Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (9): 2944-2958.doi: 10.19799/j.cnki.2095-4239.2022.0295
• Special Issue for the 10th Anniversary • Previous Articles Next Articles
Zhizhang YUAN1( ), Zonghao LIU2, Xianfeng LI1(
), Zonghao LIU2, Xianfeng LI1( )
)
Received:2022-05-31
Revised:2022-06-17
Online:2022-09-05
Published:2022-08-30
Contact:
Xianfeng LI
E-mail:yuanzhizhang@dicp.ac.cn;lixianfeng@dicp.ac.cn
CLC Number:
Zhizhang YUAN, Zonghao LIU, Xianfeng LI. Research progress of flow battery technologies[J]. Energy Storage Science and Technology, 2022, 11(9): 2944-2958.
Table 1
Flow batteries using quinone-based anolyte and Fe(CN)64-/Fe(CN)63- catholyte"
| 负极活性物质 | 支持电解质 | 运行环境 | 电流密度/(mA/cm2) | 效率 | 循环 | 容量保持率 | 参考文献 |
|---|---|---|---|---|---|---|---|
 | 1 mol/L KOH | 高纯Ar | 100 | CE约99% EE约84% | 100 | 99.9% | [ |
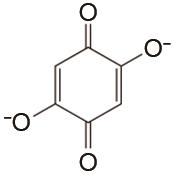 | 1 mol/L KOH | 高纯Ar | 100 | CE约99% EE约65% | 150 | 99.76% | [ |
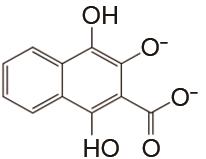 | 1 mol/L KOH | 高纯N2 | 100 | CE约100% EE约69% | 100 | 94.7% | [ |
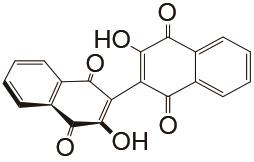 | 1 mol/L KOH | N2 | 100 | CE约99% EE约77% | <300 | 99.992% | [ |
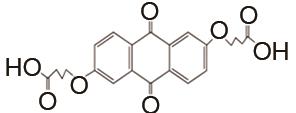 | 1 mol/L KOH | N2 | 100 | CE约99% | 250 | 99.999% | [ |
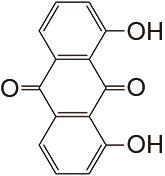 | 1 mol/L KOH | N2 | 80 | CE约99% EE约77% | 100 | 99.88% | [ |
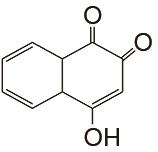 | 1 mol/L KOH | 未提供 | 100 | CE约99% EE约55% | 200 | 99.994% | [ |
 | 1 mol/L KOH | 高纯N2 | 80 | CE约99% EE约76% | 140 | >99% | [ |
| 1 | THALLER L. Electrically rechargeable REDOX flow cell [M]. (NASA TM X-71540) 1974. |
| 2 | 李先锋, 张洪章, 郑琼, 等. 能源革命中的电化学储能技术[J]. 中国科学院院刊, 2019, 34(4): 443-449. |
| LI X F, ZHANG H Z, ZHENG Q, et al. Electrochemical energy storage technology in energy revolution[J]. Bulletin of Chinese Academy of Sciences, 2019, 34(4): 443-449. | |
| 3 | WANG F, HARINDINTWALI J D, YUAN Z Z, et al. Technologies and perspectives for achieving carbon neutrality[J]. The Innovation, 2021, 2(4): doi: 10.1016/j.xinn.2021.100180. |
| 4 | YUAN Z Z, YIN Y B, XIE C X, et al. Advanced materials for zinc-based flow battery: Development and challenge[J]. Advanced Materials, 2019, 31(50): doi: 10.1002/adma.201902025. |
| 5 | KANQRO W. Verfahren zur speicherung von elektrischer energie: DE914264C [P].1949-06-28. |
| 6 | ZHANG H, TAN Y, LI J Y, et al. Studies on properties of rayon- and polyacrylonitrile-based graphite felt electrodes affecting Fe/Cr redox flow battery performance[J]. Electrochimica Acta, 2017, 248: 603-613. |
| 7 | ZHANG H, CHEN N, SUN C Y, et al. Investigations on physicochemical properties and electrochemical performance of graphite felt and carbon felt for iron-chromium redox flow battery[J]. International Journal of Energy Research, 2020, 44(5): 3839-3853. |
| 8 | INOUE M, TSUZUKI Y, IIZUKA Y, et al. Carbon fiber electrode for redox flow battery[J]. Journal of the Electrochemical Society, 1987, 134(3): 756-757. |
| 9 | WATERS S E, ROBB B H, MARSHAK M P. Effect of chelation on iron-chromium redox flow batteries[J]. ACS Energy Letters, 2020, 5(6): 1758-1762. |
| 10 | Rodes A, Feliu J M, Aldaz A, et al. The influence of polyoriented gold electrodes modified by reversibly and irreversibly adsorbed ad-atoms on the redox behaviour of the Cr(Ⅲ)/Cr(Ⅱ) [J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1989, 271(1): 127-139. |
| 11 | ZENG Y K, ZHOU X L, AN L, et al. A high-performance flow-field structured iron-chromium redox flow battery[J]. Journal of Power Sources, 2016, 324: 738-744. |
| 12 | Sum E, Skyllas-Kazacos M. A study of the V(Ⅱ)/V(Ⅲ) redox couple for redox flow cell applications [J]. Journal of Power Sources, 1985, 15(2): 179-190. |
| 13 | SKYLLAS-KAZACOS M, RYCHCIK M, ROBINS R G, et al. New all-vanadium redox flow cell[J]. Journal of the Electrochemical Society, 1986, 133(5): 1057-1058. |
| 14 | HUANG K L, LI X G, LIU S Q, et al. Research progress of vanadium redox flow battery for energy storage in China[J]. Renewable Energy, 2008, 33(2): 186-192. |
| 15 | YUAN Z Z, ZHANG H M, LI X F. Ion conducting membranes for aqueous flow battery systems[J]. Chemical Communications (Cambridge, England), 2018, 54(55): 7570-7588. |
| 16 | DAI Q, XING F, LIU X N, et al. High-performance PBI membranes for flow batteries: From the transport mechanism to the pilot plant[J]. Energy & Environmental Science, 2022, 15(4): 1594-1600. |
| 17 | LI T Y, XING F, LIU T, et al. Cost, performance prediction and optimization of a vanadium flow battery by machine-learning[J]. Energy & Environmental Science, 2020, 13(11): 4353-4361. |
| 18 | ZHANG J, JIANG G P, XU P, et al. An all-aqueous redox flow battery with unprecedented energy density[J]. Energy & Environmental Science, 2018, 11(8): 2010-2015. |
| 19 | GONG K, MA X Y, CONFORTI K M, et al. A zinc-iron redox-flow battery under $100 per kWh of system capital cost[J]. Energy & Environmental Science, 2015, 8(10): 2941-2945. |
| 20 | YIN Y B, WANG S N, ZHANG Q, et al. Dendrite-free zinc deposition induced by tin-modified multifunctional 3D host for stable zinc-based flow battery[J]. Advanced Materials, 2020, 32(6): doi:10.1002/adma.201906803. |
| 21 | WANG S N, YUAN C G, CHANG N N, et al. Act in contravention: A non-planar coupled electrode design utilizing "tip effect" for ultra-high areal capacity, long cycle life zinc-based batteries[J]. Science Bulletin, 2021, 66(9): 889-896. |
| 22 | WANG S N, WANG Z Y, YIN Y B, et al. A highly reversible zinc deposition for flow batteries regulated by critical concentration induced nucleation[J]. Energy & Environmental Science, 2021, 14(7): 4077-4084. |
| 23 | LI X J, LI T Y, XU P C, et al. A complexing agent to enable a wide-temperature range bromine-based flow battery for stationary energy storage[J]. Advanced Functional Materials, 2021, 31(22): doi: 10.1002/adfm.202100133. |
| 24 | LI X J, XIE C X, LI T Y, et al. Low-cost titanium-bromine flow battery with ultrahigh cycle stability for grid-scale energy storage[J]. Advanced Materials (Deerfield Beach, Fla), 2020, 32(49): doi: 10.1002/adma.202005036. |
| 25 | LU W J, XU P C, SHAO S Y, et al. Multifunctional carbon felt electrode with N-rich defects enables a long-cycle zinc-bromine flow battery with ultrahigh power density[J]. Advanced Functional Materials, 2021, 31(30): doi: 10.1002/adfm.202102913. |
| 26 | HUA L, LU W, LI T, et al. A highly selective porous composite membrane with bromine capturing ability for a bromine-based flow battery[J]. Materials Today Energy, 2021, 21: doi:10.1016/j.mtener.2021.100763. |
| 27 | ADAMS G B. Electrically rechargeable battery: US4180623[P]. 1979-12-25. |
| 28 | HU J, YUE M, ZHANG H M, et al. A boron nitride nanosheets composite membrane for a long-life zinc-based flow battery[J]. Angewandte Chemie (International Ed in English), 2020, 59(17): 6715-6719. |
| 29 | WU J E, YUAN C G, LI T Y, et al. Dendrite-free zinc-based battery with high areal capacity via the region-induced deposition effect of turing membrane[J]. Journal of the American Chemical Society, 2021, 143(33): 13135-13144. |
| 30 | YUAN Z Z, LIU X Q, XU W B, et al. Negatively charged nanoporous membrane for a dendrite-free alkaline zinc-based flow battery with long cycle life[J]. Nature Communications, 2018, 9: 3731. |
| 31 | HU J, ZHANG H M, XU W B, et al. Mechanism and transfer behavior of ions in Nafion membranes under alkaline media[J]. Journal of Membrane Science, 2018, 566: 8-14. |
| 32 | HU J, TANG X M, DAI Q, et al. Layered double hydroxide membrane with high hydroxide conductivity and ion selectivity for energy storage device[J]. Nature Communications, 2021, 12: 3409. |
| 33 | YUAN Z Z, LIANG L X, DAI Q, et al. Low-cost hydrocarbon membrane enables commercial-scale flow batteries for long-duration energy storage[J]. Joule, 2022, 6(4): 884-905. |
| 34 | WINSBERG J, STOLZE C, SCHWENKE A, et al. Aqueous 2, 2, 6, 6-tetramethylpiperidine-N-oxyl catholytes for a high-capacity and high current density oxygen-insensitive hybrid-flow battery[J]. ACS Energy Letters, 2017, 2(2): 411-416. |
| 35 | ULAGANATHAN M, SURESH S, MARIYAPPAN K, et al. New zinc-vanadium (Zn-V) hybrid redox flow battery: High-voltage and energy-efficient advanced energy storage system[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(6): 6053-6060. |
| 36 | XIE C X, LI T Y, DENG C Z, et al. A highly reversible neutral zinc/manganese battery for stationary energy storage[J]. Energy & Environmental Science, 2020, 13(1): 135-143. |
| 37 | XIE C X, DUAN Y Q, XU W B, et al. A low-cost neutral zinc-iron flow battery with high energy density for stationary energy storage[J]. Angewandte Chemie (International Ed in English), 2017, 56(47): 14953-14957. |
| 38 | XIE C X, LIU Y, LU W J, et al. Highly stable zinc-iodine single flow batteries with super high energy density for stationary energy storage[J]. Energy & Environmental Science, 2019, 12(6): 1834-1839. |
| 39 | WENG G M, LI Z J, CONG G T, et al. Unlocking the capacity of iodide for high-energy-density zinc/polyiodide and lithium/polyiodide redox flow batteries[J]. Energy & Environmental Science, 2017, 10(3): 735-741. |
| 40 | LIU Z, CUI T, PULLETIKURTHI G, et al. Dendrite-free nanocrystalline zinc electrodeposition from an ionic liquid containing nickel triflate for rechargeable Zn-based batteries[J]. Angewandte Chemie (International Ed in English), 2016, 55(8): 2889-2893. |
| 41 | PARKER J F, CHERVIN C N, NELSON E S, et al. Wiring zinc in three dimensions re-writes battery performance-dendrite-free cycling[J]. Energy Environ Sci, 2014, 7(3): 1117-1124. |
| 42 | BANIK S J, AKOLKAR R. Suppressing dendrite growth during zinc electrodeposition by PEG-200 additive[J]. Journal of the Electrochemical Society, 2013, 160(11): D519-D523. |
| 43 | FU J, CANO Z P, PARK M G, et al. Electrically rechargeable zinc-air batteries: Progress, challenges, and perspectives[J]. Advanced Materials, 2017, 29(7): doi: 10.1002/adma.201604685. |
| 44 | PARKER J F, CHERVIN C N, PALA I R, et al. Rechargeable nickel-3D zinc batteries: An energy-dense, safer alternative to lithium-ion[J]. Science, 2017, 356(6336): 415-418. |
| 45 | KNEHR K W, BISWAS S, STEINGART D A. Quantification of the voltage losses in the minimal architecture zinc-bromine battery using GITT and EIS[J]. Journal of the Electrochemical Society, 2017, 164(13): A3101-A3108. |
| 46 | HUSKINSON B, MARSHAK M P, SUH C, et al. A metal-free organic-inorganic aqueous flow battery[J]. Nature, 2014, 505(7482): 195-198. |
| 47 | KHATAEE A, WEDEGE K, DRAŽEVIĆ E, et al. Differential pH as a method for increasing cell potential in organic aqueous flow batteries[J]. J Mater Chem A, 2017, 5(41): 21875-21882. |
| 48 | LIN K X, CHEN Q, GERHARDT M R, et al. Alkaline quinone flow battery[J]. Science, 2015, 349(6255): 1529-1532. |
| 49 | YANG Z J, TONG L C, TABOR D P, et al. Alkaline benzoquinone aqueous flow battery for large-scale storage of electrical energy[J]. Advanced Energy Materials, 2018, 8(8): doi: 10.1002/aenm.201870034. |
| 50 | LIU W Q, ZHAO Z M, LI T Y, et al. A high potential biphenol derivative cathode: Toward a highly stable air-insensitive aqueous organic flow battery[J]. Science Bulletin, 2021, 66(5): 457-463. |
| 51 | WANG C X, YANG Z, WANG Y R, et al. High-performance alkaline organic redox flow batteries based on 2-hydroxy-3-carboxy-1, 4-naphthoquinone[J]. ACS Energy Letters, 2018, 3(10): 2404-2409. |
| 52 | TONG L C, GOULET M A, TABOR D P, et al. Molecular engineering of an alkaline naphthoquinone flow battery[J]. ACS Energy Letters, 2019, 4(8): 1880-1887. |
| 53 | KWABI D G, LIN K X, JI Y L, et al. Alkaline quinone flow battery with long lifetime at pH 12[J]. Joule, 2018, 2(9): 1894-1906. |
| 54 | CAO J Y, TAO M, CHEN H P, et al. A highly reversible anthraquinone-based anolyte for alkaline aqueous redox flow batteries[J]. Journal of Power Sources, 2018, 386: 40-46. |
| 55 | LEE W, PARK G, KWON Y. Alkaline aqueous organic redox flow batteries of high energy and power densities using mixed naphthoquinone derivatives[J]. Chemical Engineering Journal, 2020, 386: doi: 10.1016/j.cej.2019.123985. |
| 56 | CHEN D J, DUAN W Q, HE Y Y, et al. Porous membrane with high selectivity for alkaline quinone-based flow batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(43): 48533-48541. |
| 57 | LIN K X, GÓMEZ-BOMBARELLI R, BEH E S, et al. A redox-flow battery with an alloxazine-based organic electrolyte[J]. Nature Energy, 2016, 1: 16102. |
| 58 | ORITA A, VERDE M G, SAKAI M, et al. A biomimetic redox flow battery based on flavin mononucleotide[J]. Nature Communications, 2016, 7: 13230. |
| 59 | HOLLAS A, WEI X L, MURUGESAN V, et al. A biomimetic high-capacity phenazine-based anolyte for aqueous organic redox flow batteries[J]. Nature Energy, 2018, 3(6): 508-514. |
| 60 | WANG C, LI X, YU B, et al. Molecular disign of fused-ring phenazine derivatives for long-cycling alkaline redox flow batteries [J]. ACS Energy Letters, 2020, (2): 411-417. |
| 61 | PANG S, WANG X Y, WANG P, et al. Biomimetic amino acid functionalized phenazine flow batteries with long lifetime at near-neutral pH[J]. Angewandte Chemie (International Ed in English), 2021, 60(10): 5289-5298. |
| 62 | XU J C, PANG S, WANG X Y, et al. Ultrastable aqueous phenazine flow batteries with high capacity operated at elevated temperatures[J]. Joule, 2021, 5(9): 2437-2449. |
| 63 | ZHANG C K, NIU Z H, PENG S S, et al. Phenothiazine-based organic catholyte for high-capacity and long-life aqueous redox flow batteries[J]. Advanced Materials, 2019, 31(24): doi: 10.1002/adma.201901052. |
| 64 | LIU T B, WEI X L, NIE Z M, et al. A total organic aqueous redox flow battery employing a low cost and sustainable methyl viologen anolyte and 4-HO-TEMPO catholyte[J]. Advanced Energy Materials, 2016, 6(3): doi: 10.1002/aenm.201501449. |
| 65 | JANOSCHKA T, MARTIN N, HAGER M D, et al. An aqueous redox-flow battery with high capacity and power: The TEMPTMA/MV system[J]. Angewandte Chemie (International Ed in English), 2016, 55(46): 14427-14430. |
| 66 | WINSBERG J, STOLZE C, MUENCH S, et al. TEMPO/phenazine combi-molecule: A redox-active material for symmetric aqueous redox-flow batteries[J]. ACS Energy Letters, 2016, 1(5): 976-980. |
| 67 | JANOSCHKA T, MARTIN N, MARTIN U, et al. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials[J]. Nature, 2015, 527(7576): 78-81. |
| 68 | HU B, FAN H, LI H B, et al. Five-membered ring nitroxide radical: A new class of high-potential, stable catholytes for neutral aqueous organic redox flow batteries[J]. Advanced Functional Materials, 2021, 31(35): doi: 10.1002/adfm.202102734. |
| 69 | FAN H, HU B, LI H B, et al. Conjugate-driven electron density delocalization of piperidine nitroxyl radical for stable aqueous zinc hybrid flow batteries[J]. Angewandte Chemie (International Ed in English), 2022, 61(17): doi: 10.1002/anie.202115908. |
| 70 | DEBRULER C, HU B, MOSS J, et al. Designer two-electron storage viologen anolyte materials for neutral aqueous organic redox flow batteries[J]. Chem, 2017, 3(6): 961-978. |
| 71 | HU B, DEBRULER C, RHODES Z, et al. Long-cycling aqueous organic redox flow battery (AORFB) toward sustainable and safe energy storage[J]. Journal of the American Chemical Society, 2017, 139(3): 1207-1214. |
| 72 | BEH E S, DE PORCELLINIS D, GRACIA R L, et al. A neutral pH aqueous organic-organometallic redox flow battery with extremely high capacity retention[J]. ACS Energy Letters, 2017, 2(3): 639-644. |
| 73 | LUO J, HU B, DEBRULER C, et al. A π-conjugation extended viologen as a two-electron storage anolyte for total organic aqueous redox flow batteries[J]. Angewandte Chemie, 2018, 57(1): 231-235. |
| 74 | HU S Z, LI T Y, HUANG M B, et al. Phenylene-bridged bispyridinium with high capacity and stability for aqueous flow batteries[J]. Advanced Materials, 2021, 33(7): doi: 10.1002/adma.202005839. |
| 75 | LIU W Q, LIU Y, ZHANG H M, et al. A highly stable neutral viologen/bromine aqueous flow battery with high energy and power density[J]. Chemical Communications, 2019, 55(33): 4801-4804. |
| 76 | HUANG J H, HU S Z, YUAN X Z, et al. Radical stabilization of a tripyridinium-triazine molecule enables reversible storage of multiple electrons[J]. Angewandte Chemie, 2021, 60(38): 20921-20925. |
| 77 | HUANG M B, HU S Z, YUAN X Z, et al. Five-membered-heterocycle bridged viologen with high voltage and superior stability for flow battery[J]. Advanced Functional Materials, 2022, 32(16): doi: 10.1002/adfm.202111744. |
| [1] | Zhu JIANG, Boyang ZOU, Lin CONG, Chunping XIE, Chuan LI, Geng QIAO, Yanqi ZHAO, Binjian NIE, Tongtong ZHANG, Zhiwei GE, Hongkun MA, Yi JIN, Yongliang LI, Yulong DING. Recent progress and outlook of thermal energy storage technologies [J]. Energy Storage Science and Technology, 2022, 11(9): 2746-2771. |
| [2] | Huamin ZHANG. Development, cost analysis considering various durations, and advancement of vanadium flow batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2772-2780. |
| [3] | Junlei WANG, Diling ZHANG, Kun WANG, Dongdong XU, Xianggui XU, Hua YAO, Wenwei LIU, Yun HUANG. Carbonates/blast furnace slag form-stable phase change materials [J]. Energy Storage Science and Technology, 2022, 11(9): 3028-3034. |
| [4] | Hong LI, Qiang ZHANG. A review of energy storage science and technology projects supported by national key R&D program [J]. Energy Storage Science and Technology, 2022, 11(9): 2691-2701. |
| [5] | Xiangjun LI, Yibiao GUAN, Juan HU, Xiaokang LAI. Review of energy storage application in China from 2012 to 2022 [J]. Energy Storage Science and Technology, 2022, 11(9): 2702-2712. |
| [6] | Jinzhi WANG, Xiaolei HAN, Chaofeng XU, Jingwen ZHAO, Yue TANG, Guanglei CUI. Research progress of sodium energy storage batteries using oxide solid-state electrolytes [J]. Energy Storage Science and Technology, 2022, 11(9): 2834-2846. |
| [7] | Chengshan XU, Borui LU, Mengqi ZHANG, Huaibin WANG, Changyong JIN, Minggao OUYANG, Xuning FENG. Study on thermal runaway gas evolution in the lithium-ion battery energy storage cabin [J]. Energy Storage Science and Technology, 2022, 11(8): 2418-2431. |
| [8] | Liang TANG, Xiaobo YIN, Houfu WU, Pengjie LIU, Qingsong WANG. Demand for safety standards in the development of the electrochemical energy storage industry [J]. Energy Storage Science and Technology, 2022, 11(8): 2645-2652. |
| [9] | Liping HUO, Weiling LUAN, Zixian ZHUANG. Development trend of lithium-ion battery safety technology for energy storage [J]. Energy Storage Science and Technology, 2022, 11(8): 2671-2680. |
| [10] | Congjia ZHANG, Minda SHI, Chen XU, Zhenyu HUANG, Song CI. Intrinsic safety mechanism and case analysis of energy storage systems based on dynamically reconfigurable battery network [J]. Energy Storage Science and Technology, 2022, 11(8): 2442-2451. |
| [11] | Zhicheng CAO, Kaiyun ZHOU, Jiali ZHU, Gaoming LIU, Min YAN, Shun TANG, Yuancheng CAO, Shijie CHENG, Weixin ZHANG. Patent analysis of fire-protection technology of lithium-ion energy storage system [J]. Energy Storage Science and Technology, 2022, 11(8): 2664-2670. |
| [12] | Yong XIAO, Jun XU. Risk assessment of battery safe operation in energy storage power station based on combination weighting and TOPSIS [J]. Energy Storage Science and Technology, 2022, 11(8): 2574-2584. |
| [13] | Mingfei LI, Mumin RAO, Wanmei SUN, Shuxin CUI, Wei CHEN. Analysis method based on porous medium modeling for thermal management system of large capacity battery energy storage [J]. Energy Storage Science and Technology, 2022, 11(8): 2526-2536. |
| [14] | Shuang SHI, Nawei LYU, Jingxuan MA, Kangyong YIN, Lei SUN, Ning ZHANG, Yang JIN. Comparative study on the effectiveness of different types of gas detection on the overcharge safety early warning of a lithium iron phosphate battery energy storage compartment [J]. Energy Storage Science and Technology, 2022, 11(8): 2452-2462. |
| [15] | Kangyong YIN, Fengbo TAO, Wei LIANG, Zhiyuan NIU. Simulation of thermal runaway gas explosion in double-layer prefabricated cabin lithium iron phosphate energy storage power station [J]. Energy Storage Science and Technology, 2022, 11(8): 2488-2496. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
