Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (7): 2075-2082.doi: 10.19799/j.cnki.2095-4239.2022.0040
• Energy Storage Materials and Devices • Previous Articles Next Articles
Yingwei PEI( ), Hong ZHANG(
), Hong ZHANG( ), Xinghui WANG(
), Xinghui WANG( )
)
Received:2022-01-20
Revised:2022-01-28
Online:2022-07-05
Published:2022-06-29
Contact:
Hong ZHANG, Xinghui WANG
E-mail:N191120044@fzu.edu.cn;zhanghong@fzu.edu.cn;seaphy23@fzu.edu.cn
CLC Number:
Yingwei PEI, Hong ZHANG, Xinghui WANG. Recent advances in the electrolytes of rechargeable zinc-ion batteries[J]. Energy Storage Science and Technology, 2022, 11(7): 2075-2082.

Fig. 2
(a) Structure and CV of Zn in 1 mol/L Zn(CF3SO3)2. (b) CV of Zn in 1 mol/L aqueous ZnCl2 electrolyte. Reproduced with permission[9]. Copyright 2016 American Chemical Society. (c) Schematic diagram: Dual function of Na2SO4 additive. Reproduced with permission[26]. Copyright 2018 Nature Publishing Group"
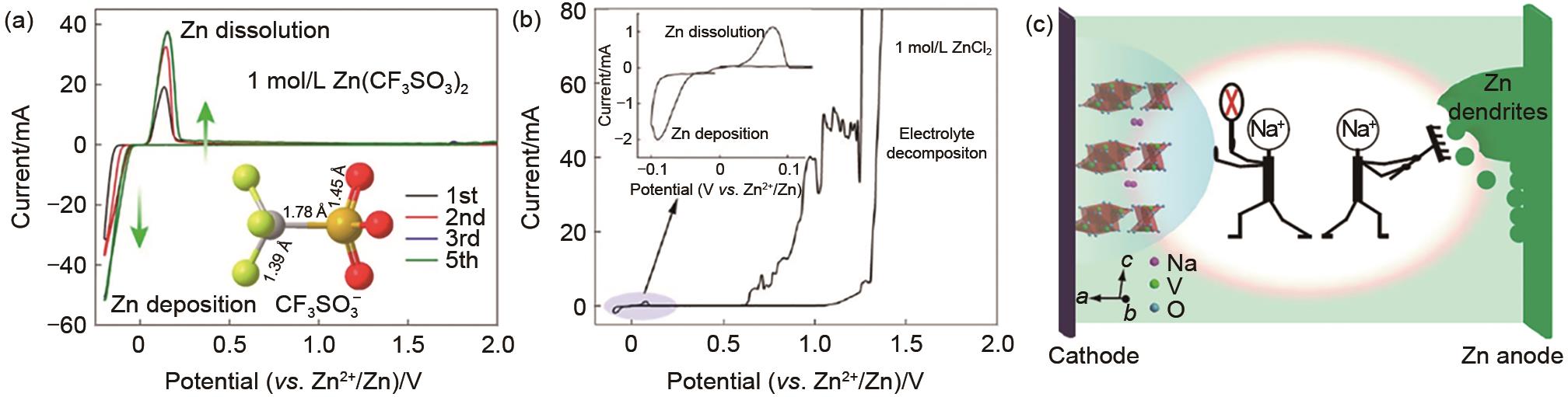

Fig. 3
(a) Schematic illustration of the coaxial fiber battery based on ZnSO4/CMC electrolyte. Reproduced with permission[34], Copyright 2019 American Chemical Society; (b) LED powered by flexible pouch Zn/NVO batteries. Reproduced with permission[26], Copyright 2018 Nature Publishing Group; (c) schematic illustration of the PAM based electrolyte. Reproduced with permission[40], Copyright 2018 American Chemical Society"
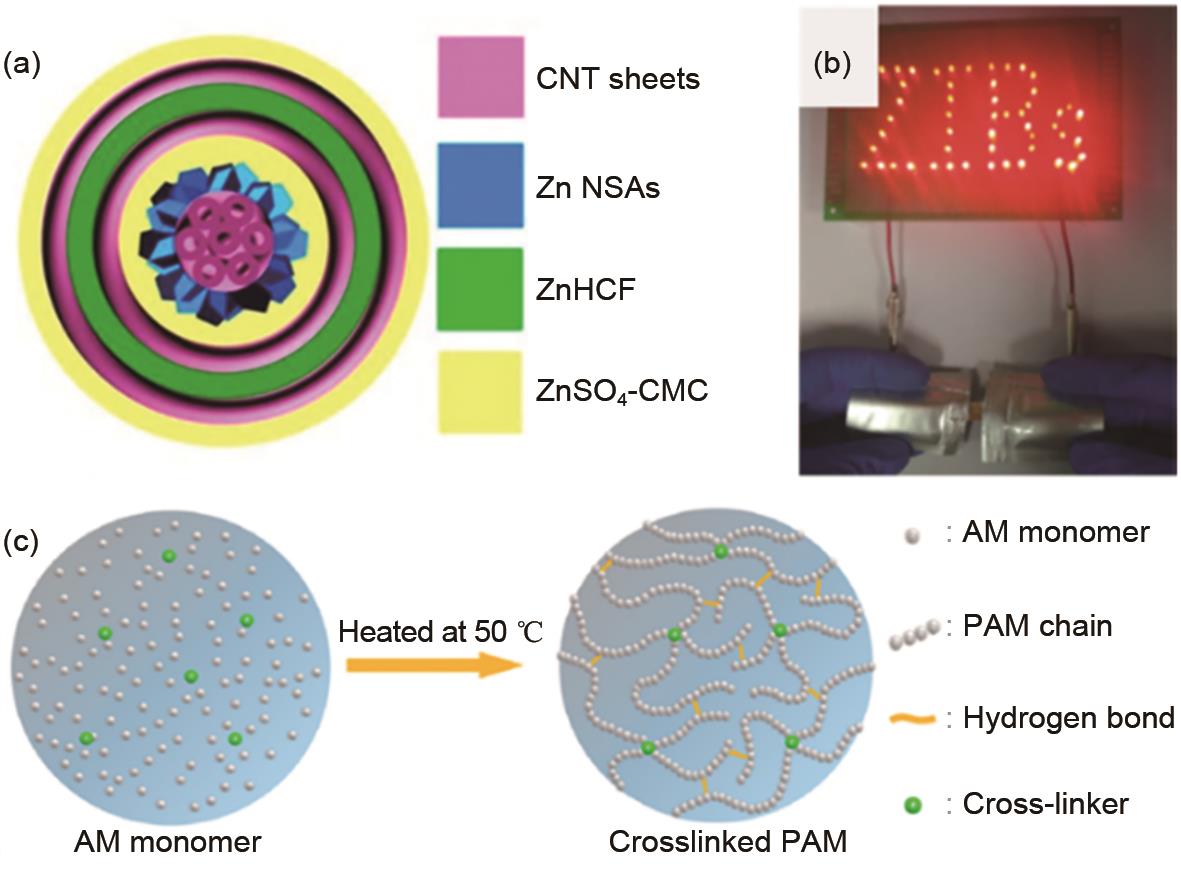
Table 1
The comparison of the electrolytes of rechargeable zinc-ion batteries"
| 分类 | 电解质 | 正极 | 离子电导率 /(mS/cm) | 电化学稳定电势窗 | 成本 | 参考文献 |
|---|---|---|---|---|---|---|
| 水溶液 | 2 mol/L ZnSO4+0.2 mol/L MnSO4 | (Akhtenskite)MnO2@CFP | — | 1.0~1.8 V | 低 | [ |
| 0.5 mol/L Zn(CH3COO)2 | Na3V2(PO4)3/C | — | 0.8~1.7 V | 低 | [ | |
| 3 mol/L Zn(CF3SO3)2 | (Spinel)ZnMn2O4/carbon | 3470 | 0.8~1.9 V | 高 | [ | |
| 0.3 mol/L Zn(TFSI)2 | Fe5V15O39(OH)9∙9H2O | — | 0.4~1.6 V | 高 | [ | |
| 20 mol/kg LiTFSI+1 mol/kg Zn(TFSI)2 | LiMn2O4 | — | 0.8~2.1 V | 高 | [ | |
| 21 mol/L LiTFSI + 1 mol/L Zn(CF3SO3)2 | VOPO4 | — | 0.8~2.1 V | 高 | [ | |
30 mmol/L ZnCl 1 mol/L ZnCl | Ca0.20V2O5∙0.80H2O | 12.7 101 | 0.25~2.0 V 0.25~1.3 V | 高 低 | [ | |
| 2 mol/L ZnSO4(原)+50%体积的甲醇 | 聚苯胺(PANI) | 16.8 (原56.9) | 0.6~1.6 V | 低 低 | [ | |
| 1 mol/L ZnSO4(原)+10 mmol/L葡萄糖 | MnO2 | 45.3~45.7 (原43.5~43.8) | 1.0~1.9 V | 低 低 | [ | |
| 3 mol/L Zn(CF3SO3)2+0.1 mol/L Mn(CF3SO3)2 | β-MnO2 | 6000 | 0.8~1.9 V | 高 | [ | |
| 1 mol/L ZnSO4+1 mol/L Na2SO4 | NaV3O8∙1.5H2O | — | 0.3~1.25 V | 低 | [ | |
| 有机溶液 | 0.5 mol/L AN-Zn(TFSI)2 | 双层水合V2O5 | — | 0.3~1.5 V | 高 | [ |
| 0.5 mol/L Zn(OTf)2/TMP-DMC(体积比=1∶1) | VS2 | 4.90 | 0.3~1.0 V | 高 | [ | |
| 0.5 mol/L ZnTFMS/DMF | PQ-MCT | 18.9 | 0.1~1.7 V | 较高 | [ | |
| * | 0.5 mol/L Zn(CF3SO3)2-TEP∶H2O(体积比=7∶3) | KCuHCf | 6.48 | 1.3~2.0 V | 较高 | [ |
| 凝胶 | 约0.44 mol/L ZnSO4/CMC(CMC∶水=3:80) | ZnHCF | — | 1.0~2.1 V | 较低 | [ |
| 2 mol/L ZnSO4+0.1 mol/L MnSO4/gum(gum∶水=1∶5) | MnO2 | 14.6 | 1.0~1.8 V | 较低 | [ | |
| 3 mol/L LiCl+ 2 mol/L ZnCl2+0.4 mol/L MnSO4/ PVA(PVA∶水=1∶10) | MnO2@PEDOT | — | 1.0~1.8 V | 偏低 | [ | |
| 1 mol/L ZnSO4/gelatin(gelatin∶水=1∶4) | NaV3O8∙1.5H2O | — | 0.3~1.25 V | 较低 | [ | |
| 2 mol/L ZnSO4+0.1 mol/L MnSO4/PAM(AM∶水=1∶10) | MnO2 | 17.3 | 0.8~1.85 V | 较低 | [ | |
| 全固态 | 4 mol/L Zn(BF4)2+2 mmol/L Al(OTf)3/poly(1,3-dioxolane) | CoHCF | 19.6 | 0.5~2.05 V | 较高 | [ |
| 1 | DUNN B, KAMATH H, TARASCON J M. Electrical energy storage for the grid: A battery of choices[J]. Science, 2011, 334(6058): 928-935. |
| 2 | LIAO M, YE L, ZHANG Y, et al. The recent advance in fiber-shaped energy storage devices[J]. Advanced Electronic Materials, 2019, 5(1): 1800456. |
| 3 | YU P, ZENG Y X, ZHANG H Z, et al. Flexible Zn-ion batteries: Recent progresses and challenges[J]. Small, 2019, 15(7): 1804760. |
| 4 | YAMAMOTO T, SHOJI T. Rechargeable Zn∣ZnSO4∣MnO2-type cells[J]. Inorganica Chimica Acta, 1986, 117(2): L27-L28. |
| 5 | XU C J, LI B H, DU H D, et al. Energetic zinc ion chemistry: The rechargeable zinc ion battery[J]. Angewandte Chemie (International Ed in English), 2012, 51(4): 933-935. |
| 6 | KORDESH K, WEISSENBACHER M. Rechargeable alkaline manganese dioxide/zinc batteries[J]. Journal of Power Sources, 1994, 51(1/2): 61-78. |
| 7 | PAN H L, SHAO Y Y, YAN P F, et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions[J]. Nature Energy, 2016, 1: 16039. |
| 8 | SUN W, WANG F, HOU S, et al. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion[J]. Journal of the American Chemical Society, 2017, 139(29): 9775-9778. |
| 9 | ZHANG N, CHENG F Y, LIU Y C, et al. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery[J]. Journal of the American Chemical Society, 2016, 138(39): 12894-12901. |
| 10 | ZHOU J, SHAN L T, WU Z X, et al. Investigation of V2O5 as a low-cost rechargeable aqueous zinc ion battery cathode[J]. Chemical Communications (Cambridge, England), 2018, 54(35): 4457-4460. |
| 11 | ZHANG L Y, CHEN L, ZHOU X F, et al. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: The zinc/zinc hexacyanoferrate system[J]. Advanced Energy Materials, 2015, 5(2): 1400930. |
| 12 | LI W, WANG K L, CHENG S J, et al. An ultrastable presodiated titanium disulfide anode for aqueous "rocking-chair" zinc ion battery[J]. Advanced Energy Materials, 2019, 9(27): 1900993. |
| 13 | YAN L J, ZENG X M, LI Z H, et al. An innovation: Dendrite free quinone paired with ZnMn2O4 for zinc ion storage[J]. Materials Today Energy, 2019, 13: 323-330. |
| 14 | SONG M, TAN H, CHAO D L, et al. Recent advances in Zn-ion batteries[J]. Advanced Functional Materials, 2018, 28(41): 1802564. |
| 15 | HUANG J H, GUO Z W, MA Y Y, et al. Recent progress of rechargeable batteries using mild aqueous electrolytes[J]. Small Methods, 2019, 3(1): 1800272. |
| 16 | LI G L, YANG Z, JIANG Y, et al. Towards polyvalent ion batteries: A zinc-ion battery based on NASICON structured Na3V2(PO4)3[J]. Nano Energy, 2016, 25: 211-217. |
| 17 | PENG Z, WEI Q L, TAN S S, et al. Novel layered iron vanadate cathode for high-capacity aqueous rechargeable zinc batteries[J]. Chemical Communications (Cambridge, England), 2018, 54(32): 4041-4044. |
| 18 | WANG F, BORODIN O, GAO T, et al. Highly reversible zinc metal anode for aqueous batteries[J]. Nature Materials, 2018, 17(6): 543-549. |
| 19 | WAN F, ZHANG Y, ZHANG L L, et al. Reversible oxygen redox chemistry in aqueous zinc-ion batteries[J]. Angewandte Chemie (International Ed in English), 2019, 58(21): 7062-7067. |
| 20 | JI X L. A perspective of ZnCl2 electrolytes: The physical and electrochemical properties[J]. eScience, 2021, 1(2): 99-107. |
| 21 | ZHANG L, RODRÍGUEZ-PÉREZ I A, JIANG H, et al. ZnCl2 "water-in-salt" electrolyte transforms the performance of vanadium oxide as a Zn battery cathode[J]. Advanced Functional Materials, 2019, 29(30): 1902653. |
| 22 | 王心怡, 李维杰, 韩朝, 等. 水系锌离子电池金属负极的挑战与优化策略[J]. 储能科学与技术, 2022, 11(4): 1211-1225. |
| WANG X Y, LI W J, HAN C, et al. Challenges and optimization strategies of the anode of aqueous zinc-ion battery[J]. Energy Storage Science and Technology, 2022, 11(4): 1211-1225. | |
| 23 | HAO J N, YUAN L B, YE C, et al. Boosting zinc electrode reversibility in aqueous electrolytes by using low-cost antisolvents[J]. Angewandte Chemie (International Ed in English), 2021, 60(13): 7366-7375. |
| 24 | SUN P, MA L, ZHOU W H, et al. Simultaneous regulation on solvation shell and electrode interface for dendrite-free Zn ion batteries achieved by a low-cost glucose additive[J]. Angewandte Chemie (International Ed in English), 2021, 60(33): 18247-18255. |
| 25 | ZHANG N, CHENG F Y, LIU J X, et al. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities[J]. Nature Communications, 2017, 8: 405. |
| 26 | WAN F, ZHANG L L, DAI X, et al. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers[J]. Nature Communications, 2018, 9: 1656. |
| 27 | SENGUTTUVAN P, HAN S D, KIM S, et al. A high power rechargeable nonaqueous multivalent Zn/V2O5 battery[J]. Advanced Energy Materials, 2016, 6(24): 1600826. |
| 28 | NAVEED A, YANG H J, SHAO Y Y, et al. A highly reversible Zn anode with intrinsically safe organic electrolyte for long-cycle-life batteries[J]. Advanced Materials (Deerfield Beach, Fla), 2019, 31(36): e1900668. |
| 29 | WANG N, DONG X L, WANG B L, et al. Zinc-organic battery with a wide operation-temperature window from -70 to 150 ℃[J]. Angewandte Chemie (International Ed in English), 2020, 59(34): 14577-14583. |
| 30 | KUNDU D P, ADAMS B D, DUFFORT V, et al. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode[J]. Nature Energy, 2016, 1: 16119. |
| 31 | NAVEED A, YANG H J, YANG J, et al. Highly reversible and rechargeable safe Zn batteries based on a triethyl phosphate electrolyte[J]. Angewandte Chemie (International Ed in English), 2019, 58(9): 2760-2764. |
| 32 | HUANG S, ZHU J C, TIAN J L, et al. Recent progress in the electrolytes of aqueous zinc-ion batteries[J]. Chemistry (Weinheim an Der Bergstrasse, Germany), 2019, 25(64): 14480-14494. |
| 33 | SUN T L, KUROKAWA T, KURODA S, et al. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity[J]. Nature Materials, 2013, 12(10): 932-937. |
| 34 | ZHANG Q C, LI C W, LI Q L, et al. Flexible and high-voltage coaxial-fiber aqueous rechargeable zinc-ion battery[J]. Nano Letters, 2019, 19(6): 4035-4042. |
| 35 | ZHANG S L, YU N S, ZENG S, et al. An adaptive and stable bio-electrolyte for rechargeable Zn-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(26): 12237-12243. |
| 36 | ZENG Y, ZHANG X, MENG Y, et al. Achieving ultrahigh energy density and long durability in a flexible rechargeable quasi-solid-state Zn-MnO2 battery[J]. Advanced Materials (Deerfield Beach, Fla), 2017, 29(26): 2017Jul;29(26). |
| 37 | HUANG Yuan, ZHANG Jiyan, LIU Jiuwei, et al. Flexible and stable quasi-solid-state zinc ion battery with conductive guar gum electrolyte[J]. Materials Today Energy, 2019, 14: 100349. |
| 38 | HUANG Yuan, LIU Jiuwei, ZHANG Jiyan, et al. Flexible quasi-solid-state zinc ion batteries enabled by highly conductive carrageenan bio-polymer electrolyte[J]. RSC Advances, 2019, 9: 16313-9. |
| 39 | MCEVOY H, ROSS-MURPHY S B, CLARK A H. Large deformation and ultimate properties of biopolymer gels: 1. Single biopolymer component systems[J]. Polymer, 1985, 26: 1483-92. |
| 40 | LI Hongfei, LIU Zhuoxin, LIANG Guojin, et al. Waterproof and tailorable elastic rechargeable yarn zinc ion batteries by a cross-linked polyacrylamide electrolyte[J]. ACS Nano, 2018, 12: 3140-8. |
| 41 | YANG Qi, LI Qing, LIU Zhuoxin, et al. Dendrites in Zn-based batteries[J]. Advanced Materials, 2020, 32: 2001854. |
| 42 | MA Longtao, CHEN Shengmei, LI Xinliang, et al. Liquid-free all-solid-state zinc batteries and encapsulation-free flexible batteries enabled by in situ constructed polymer electrolyte[J]. Angewandte Chemie International Edition, 2020, 59: 23836-44. |
| [1] | Huimin ZHANG, Jing WANG, Yibo WANG, Jiaxin ZHENG, Jingyi QIU, Gaoping CAO, Hao ZHANG. Multiscale modeling of the SEI of lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(2): 366-382. |
| [2] | Jinmei DONG, Qiyuan LIU, Fang WU, Lirui JIA, Jing WEN, Chenggong CHANG, Weixin ZHENG, Xueying XIAO. Phase change characteristics and proportion adjustment of fatty acid binary energy storage materials [J]. Energy Storage Science and Technology, 2023, 12(2): 349-356. |
| [3] | Mengyu TIAN, Yida WU, Junfeng HAO, Jing ZHU, Guanjun CEN, Ronghan QIAO, Xiaoyu SHEN, Hongxiang JI, Zhou JIN, Yuanjie ZHAN, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Xuejie HUANG. Reviews of selected 100 recent papers for lithium batteries (Oct. 1, 2022 to Nov. 30, 2022) [J]. Energy Storage Science and Technology, 2023, 12(1): 1-15. |
| [4] | Limu XIAO, Xin GAO, Shihai ZHANG, Xiankui WEN. Thermodynamic analysis on the liquid air energy storage system with liquid natural gas and organic Rankine cycle [J]. Energy Storage Science and Technology, 2023, 12(1): 155-164. |
| [5] | Ziwei YUAN, Chuyuan LIN, Ziyan YUAN, Xiaoli SUN, Qingrong QIAN, Qinghua CHEN, Lingxing ZENG. The research process on low temperature performance of zinc ion batteries [J]. Energy Storage Science and Technology, 2023, 12(1): 278-298. |
| [6] | Jing ZHU, Yida WU, Junfeng HAO, Guanjun CEN, Ronghan QIAO, Xiaoyu SHEN, Mengyu TIAN, Hongxiang JI, Zhou JIN, Yuanjie ZHAN, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Xuejie HUANG. Reviews of selected 100 recent papers for lithium batteries (Jun. 1, 2022 to Jul. 31, 2022) [J]. Energy Storage Science and Technology, 2022, 11(9): 3035-3050. |
| [7] | Jun ZHANG, Qi LI, Ying TAO, Quanhong YANG. Sieving carbons for sodium-ion batteries: Origin and progress [J]. Energy Storage Science and Technology, 2022, 11(9): 2825-2833. |
| [8] | Jinghua WU, Jing YANG, Gaozhan LIU, Zhiyan WANG, Zhihua ZHANG, Hailong YU, Xiayin YAO, Xuejie HUANG. Review and prospective of solid-state lithium batteries in the past decade (2011—2021) [J]. Energy Storage Science and Technology, 2022, 11(9): 2713-2745. |
| [9] | Qunbin ZHANG, Tao DONG, Jingjing LI, Yanxia LIU, Haitao ZHANG. Research progress on the recovery and high-value utilization of spent electrolyte from lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2798-2810. |
| [10] | Pengbo ZHAI, Dongmei CHANG, Zhijie BI, Ning ZHAO, Xiangxin GUO. Research progress on key interfacial issues in lithium lanthanum zirconium oxide-based solid-state [J]. Energy Storage Science and Technology, 2022, 11(9): 2847-2865. |
| [11] | Jianxin LU, Ying ZHANG, Chuyuan MA, Kang DENG, Chunying LEI. Study on fire-extinguishing performance of hydrogel on lithium-iron-phosphate batteries [J]. Energy Storage Science and Technology, 2022, 11(8): 2637-2644. |
| [12] | Xiaoyu SHEN, Guanjun CEN, Ronghan QIAO, Jing ZHU, Hongxiang JI, Mengyu TIAN, Zhou JIN, Yong YAN, Yida WU, Yuanjie ZHAN, Hailong YU, Liubin BEN, Yanyan LIU, Xuejie HUANG. Reviews of selected 100 recent papers for lithium batteries (Apr. 1, 2022 to May 31, 2022) [J]. Energy Storage Science and Technology, 2022, 11(7): 2007-2022. |
| [13] | Sida HUO, Wendong XUE, Xinli LI, Yong LI. Visualization analysis of composite electrolytes for lithium battery based on CiteSpace [J]. Energy Storage Science and Technology, 2022, 11(7): 2103-2113. |
| [14] | ZHANG Hong, ZHANG Yang, ZHAO Yao, WANG Jiulin. Research progress of sulfur cathode in solid-solid conversion reaction [J]. Energy Storage Science and Technology, 2022, 11(6): 1919-1933. |
| [15] | ZHOU Weidong, HUANG Qiu, XIE Xiaoxin, CHEN Kejun, LI Wei, QIU Jieshan. Research progress of polymer electrolyte for solid state lithium batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1788-1805. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
