1361. |
| 24 | YAMADA Y, FURUKAWA K, SODEYAMA K, et al. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries[J]. Journal of the American Chemical Society, 2014, 136(13): 5039-5046. |
| 25 | YAO Y X, YAO N, ZHOU X R, et al. Ethylene-carbonate-free electrolytes for rechargeable Li-ion pouch cells at sub-freezing temperatures[J]. Advanced Materials, 2022, 34(45): e2206448. |
| 26 | YAO Y X, CHEN X, YAO N, et al. Unlocking charge transfer limitations for extreme fast charging of Li-ion batteries[J]. Angewandte Chemie (International Ed in English), 2023, 62(4): e202214828. |
| 27 | XU K, VON CRESCE A, LEE U. Differentiating contributions to "ion transfer" barrier from interphasial resistance and Li+ desolvation at electrolyte/graphite interface[J]. Langmuir, 2010, 26(13): 11538-11543. |
| 28 | LI Q Y, LU D P, ZHENG J M, et al. Li+-desolvation dictating lithium-ion battery's low-temperature performances[J]. ACS Applied Materials & Interfaces, 2017, 9(49): 42761-42768. |
| 29 | QIN M S, LIU M C, ZENG Z Q, et al. Rejuvenating propylene carbonate-based electrolyte through nonsolvating interactions for wide-temperature Li-ions batteries[J]. Advanced Energy Materials, 2022, 12(48): 2201801. |
| 30 | YUAN S, CAO S K, CHEN X, et al. Deshielding anions enable solvation chemistry control of LiPF6-based electrolyte toward low-temperature lithium-ion batteries[J]. Advanced Materials, 2024, 36(16): e2311327. |
| 31 | WALDMANN T, HOGG B I, KASPER M, et al. Interplay of operational parameters on lithium deposition in lithium-ion cells: Systematic measurements with reconstructed 3-electrode pouch full cells[J]. Journal of the Electrochemical Society, 2016, 163(7): A1232-A1238. |
| 32 | WENG S T, ZHANG X, YANG G J, et al. Temperature-dependent interphase formation and Li+ transport in lithium metal batteries[J]. Nature Communications, 2023, 14: 4474. |
| 33 | CHEN X, ZHANG Q. Atomic insights into the fundamental interactions in lithium battery electrolytes[J]. Accounts of Chemical Research, 2020, 53(9): 1992-2002. |
| 34 | HUANG K S, BI S, KURT B, et al. Regulation of SEI formation by anion receptors to achieve ultra-stable lithium-metal batteries[J]. Angewandte Chemie (International Ed in English), 2021, 60(35): 19232-19240. |
| 35 | YAAKOV D, GOFER Y, AURBACH D, et al. On the study of electrolyte solutions for Li-ion batteries that can work over a wide temperature range[J]. Journal of the Electrochemical Society, 2010, 157: A1383-A1391. |
| 36 | ZHANG S, XU K, JOW T. Low-temperature performance of Li-ion cells with a LiBF4-based electrolyte[J]. Journal of Solid State Electrochemistry, 2003, 7(3): 147-151. |
| 37 | MANDAL B K, PADHI A K, SHI Z, et al. New low temperature electrolytes with thermal runaway inhibition for lithium-ion rechargeable batteries[J]. Journal of Power Sources, 2006, 162(1): 690-695. |
| 38 | EIN-ELI Y, THOMAS S R, CHADHA R, et al. Li-ion battery electrolyte formulated for low-temperature applications[J]. Journal of the Electrochemical Society, 1997, 144(3): 823-829. |
| 39 | ZHAO Q P, ZHANG Y, TANG F J, et al. Mixed salts of lithium difluoro (oxalate) borate and lithium tetrafluorobotate electrolyte on low-temperature performance for lithium-ion batteries[J]. Journal of the Electrochemical Society, 2017, 164(9): A1873-A1880. |
| 40 | LI F Q, GONG Y, JIA G F, et al. A novel dual-salts of LiTFSI and LiODFB in LiFePO4-based batteries for suppressing aluminum corrosion and improving cycling stability[J]. Journal of Power Sources, 2015, 295: 47-54. |
| 41 | YANG J Z, FONSECA RODRIGUES M T, SON S B, et al. Dual-salt electrolytes to effectively reduce impedance rise of high-nickel lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(34): 40502-40512. |
| 42 | WANG Y D, ZHENG H, HONG L, et al. Lithium difluoro(bisoxalato) phosphate-based multi-salt low concentration electrolytes for wide-temperature lithium metal batteries: Experiments and theoretical calculations[J]. Chemical Engineering Journal, 2022, 445: 136802. |
| 43 | WANG Q D, ZHAO C L, WANG J L, et al. High entropy liquid electrolytes for lithium batteries[J]. Nature Communications, 2023, 14: 440. |
| 44 | TAN S, SHADIKE Z, CAI X Y, et al. Review on low-temperature electrolytes for lithium-ion and lithium metal batteries[J]. Electrochemical Energy Reviews, 2023, 6(1): 35. |
| 45 | LAI P B, HUA H M, HUANG B Y, et al. A carboxylic ester-based electrolyte with additive to improve performance of lithium batteries at ultra-low temperature[J]. Journal of the Electrochemical Society, 2022, 169(10): 100539. |
| 46 | SMART M C, RATNAKUMAR B V, SURAMPUDI S. Use of organic esters as cosolvents in electrolytes for lithium-ion batteries with improved low temperature performance[J]. Journal of the Electrochemical Society, 2002, 149(4): A361. |
| 47 | YOO D J, LIU Q, COHEN O, et al. Rational design of fluorinated electrolytes for low temperature lithium-ion batteries[J]. Advanced Energy Materials, 2023, 13(20): 2204182. |
| 48 | MO Y B, LIU G P, YIN Y, et al. Fluorinated solvent molecule tuning enables fast-charging and low-temperature lithium-ion batteries[J]. Advanced Energy Materials, 2023, 13(32): 2301285. |
| 49 | ZHANG G Z, CHANG J, WANG L G, et al. A monofluoride ether-based electrolyte solution for fast-charging and low-temperature non-aqueous lithium metal batteries[J]. Nature Communications, 2023, 14: 1081. |
| 50 | HAREGEWOIN A M, WOTANGO A S, HWANG B J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives[J]. Energy & Environmental Science, 2016, 9(6): 1955-1988. |
| 51 | ZHAO H J, YU X Q, LI J D, et al. Film-forming electrolyte additives for rechargeable lithium-ion batteries: Progress and outlook[J]. Journal of Materials Chemistry A, 2019, 7(15): 8700-8722. |
| 52 | LIAO L, CHENG X Q, MA Y L, et al. Fluoroethylene carbonate as electrolyte additive to improve low temperature performance of LiFePO4 electrode[J]. Electrochimica Acta, 2013, 87: 466-472. |
| 53 | ZHANG X Q, CHENG X B, CHEN X, et al. Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries[J]. Advanced Functional Materials, 2017, 27(10): 1605989. |
| 54 | KIM K, PARK I, HA S Y, et al. Understanding the thermal instability of fluoroethylene carbonate in LiPF6-based electrolytes for lithium ion batteries[J]. Electrochimica Acta, 2017, 225: 358-368. |
| 55 | KANG Y Y, WANG J, WANG M, et al. Multifunctional fluoroethylene carbonate for improving high-temperature performance of LiNi0.8Mn0.1Co0.1O2||SiOx@Graphite lithium-ion batteries[J]. ACS Applied Energy Materials, 2020, 3(10): 9989-10000. |
| 56 | ZHAN Y X, LIU Z Y, GENG Y Y, et al. Fluorinating solid electrolyte interphase by regulating polymer-solvent interaction in lithium metal batteries[J]. Energy Storage Materials, 2023, 60: 102799. |
| 57 | LIU B X, LI B, GUAN S Y. Effect of fluoroethylene carbonate additive on low temperature performance of Li-ion batteries[J]. Electrochemical and Solid-State Letters, 2012, 15(6): A77. |
| 58 | HOU T Z, YANG G, RAJPUT N, et al. The influence of FEC on the solvation structure and reduction reaction of LiPF6/EC electrolytes and its implication for solid electrolyte interphase formation[J]. Nano Energy, 2019, 64: 103881. |
| 59 | HE H, WANG Y, LI M, et al. Effect of fluoroethylene carbonate additive on the low-temperature performance of lithium-ion batteries[J]. Journal of Electroanalytical Chemistry, 2022, 925: 116870. |
| 60 | HU S G, ZHAO H J, QIAN Y X, et al. Improved high-temperature performance of LiNi0.5Co0.2Mn0.3O2/artificial graphite lithium ion pouch cells by difluoroethylene carbonate[J]. Journal of Energy Storage, 2023, 57: 106266. |
| 61 | SONG G, YI Z L, SU F Y, et al. New insights into the mechanism of LiDFBOP for improving the low-temperature performance via the rational design of an interphase on a graphite anode[J]. ACS Applied Materials & Interfaces, 2021, 13(33): 40042-40052. |
| 62 | WRODNIGG G H, BESENHARD J O, WINTER M. Ethylene sulfite as electrolyte additive for lithium-ion cells with graphitic anodes[J]. Journal of the Electrochemical Society, 1999, 146(2): 470-472. |
| 63 | WU Z L, LI S G, ZHENG Y Z, et al. The roles of sulfur-containing additives and their working mechanism on the temperature-dependent performances of Li-ion batteries[J]. Journal of the Electrochemical Society, 2018, 165(11): A2792-A2800. |
| 64 | WU S P, LIN Y L, XING L D, et al. Stabilizing LiCoO2/graphite at high voltages with an electrolyte additive[J]. ACS Applied Materials & Interfaces, 2019, 11(19): 17940-17951. |
| 65 | WANG K, XING L D, ZHI H Z, et al. High stability graphite/electrolyte interface created by a novel electrolyte additive: A theoretical and experimental study[J]. Electrochimica Acta, 2018, 262: 226-232. |
| 66 | QIAN Y X, CHU Y L, ZHENG Z T, et al. A new cyclic carbonate enables high power/low temperature lithium-ion batteries[J]. Energy Storage Materials, 2022, 45: 14-23. |
| 67 | GUO R D, CHE Y X, LAN G Y, et al. Tailoring low-temperature performance of a lithium-ion battery via rational designing interphase on an anode[J]. ACS Applied Materials & Interfaces, 2019, 11(41): 38285-38293. |
| 68 | YANG B W, ZHANG H, YU L, et al. Lithium difluorophosphate as an additive to improve the low temperature performance of LiNi0.5Co0.2Mn0.3O2/graphite cells[J]. Electrochimica Acta, 2016, 221: 107-114. |
| 69 | JONES J P, SMART M C, KRAUSE F C, et al. The effect of electrolyte additives upon lithium plating during low temperature charging of graphite-LiNiCoAlO2 lithium-ion three electrode cells[J]. Journal of the Electrochemical Society, 2020, 167(2): 020536. |
| 70 | TÄUBERT C, FLEISCHHAMMER M, WOHLFAHRT-MEHRENS M, et al. LiBOB as electrolyte salt or additive for lithium-ion batteries based on LiNi0.8Co0.15Al0.05O2/graphite[J]. Journal of the Electrochemical Society, 2010, 157(6): A721. |
| 71 | CHEN H X, LIU B, WANG Y, et al. Insight into wide temperature electrolyte based on lithiumdifluoro(oxalate)borate for high voltage lithium-ion batteries[J]. Journal of Alloys and Compounds, 2021, 876: 159966. |
| 72 | SHI P C, LIU F F, FENG Y Z, et al. The synergetic effect of lithium bisoxalatodifluorophosphate and fluoroethylene carbonate on dendrite suppression for fast charging lithium metal batteries[J]. Small, 2020, 16(30): e2001989. |
| 73 | XU G J, HUANG S Q, CUI Z L, et al. Functional additives assisted ester-carbonate electrolyte enables wide temperature operation of a high-voltage (5 V-Class) Li-ion battery[J]. Journal of Power Sources, 2019, 416: 29-36. |
| 74 | WANG J H, ZHENG Q F, FANG M M, et al. Concentrated electrolytes widen the operating temperature range of lithium-ion batteries[J]. Advanced Science, 2021, 8(18): e2101646. |
| 75 | CAO X, JIA H, XU W, et al. Review—localized high-concentration electrolytes for lithium batteries[J]. Journal of the Electrochemical Society, 2021, 168(1): 010522. |
| 76 | CHEN S R, ZHENG J M, MEI D H, et al. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes[J]. Advanced Materials, 2018, 30(21): e1706102. |
| 77 | LEI S, ZENG Z Q, LIU M C, et al. Balanced solvation/de-solvation of electrolyte facilitates Li-ion intercalation for fast charging and low-temperature Li-ion batteries[J]. Nano Energy, 2022, 98: 107265. |
| 78 | WU Y Q, ZHANG J, LIU J L, et al. Boosting reversible charging of Li-ion batteries at low temperatures by a synergy of propylene carbonate-based electrolyte and defective graphite[J]. Nano Research, 2024, 17(3): 1491-1499. |
| 79 | CHENG L W, WANG Y Y, YANG J, et al. An ultrafast and stable Li-metal battery cycled at -40℃[J]. Advanced Functional Materials, 2023, 33(11): 2212349. |
| 80 | WANG Z C, HAN R, HUANG D, et al. Co-intercalation-free ether-based weakly solvating electrolytes enable fast-charging and wide-temperature lithium-ion batteries[J]. ACS Nano, 2023, 17(18): 18103-18113. |
| 81 | AGUILERA L, SCHEERS J, MATIC A. Enhanced low-temperature ionic conductivity via different Li+ solvated clusters in organic solvent/ionic liquid mixed electrolytes[J]. Physical Chemistry Chemical Physics: PCCP, 2016, 18(36): 25458-25464. |
| 82 | YANG Y, DAVIES D M, YIN Y J, et al. High-efficiency lithium-metal anode enabled by liquefied gas electrolytes[J]. Joule, 2019, 3(8): 1986-2000. |
| 83 | YAO N, CHEN X, FU Z H, et al. Applying classical, Ab initio, and machine-learning molecular dynamics simulations to the liquid electrolyte for rechargeable batteries[J]. Chemical Reviews, 2022, 122(12): 10970-11021. |
| [1] | Hong ZHOU, Zhulin XIN, Hao FU, Qiang ZHANG, Feng WEI. Analysis of the key materials employed in solid-state lithium batteries based on patent data mining [J]. Energy Storage Science and Technology, 2024, 13(7): 2386-2398. |
| [2] | Yuhao WANG, Zhiyong LI, Xin GUO. Applications and challenges of polymer-based electrolytes in low-temperature solid-state lithium batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2243-2258. |
| [3] | Xiaofei ZHEN, Beibei WANG, Xiaohu ZHANG, Yiming SUN, Wenjiong CAO, Ti DONG. Study on the generation and diffusion law of thermal runaway gas in lithium battery energy storage system [J]. Energy Storage Science and Technology, 2024, 13(6): 1986-1994. |
| [4] | Baoquan LIU, Xiaoyu CAO. Accurate typical gas detection of lithium battery in early thermal runaway period [J]. Energy Storage Science and Technology, 2024, 13(6): 1995-2009. |
| [5] | Wei XIAO, Xiaowen WU, Jingling SUN, Wei CHEN. Numerical calculation of temperature field of energy storage battery module and optimization design of heat dissipation system [J]. Energy Storage Science and Technology, 2024, 13(4): 1159-1166. |
| [6] | Yaning ZHU, Zhendong ZHANG, Lei SHENG, Long CHEN, Zehua ZHU, Linxiang FU, Qing BI. Thermal runaway experiment of 21700 lithium-ion battery under different health conditions [J]. Energy Storage Science and Technology, 2024, 13(3): 971-980. |
| [7] | Qilin GUO, Liangyu TAO, Zheshu MA, Yongming GU, Yuting WANG. Numerical simulation analysis of combustion of electric sport utility vehicles [J]. Energy Storage Science and Technology, 2024, 13(3): 1000-1008. |
| [8] | Zhige TAO, Shunbing ZHU, Shuangping HOU, Ke LI, He WANG. Comprehensive research on fire and safety protection technology for lithium battery energy storage power stations [J]. Energy Storage Science and Technology, 2024, 13(2): 536-545. |
| [9] | Huan LIU, Na PENG, Qingwen GAO, Wenpeng LI, Zhirong YANG, Jingtao WANG. Crown ether-doped polymer solid electrolyte for high-performance all-solid-state lithium batteries [J]. Energy Storage Science and Technology, 2023, 12(8): 2401-2411. |
| [10] | Zhihao LIU, Tong DU, Ruirui LI, Tao DENG. Developments of wide temperature range, high voltage and safe EC-free electrolytes [J]. Energy Storage Science and Technology, 2023, 12(8): 2504-2525. |
| [11] | Minghu WU, Chengpeng YUE, Fan ZHANG, Junxiao LI, Wei HUANG, Sheng HU, Jing TANG. Combined GRU-MLR method for predicting the remaining useful life of lithium batteries via multiscale decomposition [J]. Energy Storage Science and Technology, 2023, 12(7): 2220-2228. |
| [12] | Yuling LIU, Jinhao MENG, Qiao PENG, Tianqi LIU, Yang WANG, Yongxiang CAI. NSGA-II genetic algorithm-based optimization of the lithium battery equalization index [J]. Energy Storage Science and Technology, 2023, 12(6): 1946-1956. |
| [13] | Hai WANG, Yuhua BIAN, Jiadong WANG, Zhaoyang LIU, Jie ZHANG, Jian YAO, Xuanwen GAO, Zhaomeng LIU, Wenbin LUO. Retired lithium battery recycling and battery-grade lithium carbonate preparation [J]. Energy Storage Science and Technology, 2023, 12(5): 1453-1460. |
| [14] | Xinhao ZHAO, Liang XU. Improved firefly optimization algorithm to optimize back propagation neural network for state of health estimation of power lithium ion batteries [J]. Energy Storage Science and Technology, 2023, 12(3): 934-940. |
| [15] | Yuhao ZHOU, Luoyun XÜ, Zhongping ZHANG, Lingchong LIU, Bin NAN, Haiqi ZHAO. Construction and simulation analysis of thermoelectric coupling model of lithium battery based on digital twin [J]. Energy Storage Science and Technology, 2023, 12(2): 536-543. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
