Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (2): 467-486.doi: 10.19799/j.cnki.2095-4239.2021.0483
• Energy Storage Materials and Devices • Previous Articles Next Articles
Xiaohan FENG1( ), Jie SUN1,2(
), Jie SUN1,2( ), Jianhao HE2, Yihua WEI2, Chenggang ZHOU1(
), Jianhao HE2, Yihua WEI2, Chenggang ZHOU1( ), Ruimin SUN1(
), Ruimin SUN1( )
)
Received:2021-09-15
Revised:2021-10-19
Online:2022-02-05
Published:2022-02-08
Contact:
Chenggang ZHOU,Ruimin SUN
E-mail:2215852440@qq.com;sunjie898@aliyun.com;cgzhou@cug.edu.cn;rmsun@cug.edu.cn
CLC Number:
Xiaohan FENG, Jie SUN, Jianhao HE, Yihua WEI, Chenggang ZHOU, Ruimin SUN. Research progress in LiFePO4 cathode material modification[J]. Energy Storage Science and Technology, 2022, 11(2): 467-486.

Fig. 3
(a)~(c) CV curves of LiFePO4 under different scan rates of 1.4 mV/s, 2.8 mV/s, and 4.2 mV/s at temperature ranging from 253~313 K; (d)~(f) image plot of diffraction patterns for (111), (211), (020), (311), and (121) reflections during two CV cycles under different scan rates of 1.4 mV/s, 2.8 mV/s, and 4.2 mV/s at a temperature of 293 K, corresponding current curves are plotted to right; LFP represent for LiFePO4; (g)~(i) selected individual diffraction patterns for two CV cycles at 1.4 mV/s, 2.8 mV/s, and 4.2 mV/s[14]"
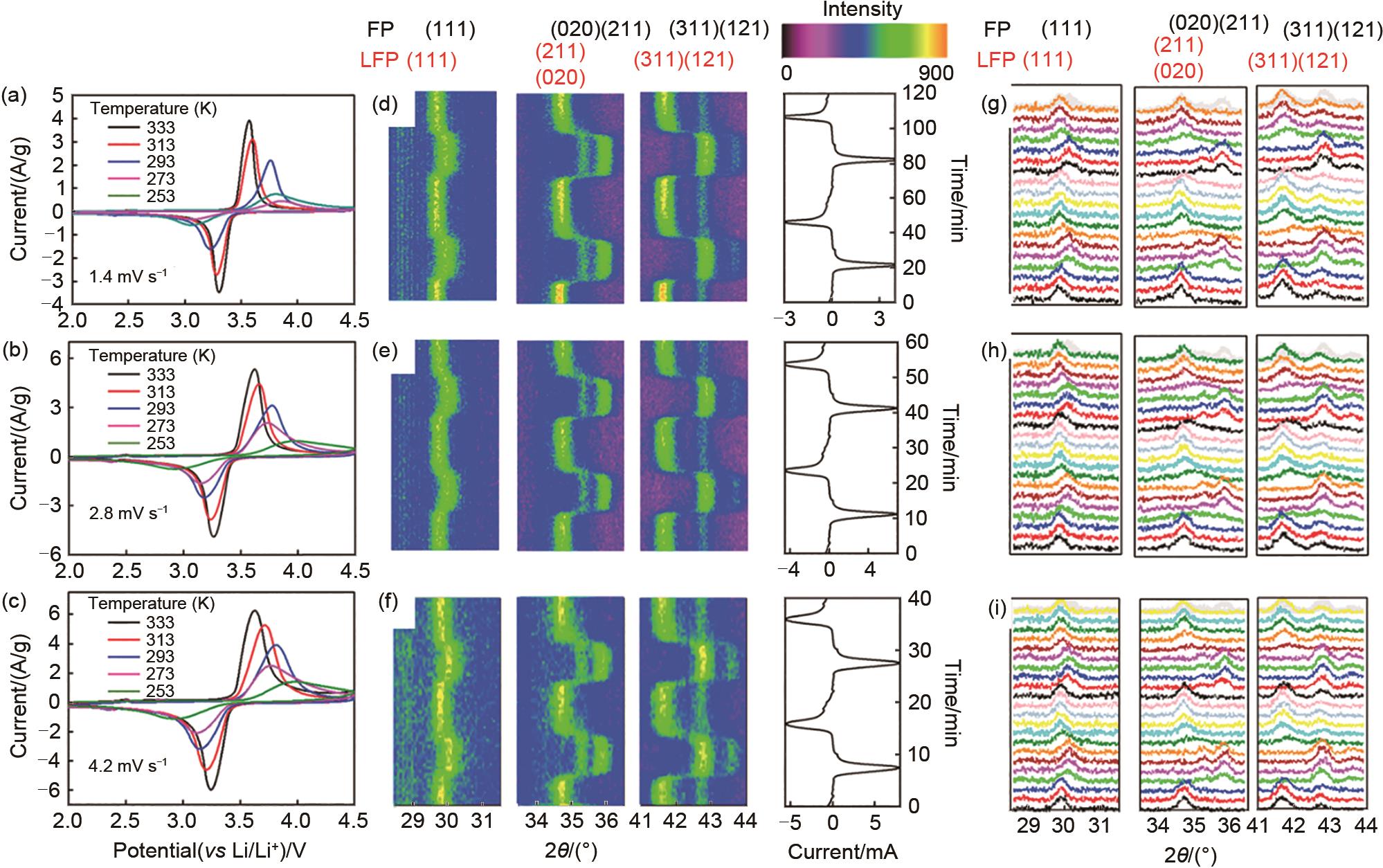
Fig. 5
(a) First charge/discharge curves of Cl-doped LFP/C and LFP/C electrodes at 0.1 C; discharge curves of different current density: (b) LFP/C; (c) Cl-doped LFP/C; (d) rate ability of LFP/C and Cl-doped LFP/C; (e) cycling performance of Cl-doped LFP/C and LFP/C at 0.1 C and 10 C, respectively[28]; (f) initial charge/discharge curves of undoped and F-doped LiFePO4/C samples at 0.1 C rate; (g) initial charge/discharge curves of LiFePO4/C and LiFePO4-xFx/C (x = 0.15) samples at 0.1C rate, (h) rate and cycle performances of undoped and F-doped LiFePO4/C samples; i) cycle performances of LiFePO4-xFx/C (x = 0.15) sample at high-rate 20 C, 30 C[29]"
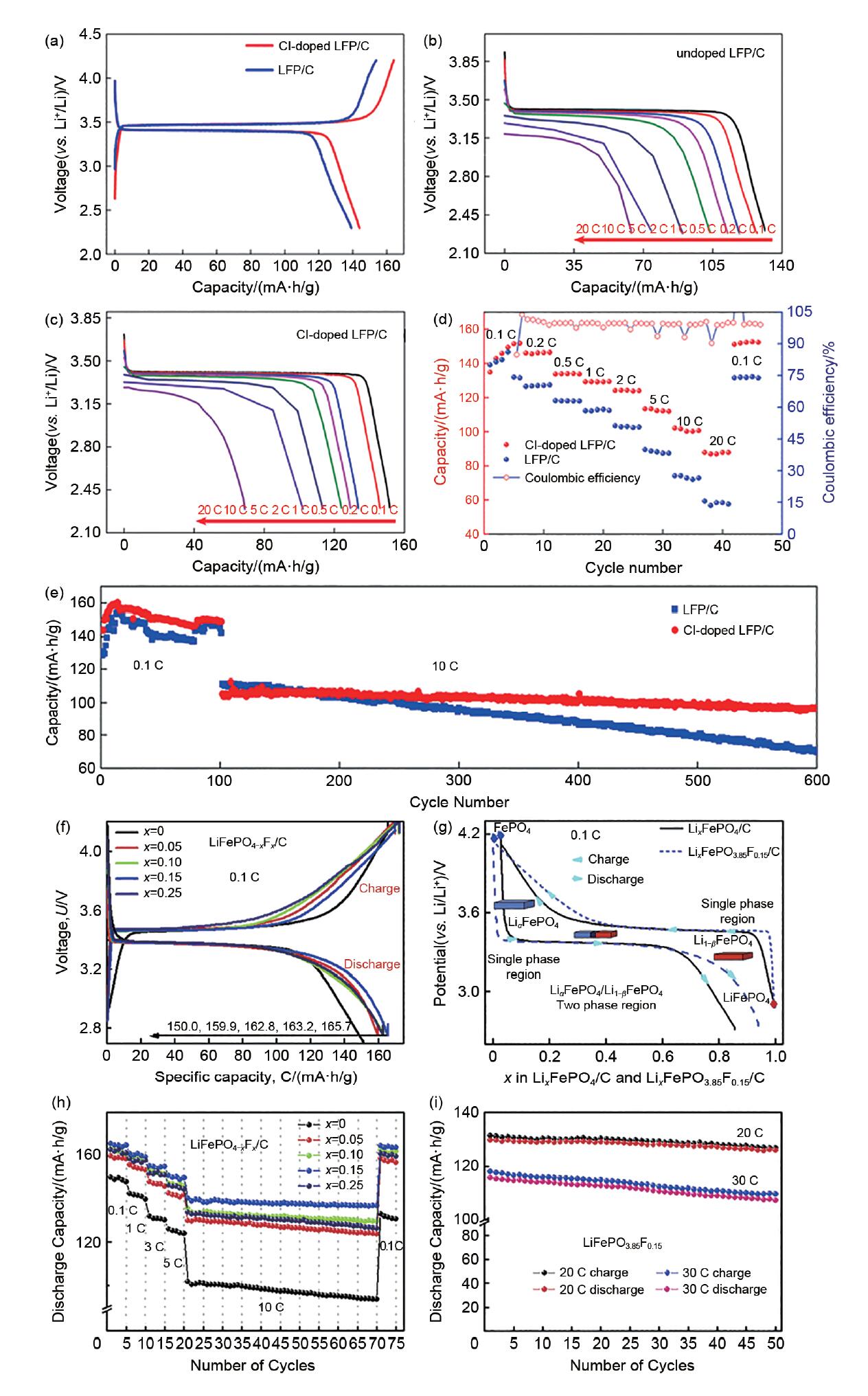
Fig. 6
(a) Schematic illustration of te synthesis process of MT-LFP materials; SEM images of the as-prepared undoped LFP (b) and (c); MT-LFP (d) and (e); Nitrogen adsorption (closed symbols)-desorption (open symbols) isotherms (f); pore diameter distribution curves (g) of as-prepared undoped LFP and MT-LFP samples; (h) charge-discharge curves of LFP and (i) MT-LFP at different rates; (j) rate capabilities of LFP and MT-LFP at various rates; cycling performance of LFP and MT-LFP cathodes at a charge/discharge rate of 0.5 C (k), 1 C (l), and 5C (m)[30]"
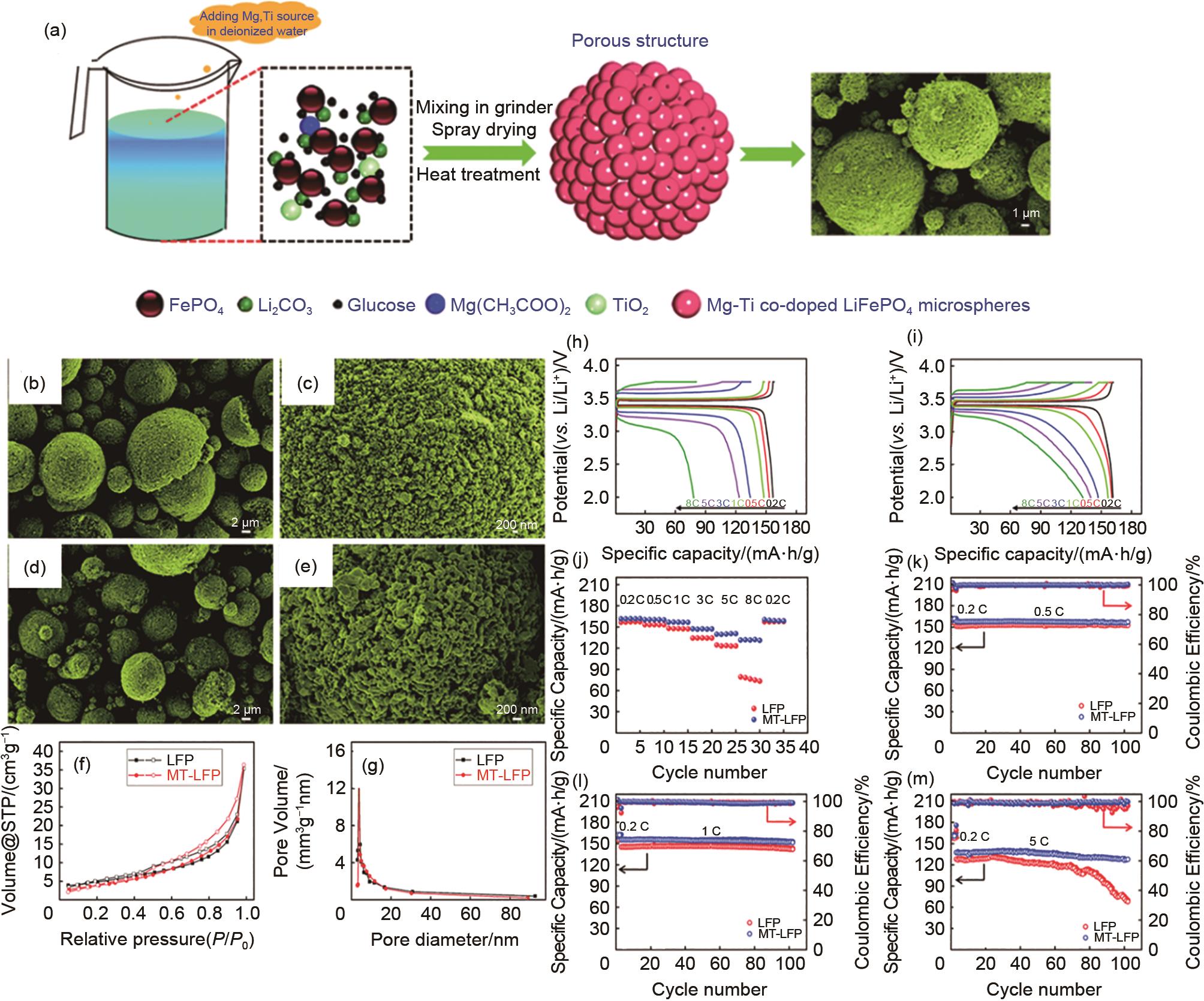
Table 1
A sum up of electrochemical performance of LiFePO4 modified by elemental doping"
| 掺杂元素 | 掺杂位点 | 最佳掺杂量 | 电化学性能(初始容量;循环性能) |
|---|---|---|---|
| Na[ | Li位 | Li0.99Na0.01FePO4 | 80.9 mA·h/g(10 C);86.7%(10 C,500圈) |
| Nb[ | Li位 | Li0.95Nb0.01FePO4 | 96.7 mA·h/g(10 C);96%(10 C,200圈) |
| Al[ | Li位 | Li0.97Al0.01FePO4 | 95 mA·h/g(0.2 C) |
| Mn[ | Fe位 | LiFe0.77Mn0.23PO4 | 80.9 mA·h/g(1 C);84%(1 C,100圈) |
| Mo[ | Fe位 | LiFe0.98Mn0.02PO4 | 141.5 mA·h/g(0.1 C);98%(0.1 C,100圈) |
| V[ | Fe位 | LiFe0.95V0.05PO4 | 119 mA·h/g(1500 mA/g);98%(1500 mA/g,100圈) |
| Ti[ | Fe位 | LiFe0.98Ti0.02PO4 | 160 mA·h/g(0.2 C);98%(0.2 C,50圈) |
| S[ | O位 | LiFePO3.78S0.22 | 112.7 mA·h/g(10 C);98%(0.2 C,50圈) |
| Cl[ | O位 | LiFePO3.98Cl0.02 | 164.1 mA·h/g(0.1 C);105.3 mA·h/g(10 C);91.5%(10 C,500圈) |
| F[ | O位 | LiFePO3.85F0.15 | 165.7 mA·h/g(0.1 C);115.7 mA·h/g(30 C);92.8%(30 C,50圈) |
| Mg&Ti[ | Mg(Fe位) Ti(Fe位) | LiFe0.985Mg0.005Ti0.01PO4 | 161.5 mA·h/g(0.2 C);139.8 mA·h/g(5 C);92.9%(5 C,100圈) |
| Zr&Co[ | Zr(Li位) Co(Fe位) | Li0.99Zr0.0025Fe0.98Co0.02PO4 | 139.9 mA·h/g(0.1 C);85%(0.1 C,50圈) |
| Ni&Mn[ | Ni(Fe位) Mn(Fe位) | LiFe0.95Ni0.02Mn0.03PO4/C | 164.3 mA·h/g(0.1 C);146 mA·h/g(1 C);98.7%(1 C,100圈) |
| V&F[ | V(Fe位) F(O位) | LiFe0.96V0.02PO3.97F0.06 | 165.7 mA·h/g(0.1 C);154.9 mA·h/g(1 C);95.7%(1 C,500圈) |
| V&Y[ | Y(Fe位) F(O位) | LiFe0.95V0.033PO3.95F0.1 | 148.6 mA·h/g(5 C);96.88%(5 C,700圈) |
| Ni&Mn&Co[ | Ni(Fe位) Mn(Fe位) Co(Fe位) | LiFe0.84Ni0.06Co0.06Mn0.04PO4 | 160.1 mA·h/g(0.1 C);110.8 mA·h/g(10 C);98.3%(10 C,50圈) |

Fig. 7
Schematic illustration of synthesis and structure of LFP/graphite composite. (a) synthesis of LFP/graphite composite by intercalating graphite with FeCl3 followed by formation of LFP within the graphite layers; (b) schematics of LFP/graphite based cathode and a commercial LFP cathode, continuous conductive network provided by graphite confers better performance on LFP/graphite electrode; structure and morphology of LFP/graphite composite: (c), (d) SEM and EDS mapping, (e), (f) SEM images, and (g), (h) TEM images of LFP/graphite composite; (i) inset in panel g is SAED of a LFP particle; (j) SEM and (k) TEM images of porous graphite particle after etching away LFP particles from LFP/graphite composite[43]"
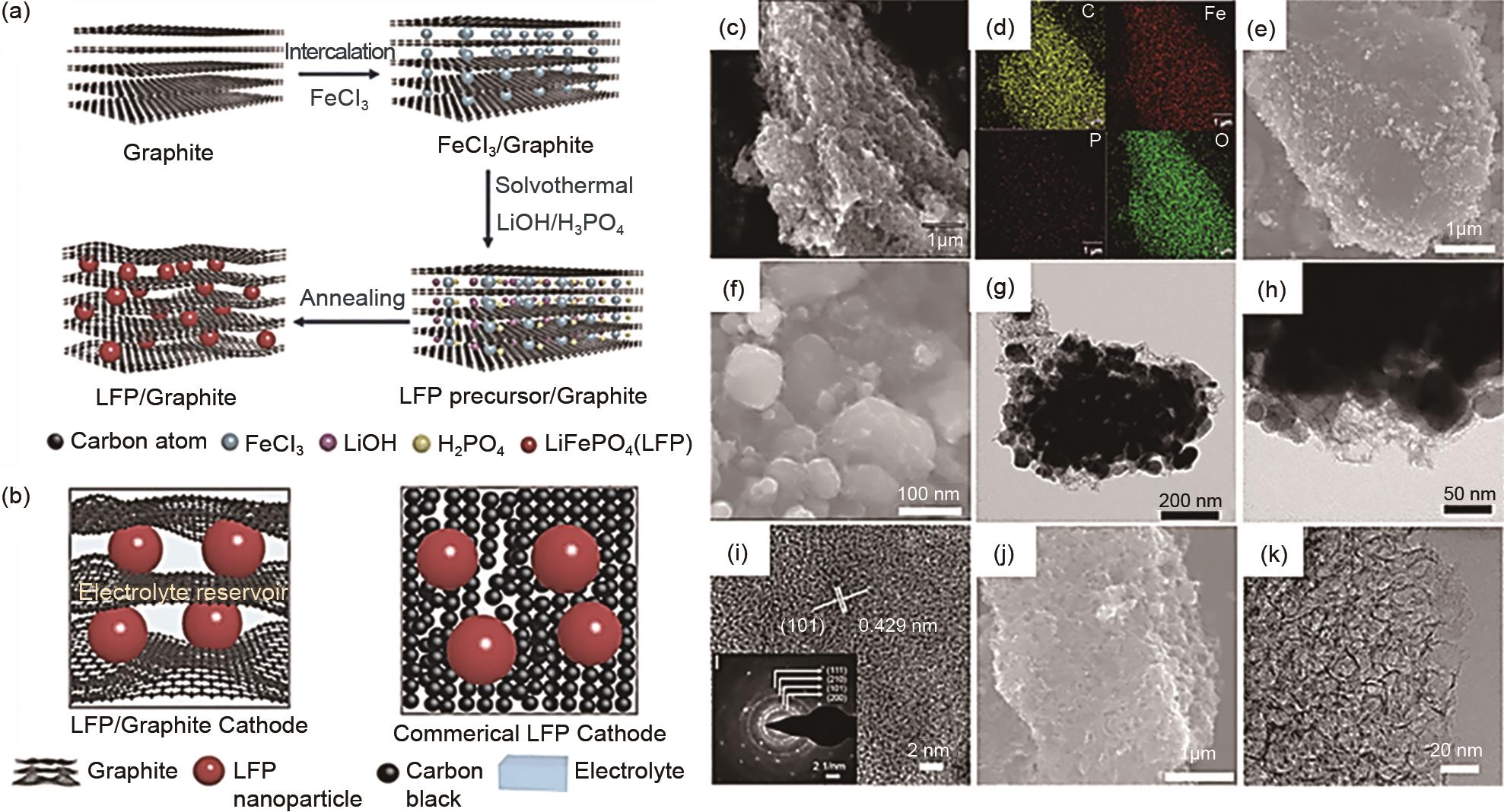

Fig. 9
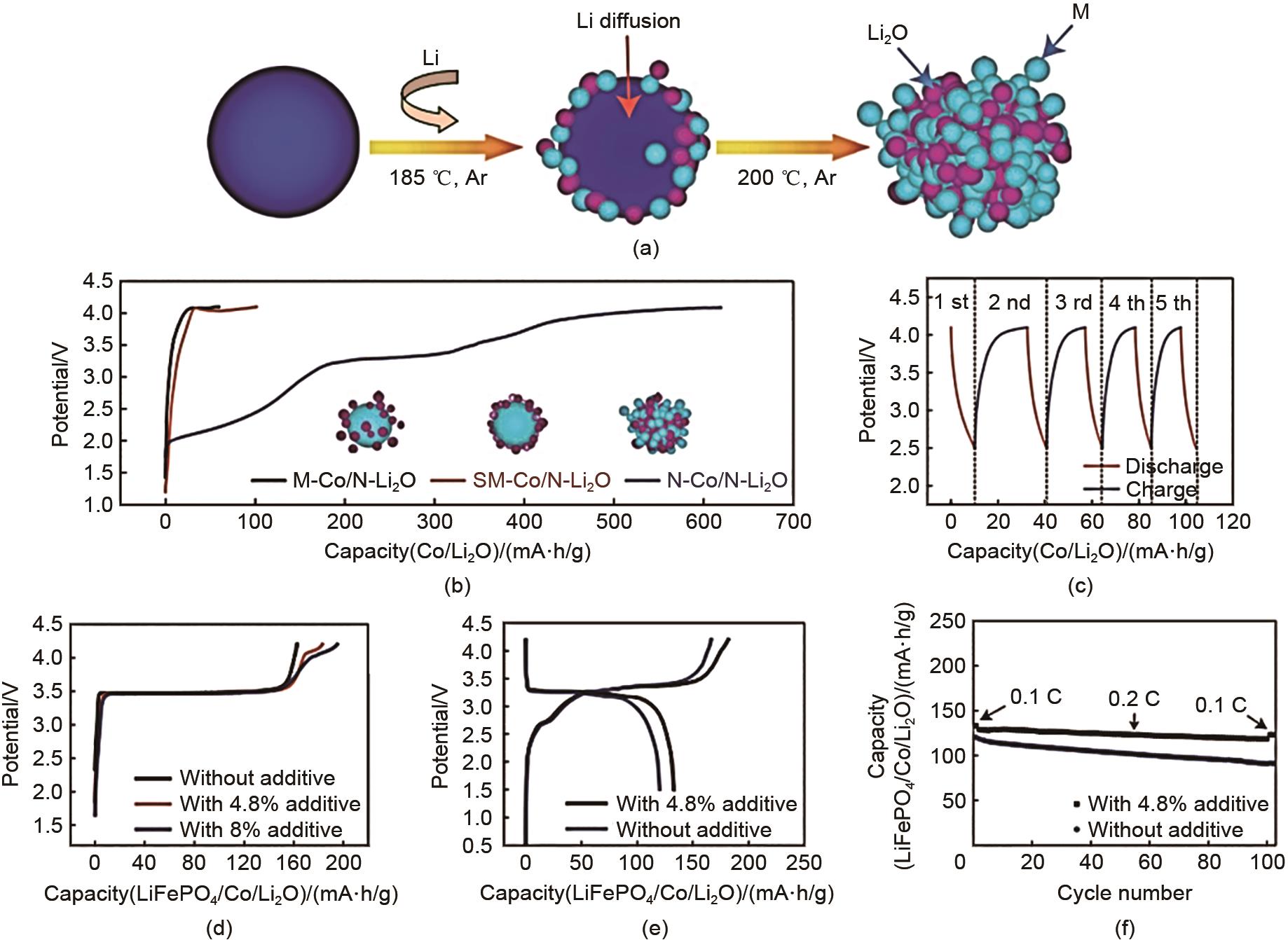
(a) Schematic of fabrication process of N-Co/N-Li2O composites; (b) initial charge potential profiles of electrodes made with various Co/Li2O nanocomposites: M-Co/N-Li2O composite, SM-Co/N-Li2O composite and N-Co/N-Li2O composite; (c) charge/discharge potential profiles of N-Co/N-Li2O electrode after first charge process; (d) initial charge potential profiles of LiFePO4 electrodes with different amounts of N-Co/N-Li2O additive in half-cell configurations; initial charge/ discharge potential profiles (e) and cycling performance (f) of LiFePO4/ graphite full cells with and without N-Co/N-Li2O additive[72]"
| 1 | LI H, PENG L, WU D B, et al. Ultrahigh-capacity and fire-resistant LiFePO4-based composite cathodes for advanced lithium-ion batteries[J]. Advanced Energy Materials, 2019, 9(10): 1802930. |
| 2 | 刘仕强, 王芳, 马天翼, 等. 磷酸铁锂动力电池备电工况寿命试验研究及分析[J]. 储能科学与技术, 2020, 9(2): 638-644. |
| LIU S Q, WANG F, MA T J, et al. Cycle life test and analysis of lithium iron phosphate based traction batteries[J]. Energy Storage Science and Technology, 2020, 9(2): 638-644. | |
| 3 | DAI Y, XU Z D, HUA D, et al. Theoretical-molar Fe3+ recovering lithium from spent LiFePO4 batteries: An acid-free, efficient, and selective process[J]. Journal of Hazardous Materials, 2020, 396: 122707. |
| 4 | 田柳文, 于华, 章文峰. 锂离子电池的明星材料磷酸铁锂: 基本性能、优化改性及未来展望[J]. 材料导报, 2019, 33(11): 3561-3579. |
| TIAN L W, YU H, ZHANG W F. The star material of Lithium ion batteries, LiFePO4 optimized modification and future prospects[J]. Materials Reports, 2019, 33(11): 3561-3579. | |
| 5 | 范茂松, 金翼, 杨凯, 等. 退役LiFePO4电池性能测评及储能应用[J]. 储能科学与技术, 2019, 8(2): 408-414. |
| FAN M S, JIN Y, YANG K, et al. Testing of the performance and energy-storage applied for retired LiFePO4 batteries[J]. Energy Storage Science and Technology, 2019, 8(2): 408-414. | |
| 6 | 李雨, 赵慧春, 白莹, 等. 高能量密度层状富锂锰基正极材料的改性研究进展[J]. 储能科学与技术, 2018, 7(3): 395-403. |
| LI Y, ZHAO H C, BAI Y, et al. Progress in the modification of lithium-rich manganese-based layered cathode materials[J]. Energy Storage Science and Technology, 2019, 8(2): 408-414. | |
| 7 | 王栋, 邓莉莉, 杜光超, 等. 锂离子电池正极材料掺杂和表面包覆研究综述[J]. 储能科学与技术, 2019, 8(1): 43-48. |
| WANG D, DEN L L, DU G C, et al. Review of doping and surface coating of cathode materials for lithium ion batteries[J]. Energy Storage Science and Technology, 2019, 8(1): 43-48. | |
| 8 | HUANG Y Y, ZHU Y C, FU H Y, et al. Mg-pillared LiCoO2: Towards stable cycling at 4.6 V[J]. Angewandte Chemie International Ed in English, 2021, 60(9): 4682-4688. |
| 9 | KOYAMA Y, UYAMA T, ORIKASA Y, et al. Hidden two-step phase transition and competing reaction pathways in LiFePO4[J]. Chemistry of Materials, 2017, 29(7): 2855-2863. |
| 10 | WANG T Z, WU X G, XU S B, et al. Performance of plug-in hybrid electric vehicle under low temperature condition and economy analysis of battery pre-heating[J]. Journal of Power Sources, 2018, 401(8): 245-254. |
| 11 | NITTA N, WU F X, LEE J T, et al. Li-ion battery materials: Present and future[J]. Materials Today, 2015, 18(5): 252-264. |
| 12 | JI Y, ZHANG Y C, WANG C Y. Li-ion cell operation at low temperatures[J]. Journal of The Electrochemical Society, 2013, 160(4): A636-A649. |
| 13 | MA S, JIANG M D, TAO P, et al. Temperature effect and thermal impact in lithium-ion batteries: A review[J]. Progress in Natural Science: Materials International, 2018, 28(6): 653-666. |
| 14 | YAN M Y, ZHANG G B, WEI Q L, et al. In operando observation of temperature-dependent phase evolution in lithium-incorporation olivine cathode[J]. Nano Energy, 2016, 22(1): 406-413. |
| 15 | WU G, LIU N, GAO X G, et al. A hydrothermally synthesized LiFePO4/C composite with superior low-temperature performance and cycle life[J]. Applied Surface Science, 2018, 435(11): 1329-1336. |
| 16 | CHUNG S Y, BLOKING J T, CHIANG Y M. Electronically conductive phospho-olivines as lithium storage electrodes[J]. Nature Materials, 2002, 1(2): 123-128. |
| 17 | LIU Y, QIN W C, ZHANG D K, et al. Effect of Na+ in situ doping on LiFePO4/C cathode material for lithium-ion batteries[J]. Progress in Natural Science: Materials International, 2021, 31(1): 14-18. |
| 18 | JOHNSON I D, BLAGOVIDOVA E, DINGWALL P A, et al. High power Nb-doped LiFePO4 Li-ion battery cathodes; pilot-scale synthesis and electrochemical properties[J]. Journal of Power Sources, 2016, 326(6): 476-481. |
| 19 | KULKA A, BRAUN A, HUANG T W, et al. Evidence for Al doping in lithium sublattice of LiFePO4[J]. Solid State Ion, 2015, 270(12): 33-38. |
| 20 | SŁAWIŃSKI W A, PLAYFORD H Y, HULL S, et al. Neutron pair distribution function study of FePO4 and LiFePO4[J]. Chemistry of Materials, 2019, 31(14): 5024-5034. |
| 21 | GOONETILLEKE D, FAULKNER T, PETERSON V K, et al. Structural evidence for Mg-doped LiFePO4 electrode polarisation in commercial Li-ion batteries[J]. Journal of Power Sources, 2018, 394(5): 1-8. |
| 22 | LEE H, KIM S, PARMAR N S, et al. Carbon-free Mn-doped LiFePO4 cathode for highly transparent thin-film batteries[J]. Journal of Power Sources, 2019, 434(10): 1-8. |
| 23 | GUO X P, WANG M, HUANG X L, et al. Direct evidence of antisite defects in LiFe0.5Mn0.5PO4via atomic-level HAADF-EELS[J]. Journal of Materials Chemistry A, 2013, 1(31): 8775-8781. |
| 24 | ZHANG Y, SHAO Z C, ZHANG Y. Preparation of Mo-doping LiFePO4/C by carbon reduction method[J]. Materials and Manufacturing Processes, 2021, 36(4): 419-425. |
| 25 | JOHNSON I D, LÜBKE M, WU O Y, et al. Pilot-scale continuous synthesis of a vanadium-doped LiFePO4/C nanocomposite high-rate cathodes for lithium-ion batteries[J]. Journal of Power Sources, 2016, 302(10): 410-418. |
| 26 | KIM S, MATHEW V, KANG J, et al. High rate capability of LiFePO4 cathodes doped with a high amount of Ti[J]. Ceramics International, 2016, 42(6): 7230-7236. |
| 27 | OKADA K, KIMURA I, MACHIDA K. High rate capability by sulfur-doping into LiFePO4 matrix[J]. RSC Advances, 2018, 8(11): 5848-5853. |
| 28 | LIU H, LUO S H, YAN S X, et al. A novel and low-cost iron source for synthesizing Cl-doped LiFePO4/C cathode materials for lithium-ion batteries[J]. Journal of Electroanalytical Chemistry, 2019, 850: 113434. |
| 29 | GAO C, ZHOU J, LIU G Z, et al. Synthesis of F-doped LiFePO4/C cathode materials for high performance lithium-ion batteries using co-precipitation method with hydrofluoric acid source[J]. Journal of Alloys and Compounds, 2017, 727(8): 501-513. |
| 30 | TU J G, WU K, TANG H, et al. Mg-Ti co-doping behavior of porous LiFePO4 microspheres for high-rate lithium-ion batteries[J]. Journal of Materials Chemistry A, 2017, 5(32): 17021-17028. |
| 31 | GAO L B, XU Z R, ZHANG S. The Co-doping effects of Zr and Co on structure and electrochemical properties of LiFePO4 cathode materials[J]. Journal of Alloys and Compounds, 2018, 739: 529-535. |
| 32 | YUAN H, WANG X Y, WU Q, et al. Effects of Ni and Mn doping on physicochemical and electrochemical performances of LiFePO4/C[J]. Journal of Alloys and Compounds, 2016, 675: 187-194. |
| 33 | LI X T, YU L N, CUI Y H, et al. Enhanced properties of LiFePO4/C cathode materials Co-doped with V and F ions via high-temperature ball milling route[J]. International Journal of Hydrogen Energy, 2019, 44(50): 27204-27213. |
| 34 | WANG H Q, LAI A J, HUANG D Q, et al. Y-F co-doping behavior of LiFePO4/C nanocomposites for high-rate lithium-ion batteries[J]. New Journal of Chemistry, 2021, 45(12): 5695-5703. |
| 35 | LIU W M, HUANG Q Z, HU G R. A novel preparation route for multi-doped LiFePO4/C from spent electroless nickel plating solution[J]. Journal of Alloys and Compounds, 2015, 632: 185-189. |
| 36 | ZHANG Z F, WU Z J, SU S H, et al. Sustainable preparation of Li(FeM)PO4/C from converter sludge and its electrochemical performance as a cathode material for lithium ion batteries[J]. Journal of Alloys and Compounds, 2013, 574: 136-141. |
| 37 | MENG Y S, LI Y Z, XIA J, et al. F-doped LiFePO4@N/B/F-doped carbon as high performance cathode materials for Li-ion batteries[J]. Applied Surface Science, 2019, 476: 761-768. |
| 38 | LIU Y L, WANG J J, LIU J, et al. Origin of phase inhomogeneity in lithium iron phosphate during carbon coating[J]. Nano Energy, 2018, 45: 52-60. |
| 39 | SONG J J, SUN B, LIU H, et al. Enhancement of the rate capability of LiFePO4 by a new highly graphitic carbon coating method[J]. ACS Appl Mater Interfaces, 2016, 8(24): 15225-15231. |
| 40 | PARK S, OH J, KIM J M, et al. Facile preparation of cellulose nanofiber derived carbon and reduced graphene oxide co-supported LiFePO4 nanocomposite as enhanced cathode material for lithium-ion battery[J]. Electrochimica Acta, 2020, 354: 136707. |
| 41 | WANG P, ZHANG G, LI Z C, et al. Improved electrochemical performance of LiFePO4@N-doped carbon nanocomposites using polybenzoxazine as nitrogen and carbon sources[J]. ACS Applied Materials & Interfaces, 2016, 8(40): 26908-26915. |
| 42 | WANG X F, FENG Z J, HOU X L, et al. Fluorine doped carbon coating of LiFePO4 as a cathode material for lithium-ion batteries[J]. Chemical Engineering Journal, 2020, 379: 122371. |
| 43 | LI F, TAO R, TAN X Y, et al. Graphite-embedded lithium iron phosphate for high-power-energy cathodes[J]. Nano Letters, 2021, 21(6): 2572-2579. |
| 44 | LUO W-B, CHOU S-L, ZHAI Y-C, et al. Self-assembled graphene and LiFePO4 composites with superior high rate capability for lithium ion batteries[J]. Journal of Materials Chemistry A, 2014, 2(14): 4927-4931. |
| 45 | WANG B, AL ABDULLA W, WANG D, et al. A three-dimensional porous LiFePO4 cathode material modified with a nitrogen-doped graphene aerogel for high-power lithium ion batteries[J]. Energy & Environmental Science, 2015, 8(3): 869-875. |
| 46 | XU L T, LÜ W, SHI K, et al. Holey graphenes as the conductive additives for LiFePO4 batteries with an excellent rate performance[J]. Carbon, 2019, 149: 257-262. |
| 47 | FAN J M, CHEN J J, CHEN Y X, et al. Hierarchical structure LiFePO4@C synthesized by oleylamine-mediated method for low temperature applications[J]. Journal of Materials Chemistry A, 2014, 2(14): 4870-4873. |
| 48 | WU X L, GUO Y G, SU J, et al. Carbon-nanotube-decorated nano-LiFePO4@C cathode material with superior high-rate and low-temperature performances for lithium-ion batteries[J]. Advanced Energy Materials, 2013, 3(9): 1155-1160. |
| 49 | LU Z G, CHENG H, LO M F, et al. Pulsed laser deposition and electrochemical characterization of LiFePO4-Ag composite thin films[J]. Advanced Functional Materials, 2007, 17(18): 3885-3896. |
| 50 | ZHU M Y, CHENG L F, LIU Y, et al. LiFePO4/(C+Cu) composite with excellent cycling stability as lithium ion battery cathodes synthesized via a modified carbothermal reduction method[J]. Ceramics International, 2018, 44(11): 12106-12111. |
| 51 | LIN Y B, LIN Y, ZHOU T, et al. Enhanced electrochemical performances of LiFePO4/C by surface modification with Sn nanoparticles[J]. Journal of Power Sources, 2013, 226: 20-26. |
| 52 | ZIOLKOWSKA D, KORONA K P, HAMANKIEWICZ B, et al. The role of SnO2 surface coating on the electrochemical performance of LiFePO4 cathode materials[J]. Electrochimica Acta, 2013, 108: 532-539. |
| 53 | LIU S X, YIN H B, WANG H B, et al. Synthesis, characterization and electrochemical performances of MoO2 and carbon co-coated LiFePO4 cathode materials[J]. Ceramics International, 2014, 40(2): 3325-3331. |
| 54 | LIU S X, WANG H B. WO2 modified LiFePO4/C cathode materials with improved electrochemical performance synthesized by in-situ synthesis method[J]. Materials Letters, 2014, 122: 151-154. |
| 55 | ZHANG M, GARCIA-ARAEZ N, HECTOR A L, et al. A sol-gel route to titanium nitride conductive coatings on battery materials and performance of TiN-coated LiFePO4[J]. Journal of Materials Chemistry A, 2017, 5(5): 2251-2260. |
| 56 | LU J, PENG Q, WANG W Y, et al. Nanoscale coating of LiMO2 (M =Ni, Co, Mn) nanobelts with Li+-conductive Li2TiO3: Toward better rate capabilities for Li-ion batteries[J]. Journal of the American Chemical Society, 2013, 135(5): 1649-1652. |
| 57 | TANG H, XU J. Enhanced electrochemical performance of LiFePO4 coated with Li0.34La0.51TiO2.94 by rheological phase reaction method[J]. Materials Science and Engineering: B, 2013, 178(20): 1503-1508. |
| 58 | PARK K Y, PARK I, KIM H, et al. Lithium-excess olivine electrode for lithium rechargeable batteries[J]. Energy & Environmental Science, 2016, 9(9): 2902-2915. |
| 59 | CHIEN W C, JHANG J S, WU S H, et al. Preparation of LiFePO4/Li3V2(PO4)3/C composite cathode materials and their electrochemical performance analysis[J]. Journal of Alloys and Compounds, 2020, 847: 156447. |
| 60 | GUO Y, HUANG Y D, JIA D Z, et al. Preparation and electrochemical properties of high-capacity LiFePO4-Li3V2(PO4)3/C composite for lithium-ion batteries[J]. Journal of Power Sources, 2014, 246: 912-917. |
| 61 | ZHANG X P, GUO H J, LI X H, et al. Studies of fast-ion conducting Li3V2(PO4)3 coated LiFePO4via sol-gel method[J]. Solid State Ion, 2012, 212: 106-111. |
| 62 | ZHENG J C, LI X H, WANG Z X, et al. Novel synthesis of LiFePO4-Li3V2(PO4)3 composite cathode material by aqueous precipitation and lithiation[J]. Journal of Power Sources, 2010, 195(9): 2935-2938. |
| 63 | ZHONG S K, WU L, LIU J Q. Sol-gel synthesis and electrochemical properties of 9LiFePO4·Li3V2(PO4)3/C composite cathode material for lithium ion batteries[J]. Electrochimica Acta, 2012, 74: 8-15. |
| 64 | LIANG S Q, CAO X X, WANG Y P, et al. Uniform 8LiFePO4·Li3V2(PO4)3/C nanoflakes for high-performance Li-ion batteries[J]. Nano Energy, 2016, 22: 48-58. |
| 65 | IM J, HEO K, KANG S-W, et al. LiFePO4 synthesis using refined Li3PO4 from wastewater in Li-ion battery recycling process[J]. Journal of the Electrochemical Society, 2019, 166(15): A3861-A3868. |
| 66 | WANG X, WANG X Y, ZHANG R, et al. Hydrothermal preparation and performance of LiFePO4 by using Li3PO4 recovered from spent cathode scraps as Li source[J]. Waste Managment, 2018, 78: 208-216. |
| 67 | ZHAO S X, DING H, WANG Y C, et al. Improving rate performance of LiFePO4 cathode materials by hybrid coating of nano-Li3PO4 and carbon[J]. Journal of Alloys and Compounds, 2013, 566: 206-211. |
| 68 | SHU H B, CHEN M F, WEN F, et al. Li fast ion conductive La0.56Li0.33TiO3 inlaid LiFePO4/C microspheres with enhanced high-rate performance as cathode materials[J]. Electrochimica Acta, 2015, 152: 368-377. |
| 69 | SHU H B, CHEN M F, FU Y Q, et al. Improvement of electrochemical performance for spherical LiFePO4via hybrid coated with electron conductive carbon and fast Li ion conductive La0.56Li0.33TiO3[J]. Journal of Power Sources, 2014, 252: 73-78. |
| 70 | 赵鹤, 韩策, 程小露. 采用阳极预锂化技术的锂离子电池高倍率老化容量衰减机理研究[J]. 储能科学与技术, 2021, 10(2): 454-461. |
| ZHAO H, HAN C, CHEN X L. Research on the capacity fading mechanism of high rate aged lithium-ion batteries with anode prelithiation treatment[J]. Energy Storage Science and Technology, 2021, 10(2): 454-461. | |
| 71 | DIAZ-LOPEZ M, CHATER P A, BORDET P, et al. Li2O: Li-Mn-O disordered rock-Salt nanocomposites as cathode prelithiation additives for high-energy density Li-ion batteries[J]. Advanced Energy Materials, 2020, 10(7): 1902788. |
| 72 | SUN Y M, LEE H-W, SEH Z W, et al. High-capacity battery cathode prelithiation to offset initial lithium loss[J]. Nature Energy, 2016, 1: 15008. |
| 73 | SUN Y, LEE H W, ZHENG G, et al. In situ chemical synthesis of lithium fluoride/metal nanocomposite for high capacity prelithiation of cathodes[J]. Nano Letters, 2016, 16(2): 1497-1501. |
| 74 | DU J M, WANG W Y, ENG A Y S, et al. Metal/LiF/Li2O nanocomposite for battery cathode prelithiation: trade-off between capacity and stability[J]. Nano Letters, 2020, 20(1): 546-552. |
| 75 | SUN Y M, LI Y B, SUN J, et al. Stabilized Li3N for efficient battery cathode prelithiation[J]. Energy Storage Materials, 2017, 6: 119-124. |
| 76 | ZHAN Y J, YU H L, BEN L B, et al. Using Li2S to compensate for the loss of active lithium in Li-ion batteries[J]. Electrochimica Acta, 2017, 255(9): 212-219. |
| [1] | Shunmin YI, Linbo XIE, Li PENG. Remaining useful life prediction of lithium-ion batteries based on VF-DW-DFN [J]. Energy Storage Science and Technology, 2022, 11(7): 2305-2315. |
| [2] | Xiaosa ZHANG, Hongyuan WANG, Zhenbiao LI, Zhimei XIA. New process of sulfated roasting-water leaching for treating electrode material of spent lithium iron phosphate batteries [J]. Energy Storage Science and Technology, 2022, 11(7): 2066-2074. |
| [3] | Qingwei ZHU, Xiaoli YU, Qichao WU, Yidan XU, Fenfang CHEN, Rui HUANG. Semi-empirical degradation model of lithium-ion battery with high energy density [J]. Energy Storage Science and Technology, 2022, 11(7): 2324-2331. |
| [4] | Yuzuo WANG, Jin WANG, Yinli LU, Dianbo RUAN. Study on the effects of pore structure on lithium-storage performances for soft carbon [J]. Energy Storage Science and Technology, 2022, 11(7): 2023-2029. |
| [5] | Wei KONG, Jingtao JIN, Xipo LU, Yang SUN. Study on cooling performance of lithium ion batteries with symmetrical serpentine channel [J]. Energy Storage Science and Technology, 2022, 11(7): 2258-2265. |
| [6] | YAN Qiaoyi, WU Feng, CHEN Renjie, LI Li. Recovery and resource recycling of graphite anode materials for spent lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1760-1771. |
| [7] | ZHOU Wei, FU Dongju, LIU Weifeng, CHEN Jianjun, HU Zhao, ZENG Xierong. Research progress on recycling technology of waste lithium iron phosphate power battery [J]. Energy Storage Science and Technology, 2022, 11(6): 1854-1864. |
| [8] | ZHANG Yan, WANG Hai, LIU Zhaomeng, ZHANG Deliu, WANG Jiadong, LI Jianzhong, GAO Xuanwen, LUO Wenbin. Research progress of nickel-rich ternary cathode material ncm for lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1693-1705. |
| [9] | WANG Yuzuo, DENG Miao, WANG Jin, YANG Bin, LU Yinli, JIN Ge, RUAN Dianbo. Study on the effects of carbonization temperature on lithium-storage kinetics for soft carbon [J]. Energy Storage Science and Technology, 2022, 11(6): 1715-1724. |
| [10] | YU Chunhui, HE Ziying, ZHANG Chenxi, LIN Xianqing, XIAO Zhexi, WEI Fei. The analyses and suppressing strategies of silicon anode with the electrolyte [J]. Energy Storage Science and Technology, 2022, 11(6): 1749-1759. |
| [11] | WANG Can, MA Pan, ZHU Guoliang, WEI Shuimiao, YANG Zhilu, ZHANG Zhiyu. Effect of lithium acrylic-coated nature graphite on its electrochemical properties [J]. Energy Storage Science and Technology, 2022, 11(6): 1706-1714. |
| [12] | LIU Hangxin, CHEN Xiantao, SUN Qiang, ZHAO Chenxi. Cycle performance characteristics of soft pack lithium-ion batteries under vacuum environment [J]. Energy Storage Science and Technology, 2022, 11(6): 1806-1815. |
| [13] | Guangyu CHENG, Xinwei LIU, Yueni MEI, Honghui GU, Cheng YANG, Ke WANG. Capacity fading analysis of lithium-ion battery after high temperature storage [J]. Energy Storage Science and Technology, 2022, 11(5): 1339-1349. |
| [14] | Yanwen DAI, Aiqing YU. Combined CNN-LSTM and GRU based health feature parameters for lithium-ion batteries SOH estimation [J]. Energy Storage Science and Technology, 2022, 11(5): 1641-1649. |
| [15] | Chunjing LIN, Danhua LI, Haoran WEN, Tianyi MA, Hong CHANG, Peixiang CHANG, Haiqiang LI, Shiqiang LIU. Research on swelling force characteristics of power battery during charging [J]. Energy Storage Science and Technology, 2022, 11(5): 1627-1633. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
