Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (5): 1364-1379.doi: 10.19799/j.cnki.2095-4239.2023.0258
• Special Issue on Key Materials and Recycling Technologies for Energy Storage Batteries • Previous Articles Next Articles
Wenzhe HAN( ), Qingsong LAI, Xuanwen GAO(
), Qingsong LAI, Xuanwen GAO( ), Wenbin LUO
), Wenbin LUO
Received:2023-04-23
Revised:2023-05-06
Online:2023-05-05
Published:2023-05-29
Contact:
Xuanwen GAO
E-mail:2171581@stu.neu.edu.cn;gaoxuanwen@smm.neu.edu.cn
CLC Number:
Wenzhe HAN, Qingsong LAI, Xuanwen GAO, Wenbin LUO. Advances toward manganese-based layered oxide cathodes for potassium-ion batteries[J]. Energy Storage Science and Technology, 2023, 12(5): 1364-1379.
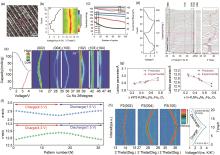
Fig. 3
(a) Enlarged HRTEM image of K0.77MnO2 ?H2O0.23[26]; (b) The first galvanostatic charge/discharge curves of the K0.77MnO2 ?H2O0.23 electrode at a current density of 20 mA/g and corresponding ex situ XRD patterns in the 2θ region of 10.5°~15.5°[26]; (c) Cycle performances and corresponding Coulombic efficiencies of the electrode at a current density of 100 mA/g for 100 cycles[26]; (d) In situ XRD characterization of K0.27(Mn0.98O2)?(H2O)0.53 electrode upon charge/discharge[43]; (e) Operando-XRD result of P2-K0.75[Ni1/3Mn2/3]O2 electrode[44]; (f) the corresponding calculated lattice parameters for o-XRD pattern[44]; (g)from experimentally calculated data and data predicted by first-principles calculations[13]; (h) Contour maps in selected 2θ ranges obtained from In operando XRD patterns of P2-K0.75MNFO2 during the charge-discharge process in the range of 1.5~3.9 V (K/K+)[13]"

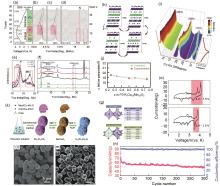
Fig. 4
(a) Typical charge/discharge profiles of P3-type K0.5MnO2 at a current rate of 2 mA/g; (b)~(d) In situ XRD pattern taken for 2 h scanning rate per pattern; (e) XRD peak comparison of as-prepared (scan #1), scan #7, and scan #14 P3-K0.5MnO2 at 18°~20°; (f) comparison with simulated XRD patterns of the O3 and P3structures[48]; (g) P3-K0.75MnO2 and P3-K0.75[Co0.5Mn0.5]O2; (h) Predicted structural change in P3-K x [Co0.5Mn0.5]O2 (0.25≤ x ≤0.75); (i) operando XRD patterns of P3-K x [Co0.5Mn0.5]O2; (j) comparison of c-lattice parameters of P3-K x [Co0.5Mn0.5]O2 obtained by first-principles calculations and operando XRD data[57]; (k) Schematic illustration of the synthesis of peanut-like P3-type K0.45Mn0.5Co0.5O2 microparticles[59]; (l), (m) SEM images of P3-type K0.45Mn0.5Co0.5O2; (n) long-term cycling performance of K0.45Mn0.5Co0.5O2 at 300 mA/g; (o) Cyclic voltammograms at a scan rate of 0.03 mV/s"

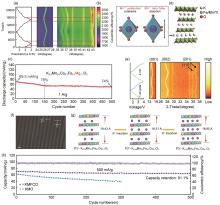
Fig. 5
(a) In situ XRD characterization of K0.5Mn0.6Co0.2Fe0.1Mg0.1O2[61]; (b) Visualization of Jahn-Teller distortion[61]; (c) Long-term cycling performance of K0.5Mn0.6Co0.2Fe0.1Mg0.1O2 at 1 A/g[61]; (e) In situ XRD patterns of K0.35Mn0.8Fe0.1Cu0.1O2[66]; (f) HRTEM images of K0.35Mn0.8Fe0.1Cu0.1O2[66]; (g) Structural changes in K x Mn0.8Fe0.1Cu0.1O2 during K+ insertion[66]; (h) Cycling performance of K0.35Mn0.8Fe0.1Cu0.1O2 and KMO at 500 mA/g[66]"
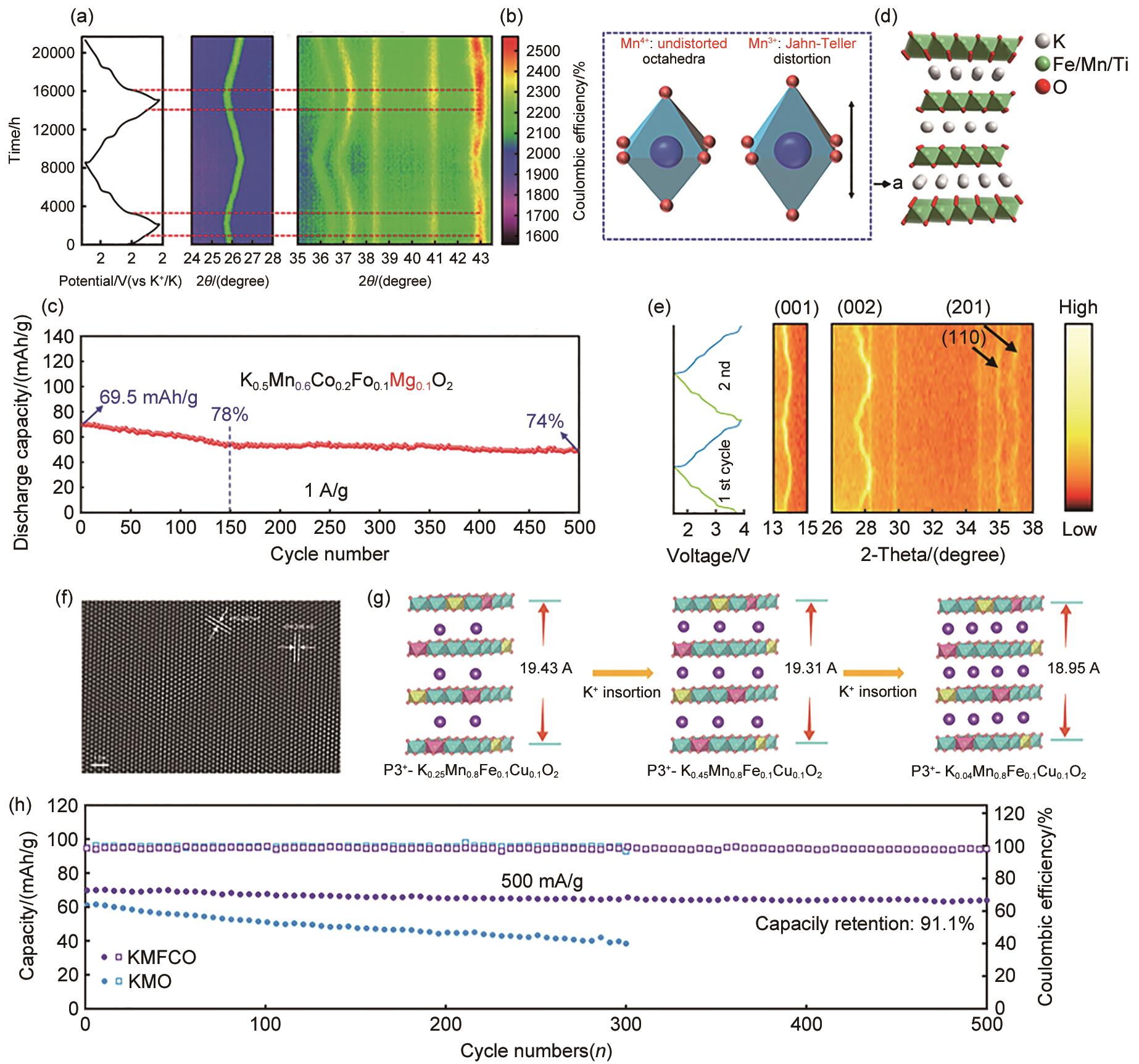
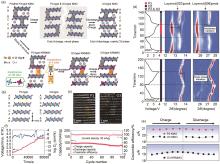
Fig. 6
(a) Schematic diagram of the effect of Rb and Mg substitution on the crystal structure; (b) The stacking of P3-type and O3-type layered structures along the c-axis direction of the TM slabs; (c) Representative HRTEM images of the K0.45Rb0.05Mn0.85Mg0.15O2 powder and K0.45Rb0.05Mn0.85Mg0.15O2 test cathode placed in the battery environment without cycling; (d) The voltage curves and representative corresponding in situ XRD patterns of K0.45MnO2 and K0.45Rb0.05Mn0.85Mg0.15O2 at 70 mA/g in the range of 1.5—3.9 V.; (e) The c-lattice parameter of K0.45MnO2 and K0.45Rb0.05Mn0.85Mg0.15O2 calculated from in situ XRD patterns; (f) Charge GITT result of K0.45Rb0.05Mn0.85Mg0.15O2 at 20 mA/g, and the corresponding DK+; (g) Cycling performance of K0.45Rb0.05Mn0.85Mg0.15O2 at 200 mA/g[69]"

| 1 | KANG B, CEDER G. Battery materials for ultrafast charging and discharging[J]. Nature, 2009, 458(7235): 190-193. |
| 2 | MANTHIRAM A. A reflection on lithium-ion battery cathode chemistry[J]. Nature Communications, 2020, 11(1): 1-9. |
| 3 | PRAMUDITA J C, SEHRAWAT D, GOONETILLEKE D, et al. An initial review of the status of electrode materials for potassium-ion batteries[J]. Advanced Energy Materials, 2017, 7(24): 1602911. |
| 4 | LARCHER D, TARASCON J M. Towards greener and more sustainable batteries for electrical energy storage[J]. Nature Chemistry, 2015, 7(1): 19-29. |
| 5 | XU Y S, DUAN S Y, SUN Y G, et al. Recent developments in electrode materials for potassium-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(9): 4334-4352. |
| 6 | KUBOTA K, DAHBI M, HOSAKA T, et al. Towards K-ion and Na-ion batteries as "beyond Li-ion"[J]. The Chemical Record, 2018, 18(4): 459-479. |
| 7 | LUO W, WAN J Y, OZDEMIR B, et al. Potassium ion batteries with graphitic materials[J]. Nano Letters, 2015, 15(11): 7671-7677. |
| 8 | WANG J, LIU Z M, ZHOU J, et al. Insights into metal/metalloid-based alloying anodes for potassium ion batteries[J]. ACS Materials Letters, 2021, 3(11): 1572-1598. |
| 9 | XUE L G, GAO H C, LI Y T, et al. Cathode dependence of liquid-alloy Na-K anodes[J]. Journal of the American Chemical Society, 2018, 140(9): 3292-3298. |
| 10 | HAN K, MENG J S, HONG X F, et al. Three-dimensional graphene-supported nickel disulfide nanoparticles promise stable and fast potassium storage[J]. Nanoscale, 2020, 12(15): 8255-8261. |
| 11 | WANG X P, XIAO Z T, HAN K, et al. Advances in fine structure optimizations of layered transition-metal oxide cathodes for potassium-ion batteries[J]. Advanced Energy Materials, 2023, 13(2): 2202861. |
| 12 | LI J Y, MANTHIRAM A. A comprehensive analysis of the interphasial and structural evolution over long-term cycling of ultrahigh-nickel cathodes in lithium-ion batteries[J]. Advanced Energy Materials, 2019, 9(45): 1902731. |
| 13 | HWANG J Y, KIM J, YU T Y, et al. A new P2-type layered oxide cathode with superior full-cell performances for K-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(37): 21362-21370. |
| 14 | XU Y S, ZHANG Q H, WANG D, et al. Enabling reversible phase transition on K5/9Mn7/9Ti2/9O2 for high-performance potassium-ion batteries cathodes[J]. Energy Storage Materials, 2020, 31: 20-26. |
| 15 | ZHANG L, ZHANG B W, WANG C R, et al. Constructing the best symmetric full K-ion battery with the NASICON-type K3V2(PO4)3[J]. Nano Energy, 2019, 60: 432-439. |
| 16 | CHONG S K, WU Y F, GUO S W, et al. Potassium nickel hexacyanoferrate as cathode for high voltage and ultralong life potassium-ion batteries[J]. Energy Storage Materials, 2019, 22: 120-127. |
| 17 | KAPAEV R R, ZHIDKOV I S, KURMAEV E Z, et al. Hexaazatriphenylene-based polymer cathode for fast and stable lithium-, sodium- and potassium-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(39): 22596-22603. |
| 18 | KIM U H, JUN D W, PARK K J, et al. Pushing the limit of layered transition metal oxide cathodes for high-energy density rechargeable Li ion batteries[J]. Energy & Environmental Science, 2018, 11(5): 1271-1279. |
| 19 | ZHANG H Y, XI K Y, JIANG K Z, et al. Enhanced K-ion kinetics in a layered cathode for potassium ion batteries[J]. Chemical Communications, 2019, 55(55): 7910-7913. |
| 20 | ZHANG H, WANG L, HE X M. Trends in a study on thermal runaway mechanism of lithium-ion battery with LiNixMnyCo1- x- yO2 cathode materials[J]. Battery Energy, 2022, 1(1): 20210011. |
| 21 | VAALMA C, GIFFIN G A, BUCHHOLZ D, et al. Non-aqueous K-ion battery based on layered K0.3MnO2 and hard carbon/carbon black[J]. Journal of the Electrochemical Society, 2016, 163(7): A1295-A1299. |
| 22 | LIU C L, LUO S H, HUANG H B, et al. Layered potassium-deficient P2- and P3-type cathode materials KxMnO2 for K-ion batteries[J]. Chemical Engineering Journal, 2019, 356: 53-59. |
| 23 | PATOUX S, DOLLÉ M, DOEFF M M. Layered Manganese oxide intergrowth electrodes for rechargeable lithium batteries. 2. substitution with Al[J]. Chemistry of Materials, 2005, 17(5): 1044-1054. |
| 24 | HUANG Z X, GU Z Y, HENG Y L, et al. Advanced layered oxide cathodes for sodium/potassium-ion batteries: Development, challenges and prospects[J]. Chemical Engineering Journal, 2023, 452: 139438. |
| 25 | SUN R, DONG S Y, XU F, et al. Co-intercalation strategy of constructing partial cation substitution of ammonium vanadate {(NH4)2V6O16} for stable zinc ion storage[J]. Dalton Transactions, 2022, 51(19): 7607-7612. |
| 26 | LIN B W, ZHU X H, FANG L Z, et al. Birnessite nanosheet arrays with high K content as a high-capacity and ultrastable cathode for K-ion batteries[J]. Advanced Materials, 2019, 31(24): 1900060. |
| 27 | KUMAKURA S, TAHARA Y, SATO S, et al. P'2-Na2/3Mn0.9Me0.1O2 (Me=Mg, Ti, Co, Ni, Cu, and Zn): Correlation between orthorhombic distortion and electrochemical property[J]. Chemistry of Materials, 2017, 29(21): 8958-8962. |
| 28 | BILLAUD J, SINGH G, ARMSTRONG A R, et al. Na0.67Mn1- xMgxO2 (0≤x≤0.2): A high capacity cathode for sodium-ion batteries[J]. Energy & Environmental Science, 2014, 7(4): 1387-1391. |
| 29 | YABUUCHI N, KUBOTA K, DAHBI M, et al. Research development on sodium-ion batteries[J]. Chemical Reviews, 2014, 114(23): 11636-11682. |
| 30 | NAM K W, KIM S, YANG E, et al. Critical role of crystal water for a layered cathode material in sodium ion batteries[J]. Chemistry of Materials, 2015, 27(10): 3721-3725. |
| 31 | JO J H, CHOI J U, KONAROV A, et al. Sodium-ion batteries: Building effective layered cathode materials with long-term cycling by modifying the surface via sodium phosphate[J]. Advanced Functional Materials, 2018, 28(14): 1705968. |
| 32 | LIU Z M, WANG J, JIA X X, et al. Graphene armored with a crystal carbon shell for ultrahigh-performance potassium ion batteries and aluminum batteries[J]. ACS Nano, 2019, 13(9): 10631-10642. |
| 33 | BAO S, LUO S H, LU J L. Preparation and optimization of ZrO2 modified P2-type Na2/3Ni1/6Co1/6Mn2/3O2 with enhanced electrochemical performance as cathode for sodium ion batteries[J]. Ceramics International, 2020, 46(10): 16080-16087. |
| 34 | YU Y, KONG W J, LI Q Y, et al. Understanding the multiple effects of TiO2 coating on NaMn0.33Fe0.33Ni0.33O2 cathode material for Na-ion batteries[J]. ACS Applied Energy Materials, 2020, 3(1): 933-942. |
| 35 | ZHANG Y, LIU L, JAMIL S, et al. Al2O3 coated Na0.44MnO2 as high-voltage cathode for sodium ion batteries[J]. Applied Surface Science, 2019, 494: 1156-1165. |
| 36 | SUN H H, HWANG J Y, YOON C S, et al. Capacity degradation mechanism and cycling stability enhancement of AlF3-coated nanorod gradient Na[Ni0.65Co0.08Mn0.27]O2 cathode for sodium-ion batteries[J]. ACS Nano, 2018, 12(12): 12912-12922. |
| 37 | 戚兴国, 王伟刚, 胡勇胜, 等. 钠离子电池层状氧化物正极材料的表面修饰研究[J]. 储能科学与技术, 2020, 9(5)1396-1401 |
| QI X G, WANG W G, HU Y S, et al. Surface modification research of layered oxide materials for sodium-ion batteries[J]. Energy Storage Science and Technology, 2020, 9(5)1396-1401 | |
| 38 | ZHENG J M, GU M, XIAO J, et al. Functioning mechanism of AlF3 coating on the Li- and Mn-rich cathode materials[J]. Chemistry of Materials, 2014, 26(22): 6320-6327. |
| 39 | ZHAO S Q, YAN K, MUNROE P, et al. Construction of hierarchical K1.39Mn3O6 spheres via AlF3 coating for high-performance potassium-ion batteries[J]. Advanced Energy Materials, 2019, 9(10): 1803757. |
| 40 | TAPIA-RUIZ N, DOSE W M, SHARMA N, et al. High voltage structural evolution and enhanced Na-ion diffusion in P2-Na2/3Ni1/3- xMgxMn2/3O2 (0≤x≤0.2) cathodes from diffraction, electrochemical and ab initio studies[J]. Energy & Environmental Science, 2018, 11(6): 1470-1479. |
| 41 | PIAO J Y, GU L, WEI Z X, et al. Phase control on surface for the stabilization of high energy cathode materials of lithium ion batteries[J]. Journal of the American Chemical Society, 2019, 141(12): 4900-4907. |
| 42 | WANG Q C, MENG J K, YUE X Y, et al. Tuning P2-structured cathode material by Na-site Mg substitution for Na-ion batteries[J]. Journal of the American Chemical Society, 2019, 141(2): 840-848. |
| 43 | GAO A, LI M, GUO N N, et al. K-birnessite electrode obtained by ion exchange for potassium-ion batteries: Insight into the concerted ionic diffusion and K storage mechanism[J]. Advanced Energy Materials, 2019, 9(1): 1802739. |
| 44 | JO J H, CHOI J U, PARK Y J, et al. P2-K0.75[Ni1/3Mn2/3]O2 cathode material for high power and long life potassium-ion batteries[J]. Advanced Energy Materials, 2020, 10(7): 1903605. |
| 45 | WANG S H, SUN C L, WANG N, et al. Ni- and/or Mn-based layered transition metal oxides as cathode materials for sodium ion batteries: Status, challenges and countermeasures[J]. Journal of Materials Chemistry A, 2019, 7(17): 10138-10158. |
| 46 | MA C Z, ALVARADO J, XU J, et al. Exploring oxygen activity in the high energy P2-type Na0.78Ni0.23Mn0.69O2 cathode material for Na-ion batteries[J]. Journal of the American Chemical Society, 2017, 139(13): 4835-4845. |
| 47 | 刘欢庆, 高旭, 陈军, 等. 钠离子电池层状氧化物正极: 层间滑移, 相变与性能[J]. 储能科学与技术, 2020, 9(5): 1327-1339 |
| LIU H Q, GAO X, CHEN J, et al. Layered oxide cathode for sodium ion batteries: Interlayer glide, phase transition and performance[J]. Energy Storage Science and Technology, 2020, 9(5): 1327-1339 | |
| 48 | KIM H, SEO D H, KIM J C, et al. Investigation of potassium storage in layered P3-type K0.5MnO2 cathode[J]. Advanced Materials, 2017, 29(37): 1702480. |
| 49 | BO S H, LI X, TOUMAR A J, et al. Layered-to-rock-salt transformation in desodiated NaxCrO2 (x=0.4)[J]. Chemistry of Materials, 2016, 28(5): 1419-1429. |
| 50 | BEZZA I, KAUS M, HEINZMANN R, et al. Mechanism of the delithiation/lithiation process in LiFe0.4Mn0.6PO4: in situ and ex situ investigations on long-range and local structures[J]. The Journal of Physical Chemistry C, 2015, 119(17): 9016-9024. |
| 51 | CHOI J U, YOON C S, ZHANG Q, et al. Understanding on the structural and electrochemical performance of orthorhombic sodium Manganese oxides[J]. Journal of Materials Chemistry A, 2019, 7(1): 202-211. |
| 52 | WANG X P, XU X M, NIU C J, et al. Earth abundant Fe/Mn-based layered oxide interconnected nanowires for advanced K-ion full batteries[J]. Nano Letters, 2017, 17(1): 544-550. |
| 53 | CHO M K, JO J H, CHOI J U, et al. Cycling stability of layered potassium Manganese oxide in nonaqueous potassium cells[J]. ACS Applied Materials & Interfaces, 2019, 11(31): 27770-27779. |
| 54 | SADA K, BARPANDA P. P3-type layered K0.48Mn0.4Co0.6O2: A novel cathode material for potassium-ion batteries[J]. Chemical Communications, 2020, 56(15): 2272-2275. |
| 55 | WENG J Y, DUAN J, SUN C L, et al. Construction of hierarchical K0.7Mn0.7Mg0.3O2 microparticles as high capacity & long cycle life cathode materials for low-cost potassium-ion batteries[J]. Chemical Engineering Journal, 2020, 392: 123649. |
| 56 | RAMASAMY H V, SENTHILKUMAR B, BARPANDA P, et al. Superior potassium-ion hybrid capacitor based on novel P3-type layered K0.45Mn0.5Co0.5O2 as high capacity cathode[J]. Chemical Engineering Journal, 2019, 368: 235-243. |
| 57 | CHOI J U, KIM J, HWANG J Y, et al. K0.54[Co0.5Mn0.5]O2: New cathode with high power capability for potassium-ion batteries[J]. Nano Energy, 2019, 61: 284-294. |
| 58 | LIU Z M, WANG J, LU B G. Plum pudding model inspired KVPO4F@3DC as high-voltage and hyperstable cathode for potassium ion batteries[J]. Science Bulletin, 2020, 65(15): 1242-1251. |
| 59 | ZHANG Z Z, SUN J L, DUAN L P, et al. Self-templated construction of peanut-like P3-type K0.45Mn0.5Co0.5O2 for highly reversible potassium storage[J]. Journal of Materials Chemistry A, 2022, 10(2): 554-560. |
| 60 | BAI P L, JIANG K Z, ZHANG X P, et al. Ni-doped layered Manganese oxide as a stable cathode for potassium-ion batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(9): 10490-10495. |
| 61 | XIAO Z T, XIA F J, XU L H, et al. Suppressing the jahn-teller effect in Mn-based layered oxide cathode toward long-life potassium-ion batteries[J]. Advanced Functional Materials, 2022, 32(14): 2108244. |
| 62 | LIU W, LI X F, XIONG D B, et al. Significantly improving cycling performance of cathodes in lithium ion batteries: The effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2[J]. Nano Energy, 2018, 44: 111-120. |
| 63 | CHOI J U, KIM J, JO J H, et al. Facile migration of potassium ions in a ternary P3-type K0.5[Mn0.8Fe0.1Ni0.1]O2 cathode in rechargeable potassium batteries[J]. Energy Storage Materials, 2020, 25: 714-723. |
| 64 | XU Y S, ZHOU Y N, ZHANG Q H, et al. Layered oxides with solid-solution reaction for high voltage potassium-ion batteries cathode[J]. Chemical Engineering Journal, 2021, 412: 128735. |
| 65 | ZHANG X Y, YU D X, WEI Z X, et al. Layered P3-type K0.4Fe0.1Mn0.8Ti0.1O2 as a low-cost and zero-strain electrode material for both potassium and sodium storage[J]. ACS Applied Materials & Interfaces, 2021, 13(16): 18897-18904. |
| 66 | LV J R, WANG B, HAO J X, et al. Single-crystalline Mn-based oxide as a high-rate and long-life cathode material for potassium-ion battery[J]. eScience, 2023, 3(1): 100081. |
| 67 | WANG P F, XIN H S, ZUO T T, et al. An abnormal 3.7 Volt O3 -Type sodium-ion battery cathode[J]. Angewandte Chemie International Edition, 2018, 57(27): 8178-8183. |
| 68 | DENG J Q, LUO W B, LU X, et al. High energy density sodium-ion battery with industrially feasible and air-stable O3 -Type layered oxide cathode[J]. Advanced Energy Materials, 2018, 8(5): 1701610. |
| 69 | CAIXIANG Z M, HAO J X, ZHOU J, et al. Interlayer-engineering and surface-substituting Manganese-based self-evolution for high-performance potassium cathode[J]. Advanced Energy Materials, 2023, 13(1): 2203126. |
| 70 | KIM H, SEO D H, URBAN A, et al. Stoichiometric layered potassium transition metal oxide for rechargeable potassium batteries[J]. Chemistry of Materials, 2018, 30(18): 6532-6539. |
| 71 | JIANG X Y, LIU X W, ZENG Z Q, et al. A nonflammable Na+-based dual-carbon battery with low-cost, high voltage, and long cycle life[J]. Advanced Energy Materials, 2018, 8(36): 1802176. |
| 72 | 黄永浩, 藏国景, 朱霨亚, 等. LiF添加剂改善含锂陶瓷隔膜与4.35 V LiNi0.8Co0.1Mn0.1O2正极的界面稳定性研究[J]. 储能科学与技术, doi: 10.19799/j.cnki.2095-4239.2023.0128. |
| HUANG Yonghao, ZANG Guojing, ZHU WeiYa, et al. Improvement in interfacial stability between lithium-containing ceramic separator and 4.35 V LiNi0.8Co0.1Mn0.1O2 cathode by LiF additives[J]. Energy Storage Science and Technology, doi: 10.19799/j.cnki.2095-4239. 2023.0128. | |
| 73 | HU Z, HAO J X, SHEN D Y, et al. Electro-spraying/spinning: A novel battery manufacturing technology[J]. Green Energy & Environment, 2022, doi: 10.1016/j.gee.2022.05.004. |
| [1] | ZHANG Haoran, CHE Haiying, GUO Kaiqiang, SHEN Zhan, ZHANG Yunlong, CHEN Hangda, ZHOU Huang, LIAO Jianping, LIU Haimei, MA Zifeng. Preparation of Sn-doped NaNi1/3Fe1/3Mn1/3-x Sn x O2 cathode materials and their electrochemical performance [J]. Energy Storage Science and Technology, 2022, 11(6): 1874-1882. |
| [2] | ZHANG Yan, WANG Hai, LIU Zhaomeng, ZHANG Deliu, WANG Jiadong, LI Jianzhong, GAO Xuanwen, LUO Wenbin. Research progress of nickel-rich ternary cathode material ncm for lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1693-1705. |
| [3] | Xiaohan FENG, Jie SUN, Jianhao HE, Yihua WEI, Chenggang ZHOU, Ruimin SUN. Research progress in LiFePO4 cathode material modification [J]. Energy Storage Science and Technology, 2022, 11(2): 467-486. |
| [4] | Can WANG, Pan MA, Guoliang ZHU, Yongchao MA, Pengcheng JI, Shuimiao WEI, Jian ZHAO, Zhishui YU. LIB long life graphite electrode: State-of-art development and perspective [J]. Energy Storage Science and Technology, 2021, 10(1): 59-67. |
| [5] | Jixian WANG, Sikan PENG, Wenzheng NAN, Xiang CHEN, Chen WANG, Shaojiu YAN, Shenglong DAI. Preparation of graphene-coated Li1.22Mn0.52Ni0.26O2 using a spray drying method for lithium-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(1): 111-117. |
| [6] | Yueyuan GU, Jucai WEI, Jindong LI, Luyang WANG, Xu WU. Overview and prospect of studies on electrochemical reduction of carbon dioxide electrolyzers [J]. Energy Storage Science and Technology, 2020, 9(6): 1691-1701. |
| [7] | Sijia REN, Leiwu TIAN, Qinjun SHAO, Jian CHEN. Synthesis of single-crystal LiNi0.8Co0.1Mn0.1O2 by flux method [J]. Energy Storage Science and Technology, 2020, 9(6): 1702-1713. |
| [8] | Xiaohui ZHU, Yuhang ZHUANG, Yang ZHAO, Mingzhu NI, Jing XU, Hui XIA. Development of layered cathode materials for sodium-ion batteries [J]. Energy Storage Science and Technology, 2020, 9(5): 1340-1349. |
| [9] | Wei ZHENG, Qiong LIU, Zhouguang LU. Modulating anionic redox reaction in layered transition metal oxides for sodium-ion batteries [J]. Energy Storage Science and Technology, 2020, 9(5): 1416-1427. |
| [10] | ZHANG Xin, KONG Lingli, GAO Tengyue, LI Haitao, YAO Xiaohui, LI Fuxuan. Analysis and improvement of cycle performance for Ni-rich lithium ion battery [J]. Energy Storage Science and Technology, 2020, 9(3): 813-817. |
| [11] | SUN Xingwei, WANG Longlong, JIANG Feng, MA Jun, ZHOU Xinhong, CUI Guanglei. Failure mechanisms and characterization techniques for solid state polymer lithium batteries [J]. Energy Storage Science and Technology, 2019, 8(6): 1024-1032. |
| [12] | LIANG Dayu, BAO Tingting, GAO Tianhui, ZHANG Jian. Analysis of cycling performance failure of NMC811/SiO-C pouch cells with high specific energy [J]. Energy Storage Science and Technology, 2018, 7(3): 459-464. |
| [13] | WU Minchang1, YU Ningbo1, QIAO Yongmin1, SUN Fangjing2, ZHANG Jie1. The evaluation of fast-charging performance of hard carbon coating artificial graphite for lithium-ion batteries [J]. Energy Storage Science and Technology, 2017, 6(S1): 15-. |
| [14] | WANG Sihui, XU Zhongling, DU Rui, MENG Huanping, LIU Yong, LIU Na, LIANG Chengdu. Degradation study of Ni-rich NCM batteries operated at high tempertures [J]. Energy Storage Science and Technology, 2017, 6(4): 770-775. |
| [15] | XIE Jia, PENG Wen, YANG Xulai. The cycle life investigation for spinel LiNi0.5Mn1.5O4 full cells [J]. Energy Storage Science and Technology, 2014, 3(6): 624-628. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
