Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (2): 366-382.doi: 10.19799/j.cnki.2095-4239.2022.0504
• Energy Storage Materials and Devices • Previous Articles Next Articles
Huimin ZHANG1( ), Jing WANG2, Yibo WANG1, Jiaxin ZHENG3, Jingyi QIU1, Gaoping CAO1, Hao ZHANG1(
), Jing WANG2, Yibo WANG1, Jiaxin ZHENG3, Jingyi QIU1, Gaoping CAO1, Hao ZHANG1( )
)
Received:2022-09-05
Revised:2022-09-15
Online:2023-02-05
Published:2023-02-24
Contact:
Hao ZHANG
E-mail:zhanghuimin_506@126.com;dr.h.zhang@hotmail.com
CLC Number:
Huimin ZHANG, Jing WANG, Yibo WANG, Jiaxin ZHENG, Jingyi QIU, Gaoping CAO, Hao ZHANG. Multiscale modeling of the SEI of lithium-ion batteries[J]. Energy Storage Science and Technology, 2023, 12(2): 366-382.
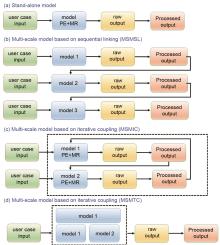
Fig. 3
Workflows of (a) stand-alone models; (b) multiscale models based on sequential linking (MSMSL); (c) multiscale models based on iterative coupling (MSMIC); and (d) multiscale models based on tight coupling (MSMTC). “PE” refers to “physical equation” (mathematical equation based on a fundamental physics theory which defines the relations between physics quantities of an entity) and “MR” to material relation (materials specific equation providing a value for a parameter in the physics equation)[13]"
Table 1
Main stream MSMs for SEI study[1]"
| Methods | MSM types | Scientific problems of SEI studied |
|---|---|---|
| First principle MD | MSMSL | Composition and decomposition reactions |
| Reactive MD | MSMSL | Decomposition reactions |
| Classical MD | MSMSL | Transport and mechanical properties |
| Hybrid MC MD | MSMIC | Formation process |
| kMC | MSMSL | Formation process, ion diffusion |
| Macroscopic models | MSMTC | Cell capacity face |
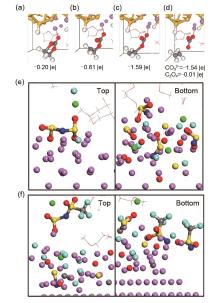
Fig. 4
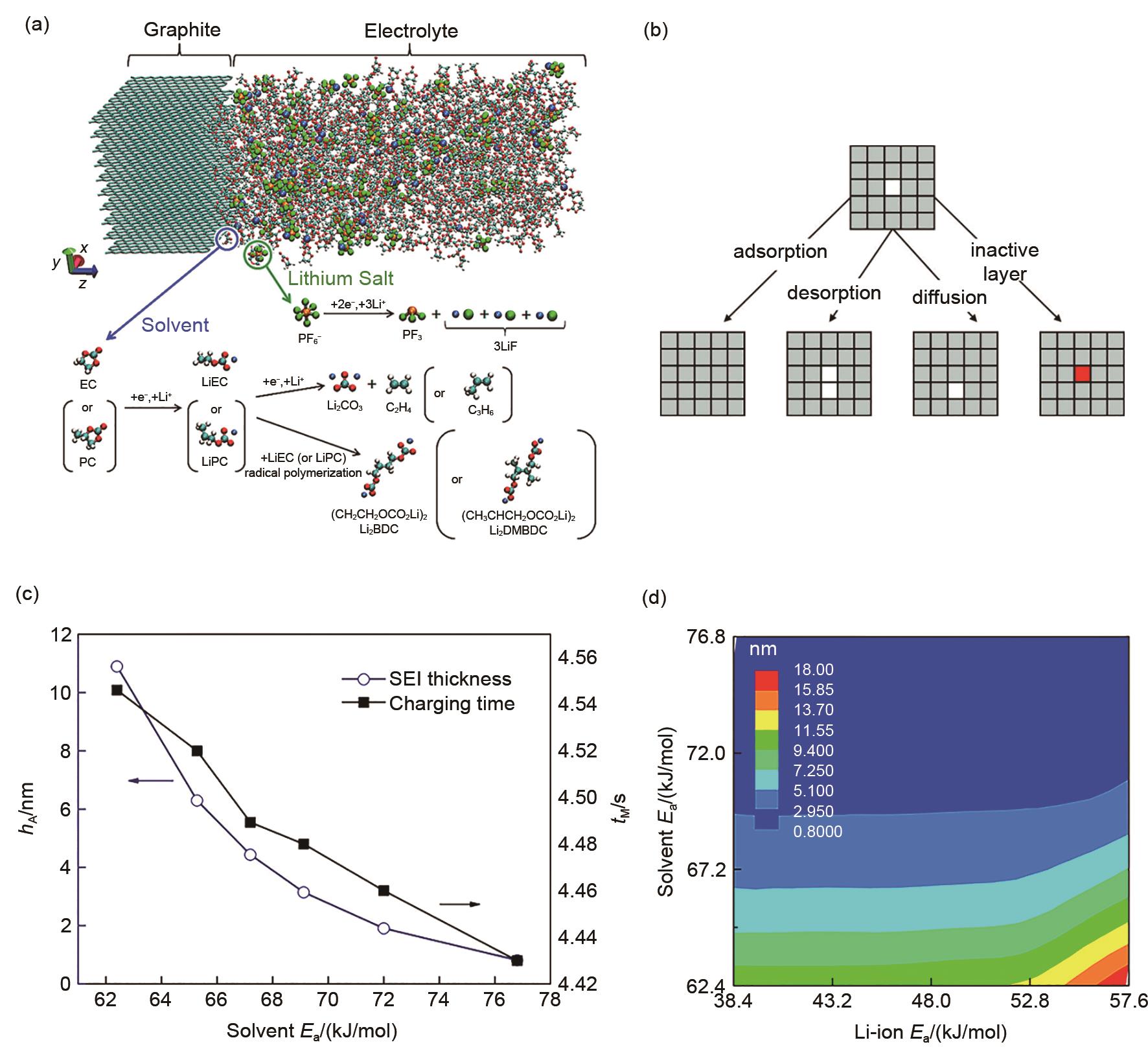
Decomposition of EC. Initially one electron is transferred from the surface to (a) Li-EC, causing a (b) Ccarbonyl —Oring bond to break. Subsequently, a second electron is transferred to (c) the EC- radical anion, triggering the breaking of a second Ccarbonyl —Oring bond and (d) generating the C2H4+CO32-pair. The net charges of the EC molecule and the CO32-/ C2H4 products are shown[43]. SEI after 16 ps of simulation using 4 mol/L (e) LiFSI or (f) LiTFSI in DME electrolytes vs. a lithium surface[49]"
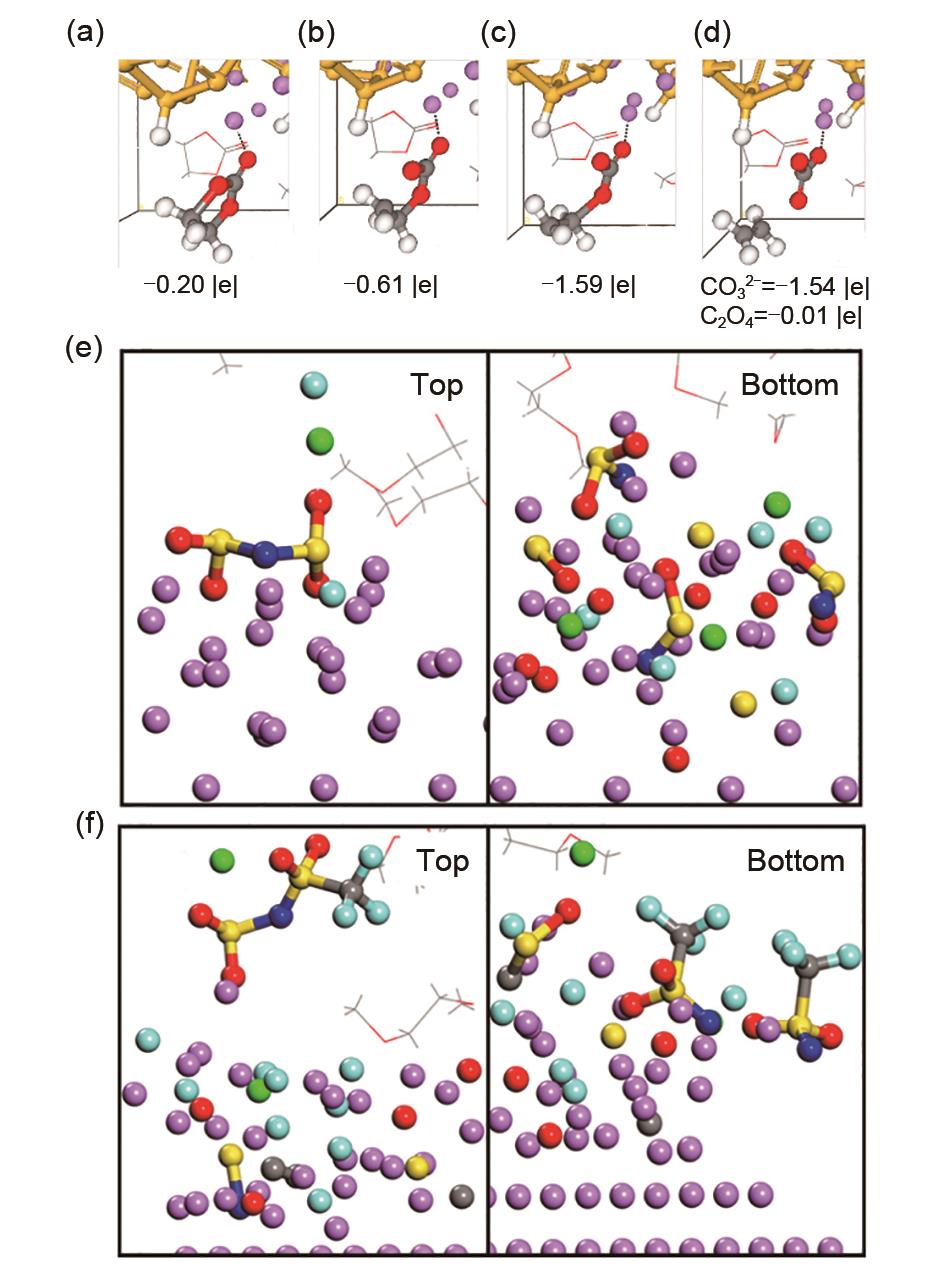
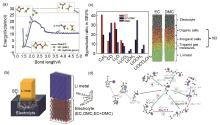
Fig. 5
(a) Energy profile for breaking C2H4 from o-EC/Li+, as obtained from QC calculations and gas phase simulations using ReaxFF at 10 K[63]; (b) Schematic of a Li metal electrode dipped in an electrolyte (left) and initial configuration of the cell (right)[64]; (c) Distribution of the SEI components for different electrolytes (left). Atomic configurations from MD simulations; the components of the SEI are identified (right)[64]; (d) Potential energy profile for the reduction of EC/Li+ and the radical termination reactions according to various pathways. ΔER and ΔEB denote the reaction energy and reaction barrier, respectively. Color scheme: cyan, carbon; white, hydrogen; red, oxygen; purple, Li+; large blue sphere, electron[70]"
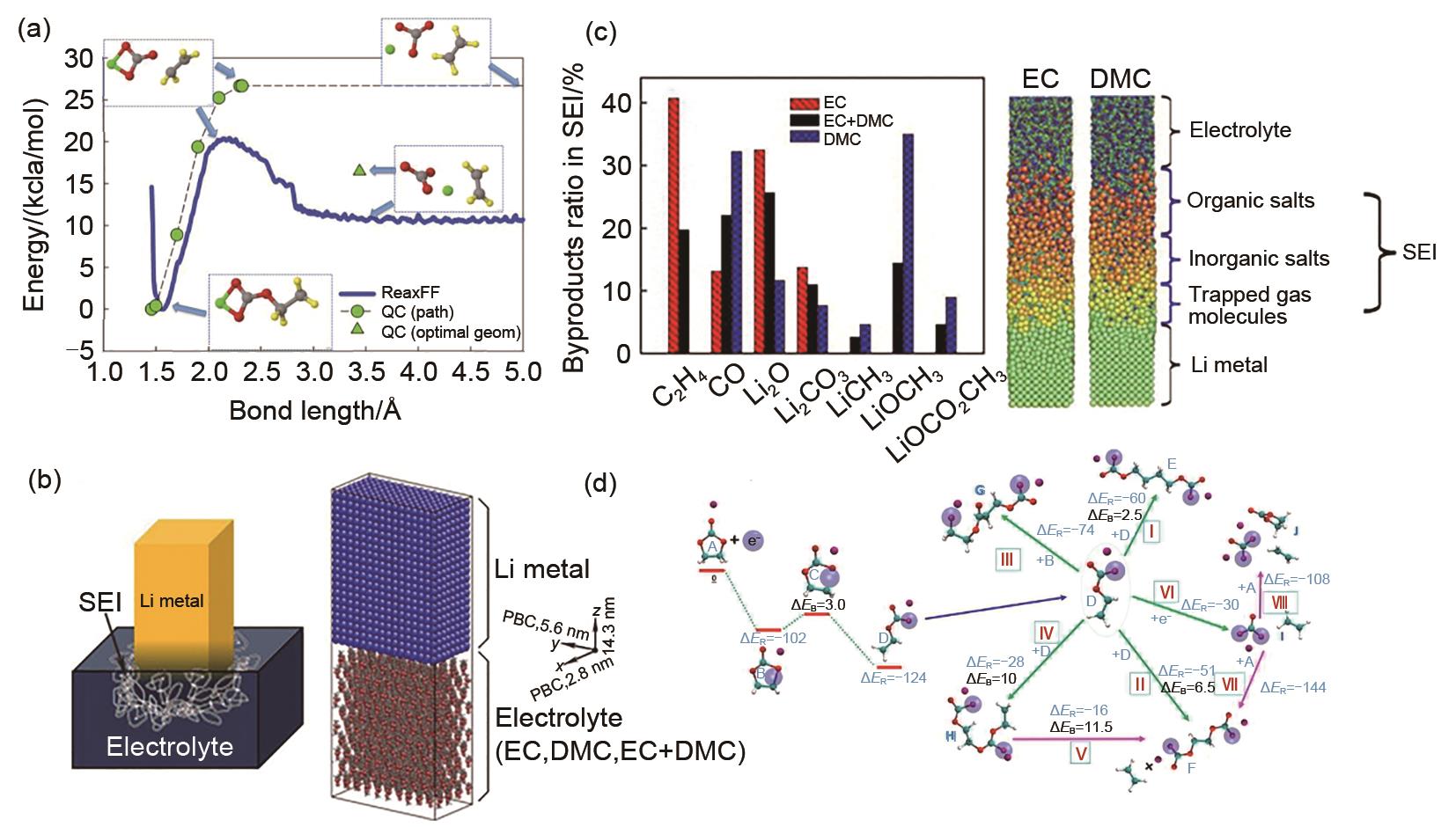

Fig. 6
(a) Li+ cation diffusion coefficients for ordered and amorphous SEI models consisting of Li2BDC and Li2EDC[54]; (b) Snapshots highlighting the Li+ distribution in ordered Li2BDC and Li2EDC, and amorphous Li2BDC, all at 393 K; Representative Li+-anion coordination in the double layer at (c) negative electrode potentials and (d) positive electrode potentials. Red and blue bars indicate the (x, y) plane of the anode and cathode surfaces, respectively[76]"
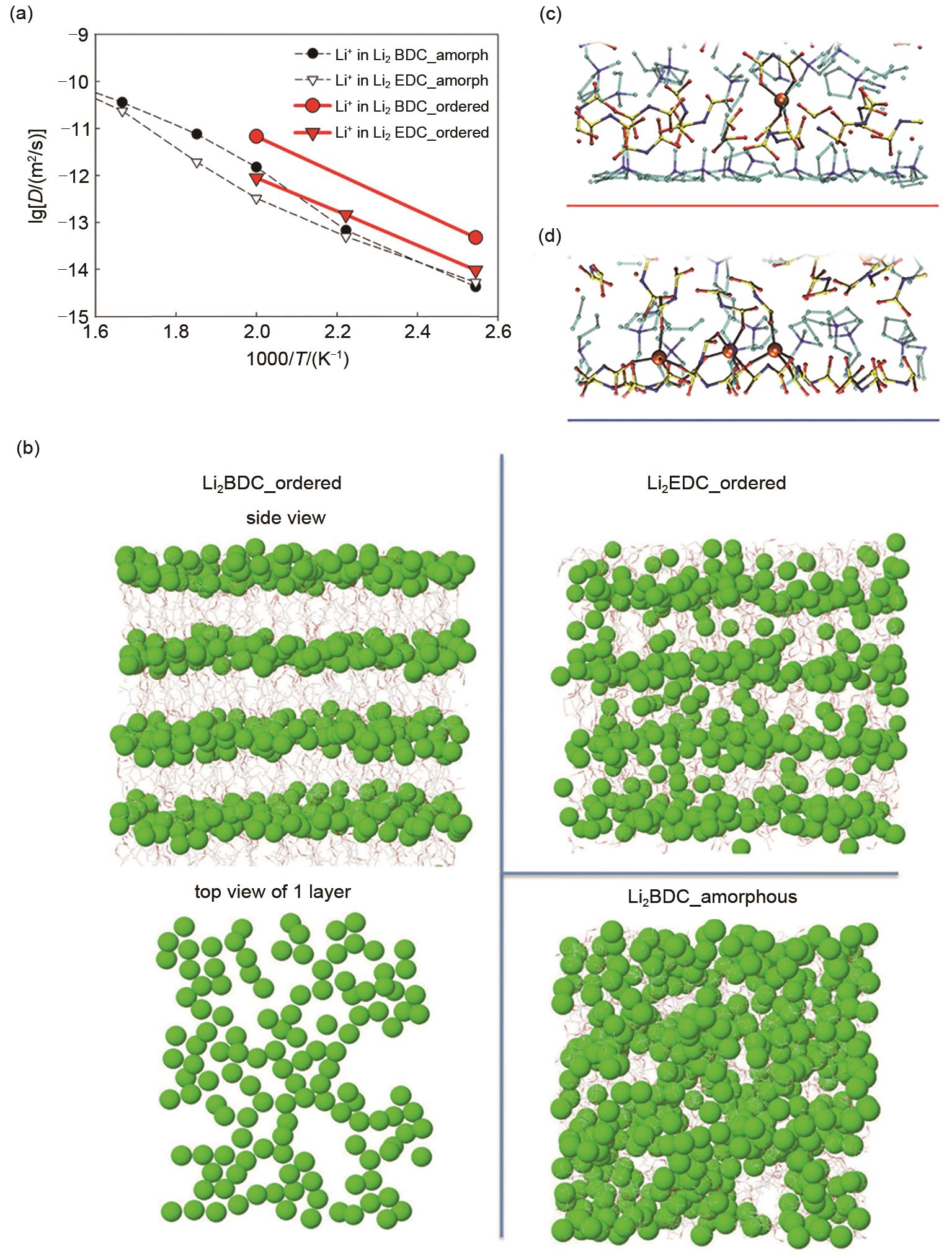
Fig. 7
(a) Model system and reaction scheme (cyan, carbon; red, oxygen; white, hydrogen; orange, phosphorus; green, fluorine; blue, lithium) [77]. (b) Schematic representation of the 4 types of events selected to occur in the kMC simulations[79]. (c, d) Effect of SEI solvent activation energy on the total charging time and SEI thickness during a full charging process [80]"
| 1 | FRANCO A A, RUCCI A, BRANDELL D, et al. Boosting rechargeable batteries R&D by multiscale modeling: Myth or reality?[J]. Chemical Reviews, 2019, 119(7): 4569-4627. |
| 2 | CHENG X B, ZHANG R, ZHAO C Z, et al. A review of solid electrolyte interphases on lithium metal anode[J]. Advanced Science (Weinheim, Baden-Wurttemberg, Germany), 2015, 3(3): doi: 10.1002/advs.201500213. |
| 3 | PINSON M B, BAZANT M Z. Theory of SEI formation in rechargeable batteries: Capacity fade, accelerated aging and lifetime prediction[J]. Journal of the Electrochemical Society, 2012, 160(2): A243-A250. |
| 4 | WU H P, JIA H, WANG C M, et al. Recent progress in understanding solid electrolyte interphase on lithium metal anodes[J]. Advanced Energy Materials, 2021, 11(5): doi: 10.1002/aenm.202003092. |
| 5 | 于沛平, 许亮, 麻冰云, 等. 多尺度模拟研究固体电解质界面[J]. 储能科学与技术, 2022, 11(3): 921-928. |
| YU P P, XU L, MA B Y, et al. Multiscale simulation of a solid electrolyte interphase[J]. Energy Storage Science and Technology, 2022, 11(3): 921-928. | |
| 6 | SUO L M, OH D, LIN Y X, et al. How solid-electrolyte interphase forms in aqueous electrolytes[J]. Journal of the American Chemical Society, 2017, 139(51): 18670-18680. |
| 7 | LI Y Z, LI Y B, PEI A, et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy[J]. Science, 2017, 358(6362): 506-510. |
| 8 | AURBACH D, EIN-ELY Y, ZABAN A. The surface chemistry of lithium electrodes in alkyl carbonate solutions[J]. Journal of the Electrochemical Society, 1994, 141(1): L1-L3. |
| 9 | PELED E, GOLODNITSKY D, ARDEL G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes[J]. Journal of the Electrochemical Society, 1997, 144(8): L208-L210. |
| 10 | XU Y B, WU H P, HE Y, et al. Atomic to nanoscale origin of vinylene carbonate enhanced cycling stability of lithium metal anode revealed by cryo-transmission electron microscopy[J]. Nano Letters, 2020, 20(1): 418-425. |
| 11 | HERMANSSON K. The European Materials Modelling Council[EB/OL]. [2022-08-01]. https://emmc.eu/. |
| 12 | BRAUNSCHWEIG D. Input-process-output Model[EB/OL]. [2022-08-01]. https://harpercollege.pressbooks.pub/programmingfundamentals/chapter/input-process-output-model/. |
| 13 | ROSSO L, BAAS A F D. What makes a material function? Let me compute the ways…Modelling in FP7 NMP programme-materials projects[M]. Luxembourg, 2012. |
| 14 | OCHTERSKI J W. Thermochemistry in Gaussian[EB/OL]. 2000[2022-08-01]. https://gaussian.com/thermo/. |
| 15 | GORDON M. Mark Gordon's quantum theory group homepage[EB/OL]. [2022-08-01]. http://www.msg.ameslab.gov/gamess/. |
| 16 | CARCÍA A, PAPIOR N, AKHTAR A, et al. Siesta: Recent developments and applications[J]. The Journal of Chemical Physics, 2020, 152: doi: 10.1016/j.jma.2020.06.021. |
| 17 | LIU Y, YU H, SUN Y, et al. Screening deep eutectic solvents for CO2 capture with COSMO-RS [J]. Frontiers in Chemisty, 2020, 8: 82. |
| 18 | TURK I. Practical MATLAB: With modeling, simulation, and processing projects[M]. 1st ed. Apress, 2019. |
| 19 | LI Z, JAFARYAR M. Nanofluid for convective heat transfer in various geometries: Nanofluid applications[M]. LAP LAMBERT Academic Publishing, 2018. |
| 20 | COMSOL Corporation. COMSOL multiphysics® modeling software[EB/OL]. [2022-08-01]. https://www.comsol.com/. |
| 21 | CAI L, WHITE R E. Mathematical modeling of a lithium ion battery with thermal effects in COMSOL Inc. Multiphysics (MP) software[J]. Journal of Power Sources, 2011, 196(14): 5985-5989. |
| 22 | SIEMENS Corporation. Engineer innovation with multiphysics computational fluid dynamics (CFD) simulation[EB/OL]. [2022-08-01]. https://mdx.plm.automation.siemens.com/star-ccm-plus/. |
| 23 | KRESSE G, FURTHMÜLLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Physical Review B, Condensed Matter, 1996, 54(16): 11169-11186. |
| 24 | DOVESI R, ERBA A, ORLANDO R, et al. Quantum-mechanical condensed matter simulations with CRYSTAL[J]. WIREs Computational Molecular Science, 2018, 8(4): e1360. |
| 25 | BLAHA P, SCHWARZ K, MADSEN G K H, et al. WIEN2k, an augmented plane wave plus local orbitals program for calculating crystal properties [R/OL]. Vienna University of Technology. 2001[2022-08-01]. https://www.researchgate.net/publication/237132866. |
| 26 | SOUTHERN S A, NAG T, KUMAR V, et al. NMR response of the tetrel bond donor[J]. Journal of Physical Chemistry C, 2022, 126(1): 851-865. |
| 27 | RATCLIFF L E, GRISANTI L, GENOVESE L, et al. Toward fast and accurate evaluation of charge on-site energies and transfer integrals in supramolecular architectures using linear constrained density functional theory (CDFT)-based methods[J]. Journal of Chemical Theory and Computation, 2015, 11(5): 2077-2086. |
| 28 | GABRIEL M A, DEUTSCH T, FRANCO A A. Fullerene-based materials as catalysts for fuel cells[J]. ECS Transactions, 2010, 25(22): 1-6. |
| 29 | VALIEV M, BYLASKA E J, GOVIND N, et al. NWChem: A comprehensive and scalable open-source solution for large scale molecular simulations[J]. Computer Physics Communications, 2010, 181(9): 1477-1489. |
| 30 | PÁLL S, ZHMUROV A, BAUER P, et al. Heterogeneous parallelization and acceleration of molecular dynamics simulations in GROMACS[J]. Journal Of Chemical Physics, 2020, 153: doi: 10.1063/5.0018516. |
| 31 | THOMPSON A P, AKTULGA H M, BERGER R, et al. LAMMPS-a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales[J]. Computer Physics Communications, 2022, 271: doi: 10.1016/j.cpc.2021.108171. |
| 32 | CASE D A, CHEATHAM T E, DARDEN III T, et al. The Amber biomolecular simulation programs[J]. Journal of Computational Chemistry, 2005, 26(16): 1668-1688. |
| 33 | KARPLUS M. Chemistry at harvard macromolecular mechanics[EB/OL]. [2022-08-01]. https://www.charmm.org/. |
| 34 | ILIAN T. Scientific computing department: the DL_POLY molecular simulation package[EB/OL]. [2022-08-01]. https://www.scd.stfc.ac.uk/Pages/DL_POLY.aspx/. |
| 35 | KNIME Corporation. Open for Innovation[EB/OL]. [2022-08-01]. https://www.knime.com/. |
| 36 | UHRIN M, HUBER S P, YU J, et al. Workflows in AiiDA: Engineering a high-throughput, event-based engine for robust and modular computational workflows[J]. Computational Materials Science, 2021, 187: doi: 10.1016/j.commatsci.2020.110086. |
| 37 | BLACK G D. Extensible Computational Chemistry Environment[EB/OL]. [2022-08-01]. https://ecce.emsl.pnl.gov/. |
| 38 | UNICORE Corporation. Distributed computing and data resources[EB/OL]. [2022-08-01]. https://www.unicore.eu/. |
| 39 | GANESH P, KENT P R C, JIANG D E. Solid-electrolyte interphase formation and electrolyte reduction at Li-ion battery graphite anodes: Insights from first-principles molecular dynamics[J]. The Journal of Physical Chemistry C, 2012, 116(46): 24476-24481. |
| 40 | LEUNG K, BUDZIEN J L. Ab initio molecular dynamics simulations of the initial stages of solid-electrolyte interphase formation on lithium ion battery graphitic anodes[J]. Physical Chemistry Chemical Physics: PCCP, 2010, 12(25): 6583-6586. |
| 41 | BRENNAN M D, BREEDON M, BEST A S, et al. Surface reactions of ethylene carbonate and propylene carbonate on the Li(001) surface[J]. Electrochimica Acta, 2017, 243: 320-330. |
| 42 | YU J M, BALBUENA P B, BUDZIEN J, et al. Hybrid DFT functional-based static and molecular dynamics studies of excess electron in liquid ethylene carbonate[J]. Journal of the Electrochemical Society, 2011, 158(4): A400. |
| 43 | MARTINEZ DE LA HOZ J M, LEUNG K, BALBUENA P B. Reduction mechanisms of ethylene carbonate on si anodes of lithium-ion batteries: Effects of degree of lithiation and nature of exposed surface[J]. ACS Applied Materials & Interfaces, 2013, 5(24): 13457-13465. |
| 44 | MORADABADI A, BAKHTIARI M, KAGHAZCHI P. Effect of anode composition on solid electrolyte interphase formation[J]. Electrochimica Acta, 2016, 213: 8-13. |
| 45 | LEUNG K, QI Y, ZAVADIL K R, et al. Using atomic layer deposition to hinder solvent decomposition in lithium ion batteries: First-principles modeling and experimental studies[J]. Journal of the American Chemical Society, 2011, 133(37): 14741-14754. |
| 46 | CHEN X, HOU T Z, LI B, et al. Towards stable lithium-sulfur batteries: Mechanistic insights into electrolyte decomposition on lithium metal anode[J]. Energy Storage Materials, 2017, 8: 194-201. |
| 47 | CAMACHO-FORERO L E, SMITH T W, BERTOLINI S, et al. Reactivity at the lithium-metal anode surface of lithium-sulfur batteries[J]. The Journal of Physical Chemistry C, 2015, 119(48): 26828-26839. |
| 48 | YILDIRIM H, HASKINS J B, BAUSCHLICHER C W Jr, et al. Decomposition of ionic liquids at lithium interfaces. 1. Ab initio molecular dynamics simulations[J]. The Journal of Physical Chemistry C, 2017, 121(51): 28214-28234. |
| 49 | CAMACHO-FORERO L E, SMITH T W, BALBUENA P B. Effects of high and low salt concentration in electrolytes at lithium-metal anode surfaces[J]. The Journal of Physical Chemistry C, 2017, 121(1): 182-194. |
| 50 | SODEYAMA K, YAMADA Y, AIKAWA K, et al. Sacrificial anion reduction mechanism for electrochemical stability improvement in highly concentrated Li-salt electrolyte[J]. The Journal of Physical Chemistry C, 2014, 118(26): 14091-14097. |
| 51 | PIPER D M, EVANS T, LEUNG K, et al. Stable silicon-ionic liquid interface for next-generation lithium-ion batteries[J]. Nature Communications, 2015, 6: 6230. |
| 52 | LEUNG K, JUNGJOHANN K L. Spatial heterogeneities and onset of passivation breakdown at lithium anode interfaces[J]. The Journal of Physical Chemistry C, 2017, 121(37): 20188-20196. |
| 53 | BENITEZ L, SEMINARIO J M. Electron transport and electrolyte reduction in the solid-electrolyte interphase of rechargeable lithium ion batteries with silicon anodes[J]. The Journal of Physical Chemistry C, 2016, 120(32): 17978-17988. |
| 54 | BEDROV D, BORODIN O, HOOPER J B. Li+ transport and mechanical properties of model solid electrolyte interphases (SEI): Insight from atomistic molecular dynamics simulations[J]. The Journal of Physical Chemistry C, 2017, 121(30): 16098-16109. |
| 55 | SHIN H, PARK J, HAN S, et al. Component-/structure-dependent elasticity of solid electrolyte interphase layer in Li-ion batteries: Experimental and computational studies. Journal of Power Sources, 2015, 277: 169-179. |
| 56 | LIU Z, QI Y, LIN Y X, et al. Interfacial study on solid electrolyte interphase at Li metal anode: Implication for Li dendrite growth[J]. Journal of the Electrochemical Society, 2016, 163(3): A592-A598. |
| 57 | SOTO F A, MA Y G, MARTINEZ DE LA HOZ J M, et al. Formation and growth mechanisms of solid-electrolyte interphase layers in rechargeable batteries[J]. Chemistry of Materials, 2015, 27(23): 7990-8000. |
| 58 | PAN J, ZHANG Q L, XIAO X C, et al. Design of nanostructured heterogeneous solid ionic coatings through a multiscale defect model[J]. ACS Applied Materials & Interfaces, 2016, 8(8): 5687-5693. |
| 59 | ZVEREVA E, CALISTE D, POCHET P. Interface identification of the solid electrolyte interphase on graphite[J]. Carbon, 2017, 111: 789-795. |
| 60 | SHI S Q, LU P, LIU Z Y, et al. Direct calculation of Li-ion transport in the solid electrolyte interphase[J]. Journal of the American Chemical Society, 2012, 134(37): 15476-15487. |
| 61 | OKUNO Y, USHIROGATA K, SODEYAMA K, et al. Decomposition of the fluoroethylene carbonate additive and the glue effect of lithium fluoride products for the solid electrolyte interphase: An ab initio study[J]. Physical Chemistry Chemical Physics: PCCP, 2016, 18(12): 8643-8653. |
| 62 | LEUNG K. First-principles modeling of Mn(II) migration above and dissolution from LixMn2O4 (001) surfaces[J]. Chemistry of Materials, 2017, 29(6): 2550-2562. |
| 63 | BEDROV D, SMITH G D, VAN DUIN A C T. Reactions of singly-reduced ethylene carbonate in lithium battery electrolytes: A molecular dynamics simulation study using the ReaxFF[J]. The Journal of Physical Chemistry A, 2012, 116(11): 2978-2985. |
| 64 | KIM S P, VAN DUIN A C T, SHENOY V B. Effect of electrolytes on the structure and evolution of the solid electrolyte interphase (SEI) in Li-ion batteries: A molecular dynamics study[J]. Journal of Power Sources, 2011, 196(20): 8590-8597. |
| 65 | PEREIRA-NABAIS C, ŚWIATOWSKA J, CHAGNES A, et al. Interphase chemistry of Si electrodes used as anodes in Li-ion batteries[J]. Applied Surface Science, 2013, 266: 5-16. |
| 66 | EDSTRÖM K, HERSTEDT M, ABRAHAM D P. A new look at the solid electrolyte interphase on graphite anodes in Li-ion batteries[J]. Journal of Power Sources, 2006, 153(2): 380-384. |
| 67 | PELED E, BAR TOW D, MERSON A, et al. Composition, depth profiles and lateral distribution of materials in the SEI built on HOPG-TOF SIMS and XPS studies[J]. Journal of Power Sources, 2001, 97/98: 52-57. |
| 68 | LU M, CHENG H, YANG Y. A comparison of solid electrolyte interphase (SEI) on the artificial graphite anode of the aged and cycled commercial lithium ion cells[J]. Electrochimica Acta, 2008, 53(9): 3539-3546. |
| 69 | YUN K S, PAI S J, YEO B C, et al. Simulation protocol for prediction of a solid-electrolyte interphase on the silicon-based anodes of a lithium-ion battery: ReaxFF reactive force field[J]. The Journal of Physical Chemistry Letters, 2017, 8(13): 2812-2818. |
| 70 | ISLAM M M, KOLESOV G, VERSTRAELEN T, et al. eReaxFF: A pseudoclassical treatment of explicit electrons within reactive force field simulations[J]. Journal of Chemical Theory and Computation, 2016, 12(8): 3463-3472. |
| 71 | CHENG T, MERINOV B V, MOROZOV S, et al. Quantum mechanics reactive dynamics study of solid Li-electrode/Li6PS5Cl-electrolyte interface[J]. ACS Energy Letters, 2017, 2(6): 1454-1459. |
| 72 | BORODIN O, ZHUANG G V, ROSS P N, et al. Molecular dynamics simulations and experimental study of lithium ion transport in dilithium ethylene dicarbonate[J]. The Journal of Physical Chemistry C, 2013, 117(15): 7433-7444. |
| 73 | BORODIN O, BEDROV D. Interfacial structure and dynamics of the lithium alkyl dicarbonate SEI components in contact with the lithium battery electrolyte[J]. The Journal of Physical Chemistry C, 2014, 118(32): 18362-18371. |
| 74 | JEONG S K, INABA M, IRIYAMA Y, et al. AFM study of surface film formation on a composite graphite electrode in lithium-ion batteries[J]. Journal of Power Sources, 2003, 119/120/121: 555-560. |
| 75 | JORN R, KUMAR R, ABRAHAM D P, et al. Atomistic modeling of the electrode-electrolyte interface in Li-ion energy storage systems: Electrolyte structuring[J]. The Journal of Physical Chemistry C, 2013, 117(8): 3747-3761. |
| 76 | HASKINS J B, WU J J, LAWSON J W. Computational and experimental study of Li-doped ionic liquids at electrified interfaces[J]. The Journal of Physical Chemistry C, Nanomaterials and Interfaces, 2016, 120(22): 11993-12011. |
| 77 | TAKENAKA N, SUZUKI Y, SAKAI H, et al. On electrolyte-dependent formation of solid electrolyte interphase film in lithium-ion batteries: Strong sensitivity to small structural difference of electrolyte molecules[J]. The Journal of Physical Chemistry C, 2014, 118(20): 10874-10882. |
| 78 | TAKENAKA N, SAKAI H, SUZUKI Y, et al. A computational chemical insight into microscopic additive effect on solid electrolyte interphase film formation in sodium-ion batteries: Suppression of unstable film growth by intact fluoroethylene carbonate[J]. The Journal of Physical Chemistry C, 2015, 119(32): 18046-18055. |
| 79 | METHEKAR R N, NORTHROP P W C, CHEN K J, et al. Kinetic Monte Carlo simulation of surface heterogeneity in graphite anodes for lithium-ion batteries: Passive layer formation[C]//Proceedings of the 2011 American Control Conference. June 29-July 1, 2011, San Francisco, CA, USA. IEEE, 2011: 1512-1517. |
| 80 | HAO F, LIU Z X, BALBUENA P B, et al. Mesoscale elucidation of solid electrolyte interphase layer formation in Li-ion battery anode[J]. The Journal of Physical Chemistry C, 2017, 121(47): 26233-26240. |
| 81 | RÖDER F, BRAATZ R D, KREWER U. Multi-scale simulation of heterogeneous surface film growth mechanisms in lithium-ion batteries[J]. Journal of the Electrochemical Society, 2017, 164(11): E3335-E3344. |
| 82 | SHINAGAWA C, USHIYAMA H, YAMASHITA K. Multiscale simulations for lithium-ion batteries: SEI film growth and capacity fading[J]. Journal of the Electrochemical Society, 2017, 164(13): A3018-A3024. |
| 83 | CHRISTENSEN J, NEWMAN J. A mathematical model for the lithium-ion negative electrode solid electrolyte interphase[J]. Journal of the Electrochemical Society, 2004, 151(11): A1977. |
| 84 | COLCLASURE A M, SMITH K A, KEE R J. Modeling detailed chemistry and transport for solid-electrolyte-interface (SEI) films in Li-ion batteries[J]. Electrochimica Acta, 2011, 58: 33-43. |
| 85 | SRIVASTAV S, XU C, EDSTRÖM K, et al. Modelling the morphological background to capacity fade in Si-based lithium-ion batteries[J]. Electrochimica Acta, 2017, 258: 755-763. |
| 86 | SAFARI M, MORCRETTE M, TEYSSOT A, et al. Multimodal physics-based aging model for life prediction of Li-ion batteries[J]. Journal of the Electrochemical Society, 2009, 156(3): A145. |
| 87 | LAWDER M T, NORTHROP P W C, SUBRAMANIAN V R. Model-based SEI layer growth and capacity fade analysis for EV and PHEV batteries and drive cycles[J]. Journal of the Electrochemical Society, 2014, 161(14): A2099-A2108. |
| [1] | Yulong ZHANG, Weiling LUAN, Senming WU. Quantitative analysis of the lithium plating-stripping process of lithium-ion batteries using external characteristic methods [J]. Energy Storage Science and Technology, 2023, 12(2): 529-535. |
| [2] | Shaojun NIU, Kai WU, Guobin ZHU, Yan WANG, Qunting QU, Honghe ZHENG. Studies on the swelling force during cycling of Si-based anodes in lithium ion batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2989-2994. |
| [3] | Qunbin ZHANG, Tao DONG, Jingjing LI, Yanxia LIU, Haitao ZHANG. Research progress on the recovery and high-value utilization of spent electrolyte from lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2798-2810. |
| [4] | Xianxi LIU, Anliang SUN, Chuan TIAN. Research on liquid cooling and heat dissipation of lithium-ion battery pack based on bionic wings vein channel cold plate [J]. Energy Storage Science and Technology, 2022, 11(7): 2266-2273. |
| [5] | Jianxiang DENG, Jinliang ZHAO, Chengde HUANG. High energy density lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(7): 2092-2102. |
| [6] | OU Yu, HOU Wenhui, LIU Kai. Research progress of smart safety electrolytes in lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1772-1787. |
| [7] | HAN Junwei, XIAO Jing, TAO Ying, KONG Debin, LV Wei, YANG Quanhong. Compact energy storage: Methodology with graphenes and the applications [J]. Energy Storage Science and Technology, 2022, 11(6): 1865-1873. |
| [8] | Lei LI, Zhao LI, Dan JI, Huichang NIU. Overcharge induced thermal runaway behaviors of pouch-type lithium-ion batteries with LFP and NCM cathodes: the differences and reasons [J]. Energy Storage Science and Technology, 2022, 11(5): 1419-1427. |
| [9] | Ce ZHANG, Siwu LI, Jia XIE. Research progress on the prelithiation technology of alloy-type anodes [J]. Energy Storage Science and Technology, 2022, 11(5): 1383-1400. |
| [10] | Nan LIN, Ulrike KREWER, Jochen ZAUSCH, Konrad STEINER, Haibo LIN, Shouhua FENG. Development and application of multiphysics models for electrochemical energy storage and conversion systems [J]. Energy Storage Science and Technology, 2022, 11(4): 1149-1164. |
| [11] | Peiping YU, Liang XU, Bingyun MA, Qintao SUN, Hao YANG, Yue LIU, Tao CHENG. Multiscale simulation of a solid electrolyte interphase [J]. Energy Storage Science and Technology, 2022, 11(3): 921-928. |
| [12] | Zhun NIU, Xueyan ZHANG, Jiawei FENG, Liguo JIN, Yonghui SHI, Jiayi YU, Zichao LI, Zhijun FENG. Preparation and electrochemical properties of FeSe2-C three-dimensional conductive composites [J]. Energy Storage Science and Technology, 2022, 11(11): 3470-3477. |
| [13] | Hongzhang ZHU, Chuanping WU, Tiannian ZHOU, Jie DENG. Thermal runaway characteristics of LiFePO4 and ternary lithium batteries with external overheating [J]. Energy Storage Science and Technology, 2022, 11(1): 201-210. |
| [14] | Lianbing LI, Sijia LI, Jie LI, Kun SUN, Zhengping WANG, Haiyue YANG, Bing GAO, Shaobo YANG. RUL prediction of lithium-ion battery based on differential voltage and Elman neural network [J]. Energy Storage Science and Technology, 2021, 10(6): 2373-2384. |
| [15] | Dajin LIU, Qiang WU, Renjie HE, Chuang YU, Jia XIE, Shijie CHENG. Research progress of biopolymers in Si anodes for lithium-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(6): 2156-2168. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
