Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (1): 86-110.doi: 10.19799/j.cnki.2095-4239.2022.0413
• Energy Storage Materials and Devices • Previous Articles Next Articles
Received:2022-07-25
Revised:2022-09-15
Online:2023-01-05
Published:2023-02-08
Contact:
Youlong XU
E-mail:zhangkai808@stu.xjtu.edu.cn;ylxu@mail.xjtu.edu.cn
CLC Number:
Kai ZHANG, Youlong XU. Research progress and development trend of sodium manganate cathode materials for sodium ion batteries[J]. Energy Storage Science and Technology, 2023, 12(1): 86-110.
Table 2
Comparison of cathode materials for sodium ion batteries"
| 正极材料 | 优势 | 劣势 |
|---|---|---|
| 过渡金属氧化物 | 工艺简单,电化学活性较高,储钠效果良好,具有更多样的结构种类,更高的比容量和其他优异的电化学性能。 | 充放电过程体积变化较大,材料循环稳定性较差,具有显著的粒径依赖性,大尺寸可逆性很差。 |
| 聚阴离子化合物 | 材料结构非常稳定,热稳定性高,倍率性能很好,工作电压较高(聚阴离子诱导效应)。 | 比容量低,能量密度低,材料中钒、钴和镍等原材料价格昂贵,成本很高。 |
| 普鲁士蓝类化合物 | 由金属与氰根配位键形成开放性框架结构,空间足够,材料价格便宜,制备工艺简单(溶热沉淀法)。 | 结构本身存在缺陷,循环稳定性能差,电池比容量很差,存在环境问题(氰根CN-)。 |

Fig. 14
(a) Schematic diagram of the fabricated Na0.7MnO2.05 hollow microsphere structure and Na0.7MnO2.05@PPy polypyrrole (PPy) covered hollow microsphere structure; (b) SEM image of the MnO2 hollow microsphere structure; (c) SEM image of the Na0.7MnO2.05 hollow microsphere structure; (d) Na0.7MnO2.05@PPy polypyrrole (PPy)-covered hollow microsphere structure SEM image[31]"
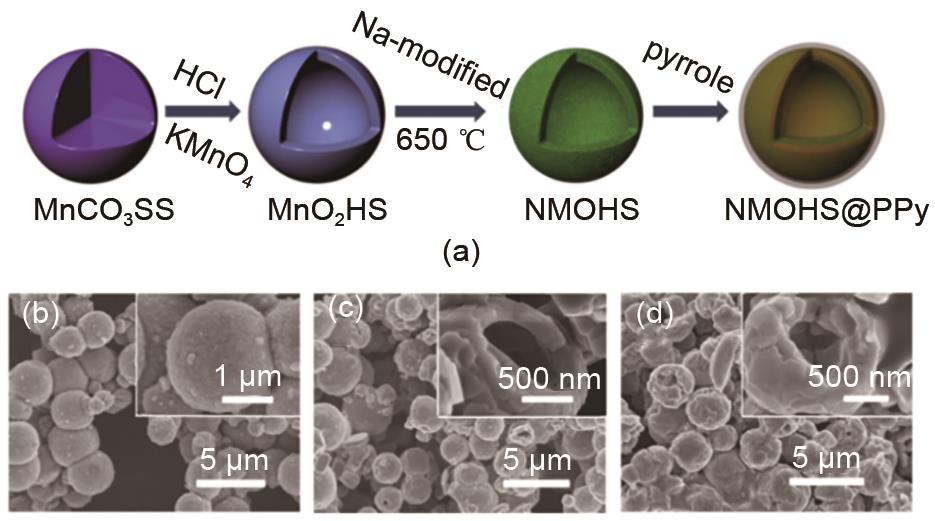
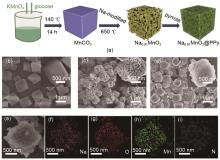
Fig. 15
(a) Preparation process of Na0.91MnO2 porous microcube structure and Na0.91MnO2@PPy polypyrrole-coated porous microcube structure, (b) SEM images of MnCO3 porous microcube, (c) Na0.91MnO2 porous microcube structure, (d, e) Na0.91MnO2@PPy polypyrrole-coated porous micro cube structure SEM images, (f—i) elemental mapping images of Na, O, Mn and N in Na0.91MnO2@PPy[32]"
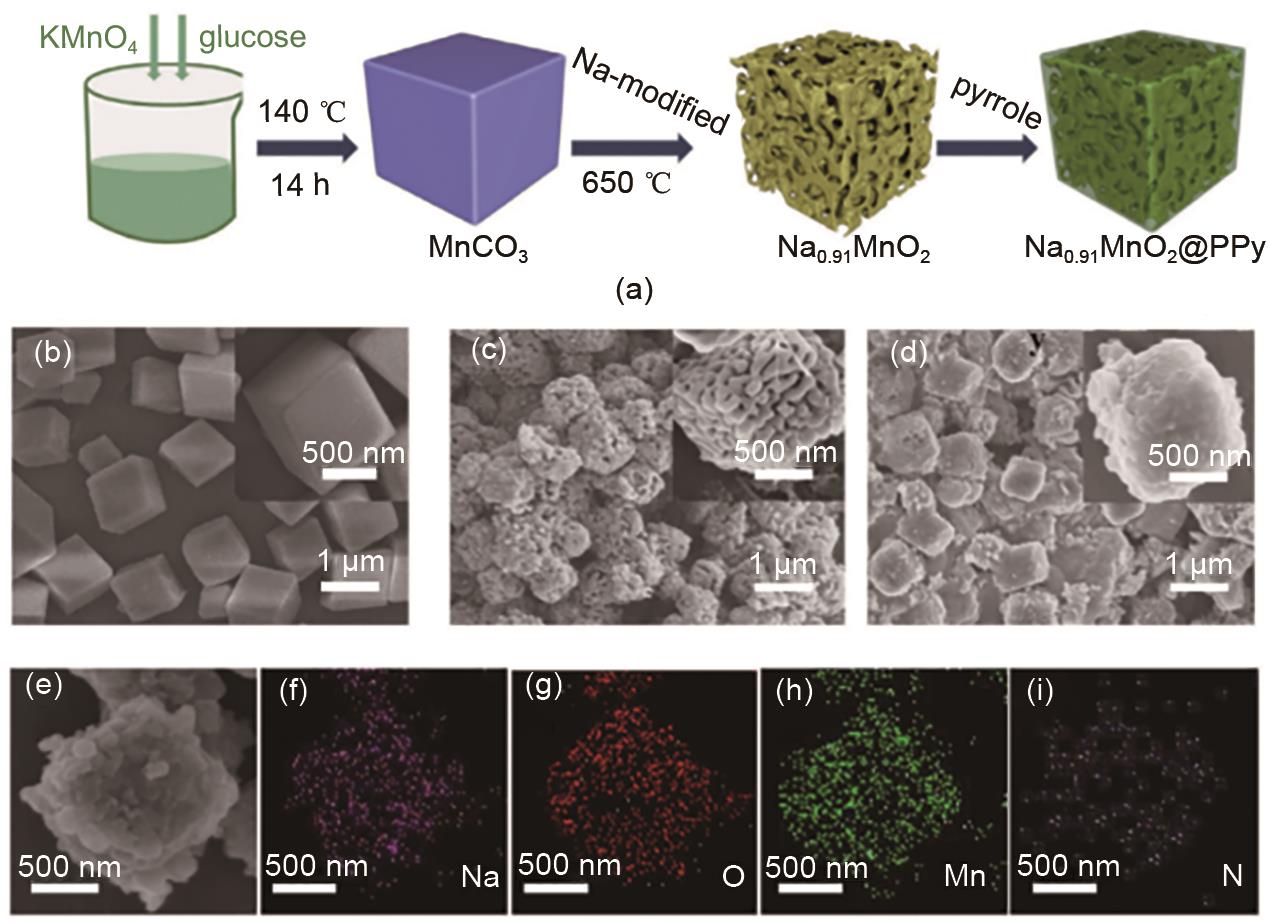
Table 3
Preparation method and electrochemical properties of Na x MnO2(x ≤0.5) and element-doped cathode materials"
| 正极材料 | 合成方法 | 电压范围/V | 比容量/(mAh/g) | 容量保持率(th表示圈数) |
|---|---|---|---|---|
| Na0.44MnO2(微棒)[ | 固相法 | 2.0~4.5 | 100.3(1 C) 90.9(包覆后1 C) 63.6(10 C) | 70.2%(1 C,400th) 86.7%(包覆后1 C, 400th) |
| Na0.44MnO2(纳米线)[ | 电纺法退火处理 | 2.0~4.0 | 120.4(0.1 C) 85.8(5 C) | 89%(5 C,3300th) |
| Na0.44MnO1.93F0.07[ | 固相法 | 2.0~4.2 | 149(0.5 C) 138(1 C) 109(5 C) | 79%(5 C,400th) |
| Na0.46Mn0.93Al0.07O1.79F0.21[ | 固相法 | 1.8~4.0 | 164.3(0.3 C) | 89.1%(5 C,500th) |
| Na0.5Ni0.25Mn0.75O2[ | 溶胶-凝胶法 | 1.5~4.4 | 210(0.1 C) | 80%(0.1 C,50th) |
| Na0.44Mn0.95Mg0.05O2[ | 固相法 | 2.0~3.8 | 105(0.2 C) | 67%(2 C,800th) 70%(20 C,800th) |
Table 4
Preparation method and electrochemical properties of Na x MnO2(x>0.5) cathode materials"
| x>0.5正极材料 | 合成方法 | 电压范围/V | 比容量/(mAh/g) | 容量保持率 |
|---|---|---|---|---|
| α-NaMnO2[ | 固相法 | 2.0~3.8 | 185(0.1 C) | 71.4%(20th) |
| Na0.7MnO2.05[ | 水热法、固相法和化学冰水浴法 | 1.8~4.4 | 165.1 | 88.6%(0.1 A/g,100th) |
| Na0.91MnO2[ | 水热法、固相法和化学冰水浴法 | 1.4~4.2 | 208(0.1 A/g) 139(2 A/g) | 86.8%(0.1 A/g,200th) |
| Na2Mn3O7[ | 固相法 | 1.5~3.5 | 160(0.05 C) | — |
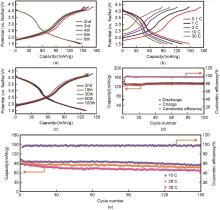
Fig. 22
(a) Charging and discharging curves at 0.1 C rate; (b) Charging and discharging curves for the first cycle at 0.1 C, 1 C, 3 C, 10 C, and 30 C rates; (c) Charging and discharging curves at 1 C rate; (d) Charging and discharging cycle performance and efficiency at 1 C rate; (e) Charging and discharging cycle performance at 10 C, 20 C, and 30 C rates and efficiency at 10 C[45]"
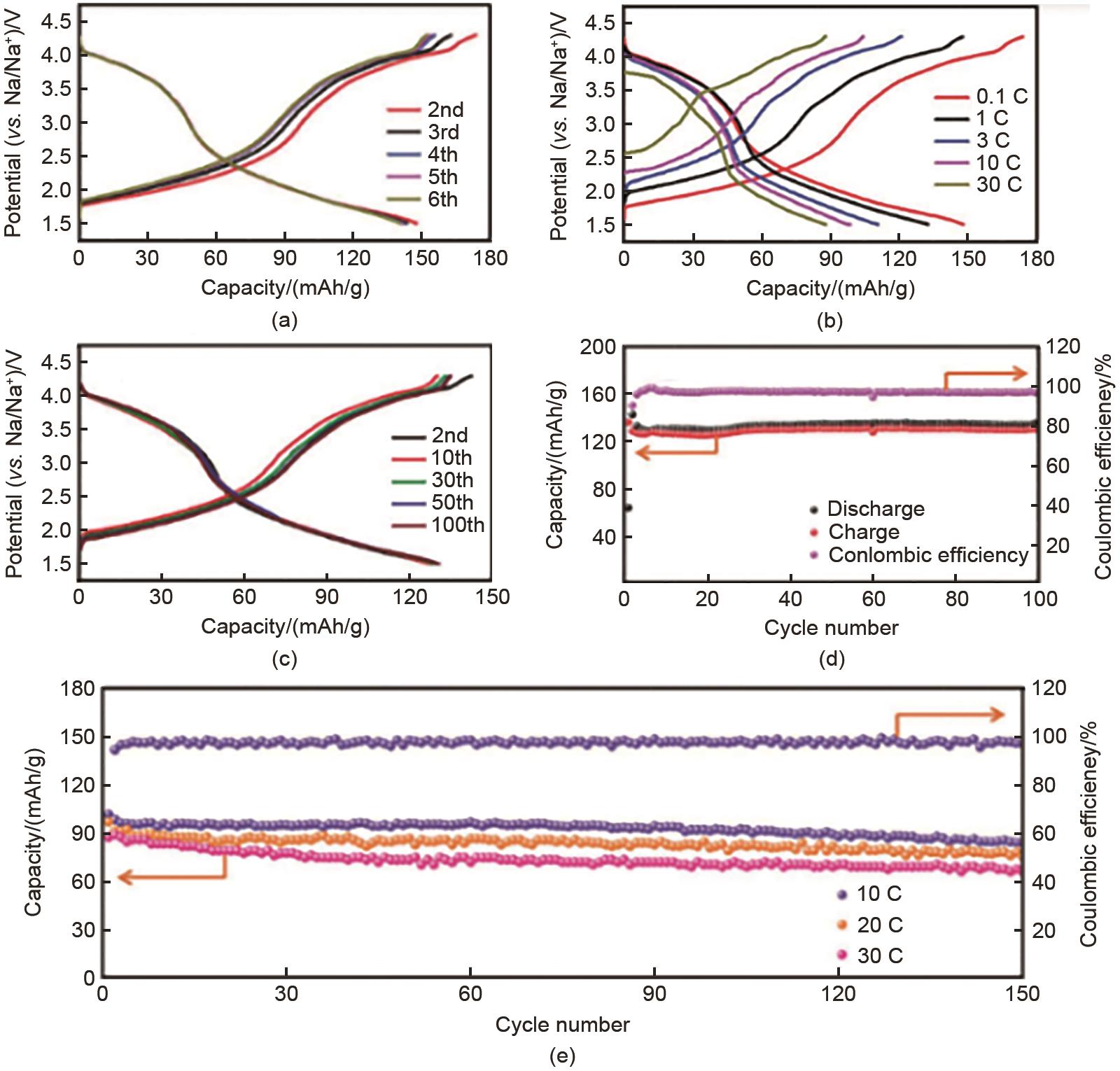
Table 5
Preparation method and electrochemical properties of Na x (Mn y M1 j )O2 (x>0.5) cathode materials"
| 锰位掺杂正极材料 | 合成方法 | 电压范围/V | 比容量/(mAh/g) | 容量保持率 |
|---|---|---|---|---|
| Na0.67Ni0.15Mn0.85O2[ | 固相法和共沉淀法 | 2.0~4.3 | 101 | 96.8%(120th) |
| Na2/3Ni1/3Mn2/3O2[ | 共沉淀法和喷雾干燥法 | 1.5~4.5① 2.0~4.0② | 228(①,0.05 C) 89(②,0.05 C) | 56%(0.5 C,100th) 97%(0.5 C,100th) |
| Na x [Fe y Mn1-y ]O2[ | 固相法 | 1.5~3.8 | 155 | |
| Na2/3[Mg0.28Mn0.72]O2[ | 固相法 | 1.5~4.4 | >200(10 mA/g) | >75%(30th) |
| Na0.67Mg0.34Mn0.66O2[ | 固相法 | 1.5~4.5 | 205(0.1 C) | 70%(0.5 C,50th) |
| Na0.67Co0.5Mn0.5O2[ | 溶胶-凝胶法 | 1.5~4.3 | 147(0.1 C) | 100%(1 C,100th) ≈50%(2000th) |
| NaNi0.5Mn0.5O2[ | 溶胶-凝胶法 | 2.0~4.0 | 141 | 90%(100th) |
| Na2/3Ni1/3Mn2/3O2[ | 固相法和液相法 | 2.5~4.3 | 162(0.1 C)、 143(0.2 C)、 121(0.5 C)、 113(1 C)、 102(2 C) | 75%(0.5 C,200th) |
| Na2/3[Ni1/3Mn2/3]O2[ | 喷雾干燥法和两步固相法 | 2.0~4.0① 1.6~3.8② | 86(0.1 C,①) 77(1 C,①) 135(0.1 C,②) 108(1 C,②) | 92%(0.1 C, 100th) 91%(1 C, 100th) |
| Na0.67Mn0.8Mg0.2O2[ | 固相法 | 1.5~4.2 | ≈150 | >96%(25th) |
| Na0.7[Mn1-x Li x ]O2+y (x=0.07)[ | 固相法 | 2.0~3.8 | ≈183 | |
| Na0.6Mn0.93Ru0.07O2[ | 固相法 | 1.5~4.5 | 206.3(0.5 C) 183.3(1 C) 163.2(2 C) 136.2(5 C) 121.1(10 C) 108.7(20 C) 97.3(50 C) | 87.4%(2 C,50th) |
| Na2/3Fe1/2Mn1/2O2[ | 共沉淀法 | 2.0~4.0 | 126(0.1 C) | 84.7%(25th) |
| Na2.4Al0.4Mn2.6O7[ | 固相法 | 1.5~4.7 | 200(0.05 C) | 90%(40th) |
| Na0.67Mn0.5Fe0.5O2[ | 溶胶-凝胶法 | 1.5~4.2 | 200 | 80%(0.1 C,50th) |
| Na2/3Mn0.75Co0.25O2[ | 固相法 | 1.8~4.0 | 164.1(0.1 C) 111.4(1 C) 85.7(5 C) 68.6(10 C) | 85.6%(0.1 C,100th) 72.3%(1 C,400th) 73.5%(5 C,1000th) 82.0%(10 C,1000th) |
| NaLi0.2Mn0.8O2[ | 固相法 | 1.5~4.0 | 160(0.1 C) 115(1 C) | 93%(0.1 C,50th) |
| Na2/3B0.11Mn0.89O2[ | 固相法 | 2.0~4.0 | 165(40 mA/g) | 82%(200th) |
| Na2/3Fe1/3Mn2/3O2[ | 固相法 | 1.5~4.3 | 193(0.05 C) | 79.3%(40th) |
| Na0.6Li0.2Mn0.8O2[ | 固相法和液相法 | 2.0~4.6 | 190(12 mA/g) | |
| NaxMg0.11Mn0.89O2[ | 共沉淀法和固相法 | 1.5~4.4 | 174(1th) 129(10th) | 93.8%(100th,对比10th) 75%(200th,对比10th) |
Table 6
Preparation method and electrochemical properties of Na x (Mn y M2 j )O2 (x>0.5) cathode materials"
| 锰位掺杂正极材料 | 合成方法 | 电压范围/V | 比容量/(mAh/g) | 容量保持率 |
|---|---|---|---|---|
| NaNi1/3Co1/3Mn1/3O2[ | 溶胶-凝胶法 | 2.0~3.75 | 120 | ≈100%(50th) |
| Na0.66Ni0.26Zn0.07Mn0.67O2[ | 固相法 | 2.2~4.25 | 132(12 mA/g) | 89%(30th) |
| Na7/9Cu2/9Fe1/9Mn2/3O2[ | 固相法 | 1.0~4.2 | 313(0.2 C,搭配硬碳负极), 能量密度195 Wh/kg | 85%(1 C,150th) |
| Na0.9Ni0.45Mn x Ti0.55-x O2(0≤x≤0.55)[ | 固相法 | 1.5~4.5,中值电压3.27 | 197,能量密度643 Wh/kg | 81%(15th) |
| Na0.67[(Mn0.78Fe0.22)0.9Ti0.1]O2[ | 超声波喷雾 热解法和固相法 | 1.4~4.2 | 180(0.1 C) | 86%(200th) |
| Na0.59Co0.20Mn0.77Mo0.03O2[ | 固相法 | 2.0~4.0 | 131.9(0.1 C) | 91.51%(100th) |
| Na0.75Ni1/3Ru1/6Mn1/2O2[ | 固相法 | 1.5~4.0 | 161.5(0.2 C) 119.5(10 C) | 79.5%(10 C,500th) |
| Na1-y (Ni1/3Fe1/3Mn1/3)O2(0≤y≤0.46)[ | 固相法 | 1.5~4.0 | 130(0.5 C) | 76%(150th) |
| NaNi1/4Fe1/2Mn1/4O2[ | 固相法 | 2.1~3.9 | 140(0.1 C) | 82.2%(100th) 76.1%(150th) |
| Na0.67Mn0.65Fe0.2Ni0.15O2[ | 溶胶-凝胶法 | 1.5~4.3 | 208(0.05 C) | 71%(50th) |
| Na[Ni0.60Co0.05Mn0.35]O2[ | 共沉淀法和 固相法 | 1.5~3.9 | 143(30 ℃,0.1 C) 147.6(55 ℃,0.1 C) 128.8(0 ℃,0.1 C) 114(-20 ℃,0.1 C) | 83%(55 ℃,0.1 C,100th) 89%(0 ℃,0.1 C,100th) 92%(-20 ℃,0.1 C,100th) |
| Na0.6Mn0.65Ni0.25Co0.10O2[ | 共沉淀法 | 2.1~4.3 | 148(0.2 C) | 43.2%(150th) |
| Na0.67[Ni0.4Co0.2Mn0.4]O2[ | 固相法 | 1.5~4.2① 1.5~4.4② | 138① 164② | 88.4%(①,100th) 70.7%(②,100th) |
| Na0.67(Fe0.5Mn0.5)1-x Co x O2(x=0,0.2,0.4,0.5)[ | 固相法和 共沉淀法 | 1.5~4.2 | 136.7(0.2 C) 81.1(5 C) | 85.5%(1 C,100th) |
| Na0.67Co0.25Mn0.705V0.045O2@ NaV6O15[ | 固相法 | 2.0~4.0 | 122.84(20 mA/g) | 88.35%(100th) |
| Na2/3Li1/9Ni2/9Mn2/3O2[ | 溶胶-凝胶法和 高温退火 | 2.5~4.2,中值电压3.3 | 97.6(0.2 C) 70(5 C ) | 78.7%(2 C,300th) |
| Na0.67Mn0.7Fe0.2Co0.1O2[ | 溶胶-凝胶法 | 1.5~4.2 | 171(0.05 C) 162(0.1 C) 145(0.2 C) 140(0.5 C) 123(1 C) 104(2 C) 75(5 C) 58(8 C) 45(10 C) | 89%(0.1 C,50th) |
| Na1.0Li0.2Mn0.7Ti0.1O2[ | 固相法 | 1.5~4.0 | 163(0.1 C) | 97%(50th) |
| Na0.8Mn0.6Co0.4-x Mg x O2(x=0,0.1,0.2)[ | 固相法 | 1.5~4.5 | 170(0.1 C) 130.1(0.5 C) 114.3(1 C) | 58.5%(x=2,0.1 C,120th) 82.5(x=2,1 C,120th) |
| Na0.67Ni0.28Mg0.05Mn0.67O2@ NaTi2(PO4)3 [ | 溶胶-凝胶法 | 2.5~4.3 | 130.4(0.1 C) 124.6(1 C) 106.8(5 C) | 77.4%(1 C,200th) |
| Na0.67Ni0.167Co0.167Mn0.67O2[ | 共沉淀法 | 2.0~4.3 | 132(0.1 C) | 87%(100th) |
| Na2/3Mn0.8Fe0.1Ti0.2O2[ | 固相法 | 2.0~4.0 | 144(0.1 C) | 95.09%(0.1 C,50th) 87.7%(1 C,300th) |
| Na0.67Mn0.65Ni0.2Co0.15O2[ | 溶胶-凝胶法 | 1.5~4.2 | 155(12 mA/h) | 85%(100th) |
| Na0.67[Mn0.61Ni0.28Sb0.11]O2[ | 溶胶-凝胶法 | 1.8~4.2 | 145(0.1 C) | 86%(200th) |
| Na0.67Ni0.33Mn0.66Sn0.01O2[ | 固相法 | 1.5~4.5 | 245(0.1 C) | 73%(50th) |
| Na0.8Li0.33Mn0.57Ti0.1O2[ | 溶胶-凝胶法 | 1.5~4.3 | 194 | 62.3%(100th) |
Table 7
Preparation method and electrochemical properties of Na x (Mn y Mmj )O2 (x>0.5) cathode materials"
| 锰位掺杂正极材料 | 合成方法 | 电压范围/V | 比容量/(mAh/g) | 容量保持率 |
|---|---|---|---|---|
| Na[Li0.05Mn0.50Ni0.30Cu0.10Mg0.05]O2[ | 共沉淀法 | 2.0~4.0 | 172(0.1 C,能量密度215 Wh/kg) | 70.4%(20 C,1000th) |
| Na0.66Li0.18Mn0.71Mg0.21Co0.08O2[ | 溶热法和固相法 | 1.5~4.5 | 166(20 mA/g) | 82%(100th) |
| Na2/3Mn1/2Fe1/4Co1/8Ni1/8O2[ | 固相法 | 2.0~4.2,中值电压3.3 | 121.7(130 mA/g),能量密度328.6 Wh/kg | 87.4%(100th) |
| Na0.6Li0.07Mn0.66Co0.17Ni0.17O2[ | 水热法和固相法 | 1.5~4.2 | 87(5 C) | 81%(100th) |
| Na[Ni0.4Fe0.2Mn0.2Ti0.2]O2[ | 热聚合法 | 2.0~4.2 | 145(0.1 C) | 84%(200th) |
| Na0.7Ni0.3Mn0.59Co0.1Cu0.01O2[ | 溶胶-凝胶法 | 1.5~4.0 | 150(0.1 C) | 94%(80th) |
| Na0.8Li0.1Mn0.6Ni0.2Cu0.1O2[ | 共沉淀法 | 1.8~4.0 | 135.1(0.1 C) | 81.7%(400th) |
| Na0.66Li0.18Mn0.71Ni0.21Co0.08O2+δ[ | 共沉淀法和固相法 | 1.5~4.5 | 200(0.1 C,能量密度640 Wh/kg)134(1 C) | 84%(0.2 C,50th) |
| Na(Mn0.25Fe0.25Co0.25Ni0.25)O2[ | 固相法 | 1.9~4.3 | 180(0.1 C),能量密度578 Wh/kg | ≈86%(20th) |
| NaLi0.07Ni0.26Mn0.4Co0.26O2[ | 共沉淀法 | 1.5~4.5 | 147(25 mA/g) 110(125 mA/g) | 87.1%(25mA/g,50th) 98%(125mA/g,50th) |
| NaTi0.03(Ni0.6Co0.2Mn0.2)0.97O2[ | 共沉淀法 | 1.5~4.1 | 154(0.1 C) | 77%(400th) |
| NaMn0.2Fe0.2Co0.2Ni0.2Ti0.2O2[ | 固相法 | 1.4~4.5 | 180(0.1 C) | |
| Na0.7Li0.03Mg0.03Ni0.27Mn0.6Ti0.07O2[ | 固相法 | 2.2~4.1,中值电压3.57 | 134(0.1 C) 110(4 C) | 82%(2 C,200th) |
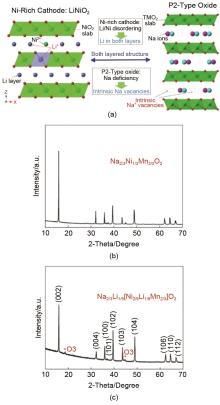
Fig. 25
(a) Schematic representation of the material structure design idea. And illustrated the Li/Ni blend as a representative of the nickel-rich cathode and the Na+ vacancies inherent in the P2 structure. XRD plots of the prepared (b) Na2/3Ni1/3Mn2/3O2 and (c) Na2/3Li1/9[Ni2/9Li1/9Mn2/3]O2 materials[100]"
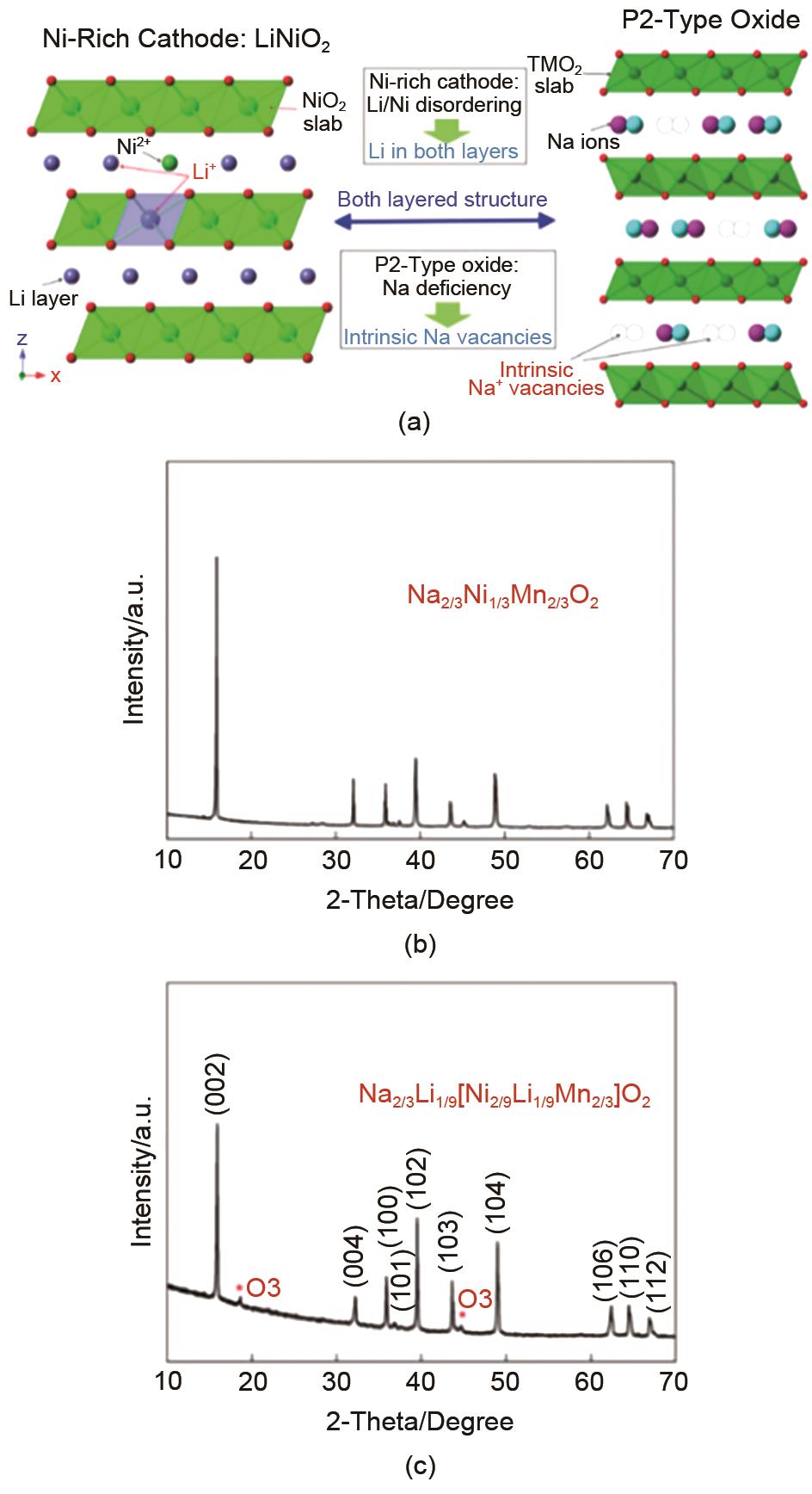
Table 8
Preparation method and electrochemical properties of (Na x A i )(Mn y M j )O2 (x>0.5) cathode materials"
| 钠位掺杂正极材料 | 合成方法 | 电压范围/V | 比容量/(mAh/g) | 容量保持率 |
|---|---|---|---|---|
| Na0.7Mg0.05[Mn0.6Ni0.2Mg0.15]O2[ | 固相法 | 1.5~4.2 | 130(0.1 C) | 73%(180th) |
| Na2/3Li1/9[Ni2/9Li1/9Mn2/3]O2[ | 固相法 | 2.0~4.2 | 64(20 C,是0.1 C时容量的58.2%) | 74.5%(1500th) |
| Na0.76Ca0.05[Ni0.23□0.08Mn0.69]O2[ | 溶胶-凝胶法 | 2.0~4.3 | 153.9(0.1 C) | |
| Na0.95Li0.15(Ni0.15Mn0.55Co0.1)O2[ | 共沉淀法 | 1.0~4.2 | 238(1th) 200(2th) | 85%(40th) |
| Na0.98Ca0.01[Ni0.5Mn0.5]O2[ | 共沉淀法 | 2.0~4.3 | 209(15 mA/g) | 75%(100th) |
Table 9
Preparation method and electrochemical properties of Na x (Mn y M j ) (O2-k nM k ) (x>0.5) cathode materials"
| 氧位掺杂正极材料 | 合成方法 | 电压范围/V | 比容量/(mAh/g) | 容量保持率 |
|---|---|---|---|---|
| Na0.6Mn0.95Ni0.05O1.95F0.05[ | 共沉淀法 | 2.5~3.9 | 80.76(2 C) | 75.0%(2 C,960th) |
| Na2/3Ni1/3Mn2/3O2-x F x (x=0,0.03,0.05,0.07)[ | 固相法 | 2.0~4.0 | 106.7(0.1 C) 104.5(0.2 C) 101.7(0.5 C) 99.1(1 C) 96(2 C) 90.9(5 C) 86.4(10 C) | 70.6%(10 C,30 ℃,2000th) 75.6%(10 C,55 ℃,2000th) |
| Na0.67Ni0.33Mn0.37Ti0.3O1.9F0.1[ | 固相法 | 2.0~4.4 | 87.7(6 C) 82.7(2C,-10 ℃) 128.1(2C,50 ℃) | 77.2%(2 C,300th) 94.2%(2 C,-10 ℃,200th) |
| Na0.67Ni0.15Fe0.2Mn0.65F0.05O1.95[ | 共沉淀法 | 1.5~4.3 | 229 100(10 C) | 87.7%(10 C,50th) |
| P2-Na0.6Mn0.7Ni0.3O2-x F x (x=0,0.03,0.05和0.07)[ | 固相法 | 1.5~3.8 | 90.5(1.0 A/g) | 78%(900th) |
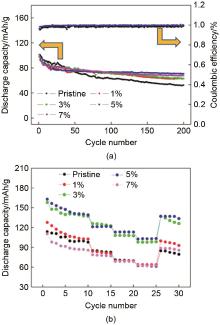
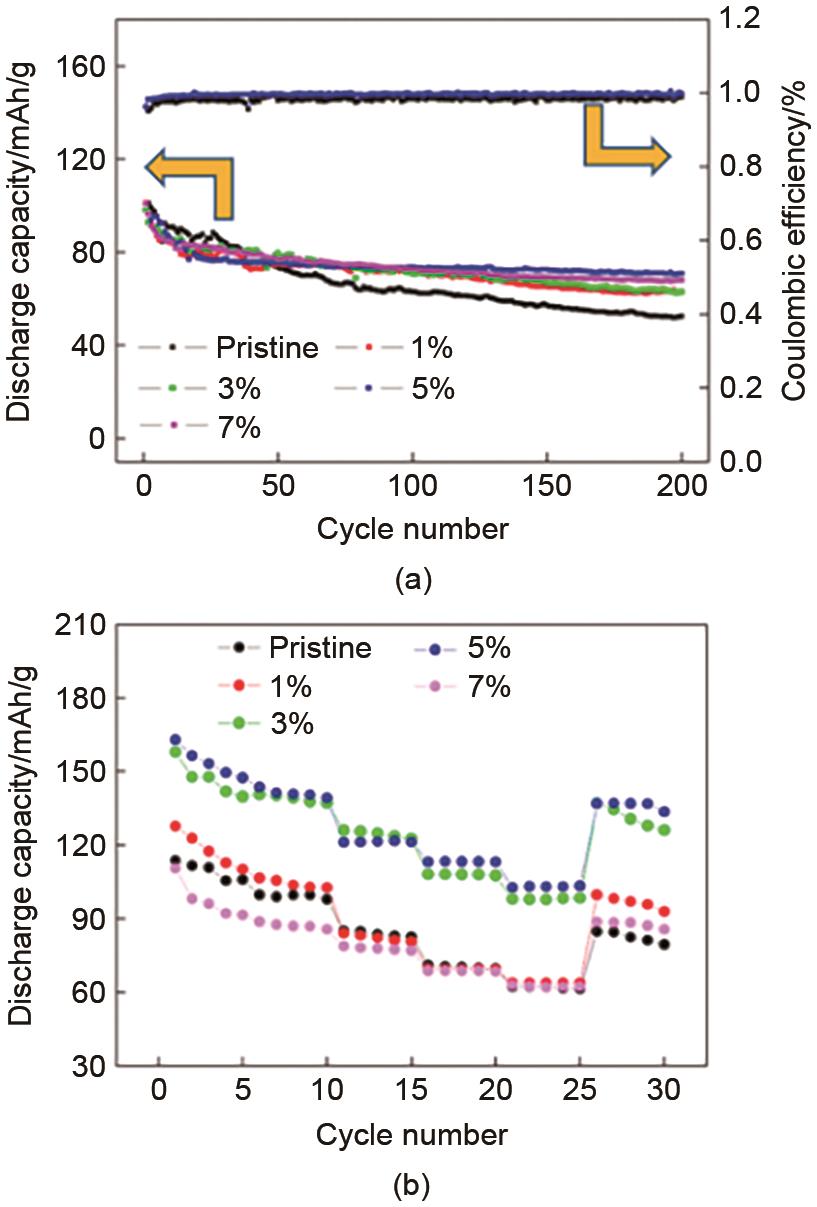
Fig. 27
Cycling performance of the original sample and samples coated with different molar concentrations of ZnO (1%, 3%, 5% and 7%) at a current density of 0.5 C; (b) rate performance of the original sample and samples coated with ZnO (1%, 3%, 5% and 7%) at different current densities (0.1, 0.2, 0.5, 1, 2 C)[47]"
| 1 | 腾讯新闻. 60GW!中信建设发布欧洲光伏组件出货强预期[EB/OL]. [2022-04-20]. https://new.qq.com/rain/a/20220420A0BIEJ00.html. |
| 2 | BP中国. BP世界能源统计年鉴2021版[EB/OL].[2021-07-08]. https://www.bp.com/zh_cn/china/home/news/reports/statistical-review-2021.html. |
| 3 | 刘英军, 刘亚奇, 张华良, 等. 我国储能政策分析与建议[J]. 储能科学与技术, 2021, 10(4): 1463-1473. |
| LIU Y J, LIU Y Q, ZHANG H L, et al. Energy storage policy analysis and suggestions in China[J]. Energy Storage Science and Technology, 2021, 10(4): 1463-1473. | |
| 4 | 朱晓辉, 庄宇航, 赵旸, 等. 钠离子电池层状正极材料研究进展[J]. 储能科学与技术, 2020, 9(5): 1340-1349. |
| ZHU X H, ZHUANG Y H, ZHAO Y, et al. Development of layered cathode materials for sodium-ion batteries[J]. Energy Storage Science and Technology, 2020, 9(5): 1340-1349. | |
| 5 | BP中国.BP世界能源统计年鉴[EB/OL].[2019-07-30].https://www.bp.com/zh_cn/china/home/news/reports/statistical-review-2019.html. |
| BP China. BP world energy statistical yearbook [EB/OL]. [2019-07-30]. https://www.bp.com/zh_cn/china/home/news/reports/statistical-review-2019.html. | |
| 6 | 袁亚琼, 司华清. 储能技术的发展前景与作用路线[J]. 技术与市场, 2019, 26(2): 77-78, 81. |
| YUAN Y Q, SI H Q. The development prospect and action route of energy storage technology[J]. Technology and Market, 2019, 26(2): 77-78, 81. | |
| 7 | 李泓, 吕迎春. 电化学储能基本问题综述[J]. 电化学, 2015, 21(5): 412-424. |
| LI H, LYU Y C. A review on electrochemical energy storage[J]. Journal of Electrochemistry, 2015, 21(5): 412-424. | |
| 8 | LI M, LU J, CHEN Z W, et al. 30 years of lithium-ion batteries[J]. Advanced Materials, 2018, 30(33): doi: 10.1002/adma.201800561. |
| 9 | 钟财富, 刘坚, 吕斌, 等. 我国新能源汽车产业锂资源需求分析及政策建议[J]. 中国能源, 2018, 40(10): 12-15, 24. |
| ZHONG C F, LIU J, LYU B, et al. Demand analysis and policy suggestions for lithium resources in China's new energy automobile industry[J]. Energy of China, 2018, 40(10): 12-15, 24. | |
| 10 | ONG S P, CHEVRIER V L, HAUTIER G, et al. Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials[J]. Energy & Environmental Science, 2011, 4(9): doi: 10.1039/c1ee01782a. |
| 11 | CHEN M Z, LIU Q N, WANG S W, et al. High-abundance and low-cost metal-based cathode materials for sodium-ion batteries: Problems, progress, and key technologies[J]. Advanced Energy Materials, 2019, 9(14): doi: 10.1002/aenm.201803609. |
| 12 | DENG J Q, LUO W B, CHOU S L, et al. Sodium-ion batteries: From academic research to practical commercialization[J]. Advanced Energy Materials, 2018, 8(4): doi: 10.1002/aenm.201701428. |
| 13 | BOMMIER C, JI X L. Electrolytes, SEI formation, and binders: A review of nonelectrode factors for sodium-ion battery anodes[J]. Small, 2018, 14(16): doi: 10.1002/smll.201703576. |
| 14 | PAN H L, HU Y S, CHEN L Q. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage[J]. Energy & Environmental Science, 2013, 6(8): doi: 10.1039/c3ee40847g. |
| 15 | YABUUCHI N, KUBOTA K, DAHBI M, et al. Research development on sodium-ion batteries[J]. Chemical Reviews, 2014, 114(23): 11636-11682. |
| 16 | CHEN L, FIORE M, WANG J E, et al. Readiness level of sodium-ion battery technology: A materials review[J]. Advanced Sustainable Systems, 2018, 2(3): doi: 10.1002/adsu.201700153. |
| 17 | SKUNDIN A M, KULOVA T L, YAROSLAVTSEV A B. Sodium-ion batteries (a review)[J]. Russian Journal of Electrochemistry, 2018, 54(2): 113-152. |
| 18 | LIANG Y R, LAI W H, MIAO Z C, et al. Nanocomposite materials for the sodium-ion battery: A review[J]. Small, 2018, 14(5): doi: 10.1002/smll.201702514. |
| 19 | KUBOTA K, DAHBI M, HOSAKA T, et al. Towards K-ion and Na-ion batteries as“beyond Li-ion”[J]. The Chemical Record, 2018, 18(4): 459-479. |
| 20 | 王勇, 刘雯, 郭瑞, 等. 钠离子电池正极材料研究进展[J]. 化工进展, 2018, 37(8): 3056-3066. |
| WANG Y, LIU W, GUO R, et al. Recent development of cathode materials for sodium-ion batteries[J]. Chemical Industry and Engineering Progress, 2018, 37(8): 3056-3066. | |
| 21 | HAN D W, KU J H, KIM R H, et al. Aluminum manganese oxides with mixed crystal structure: High-energy-density cathodes for rechargeable sodium batteries[J]. ChemSusChem, 2014, 7(7): 1870-1875. |
| 22 | 张玉婷, 徐天野, 王振华, 等. 钠离子电池关键电极材料研究进展[J]. 电子元件与材料, 2020, 39(11): 21-32. |
| ZHANG Y T, XU T Y, WANG Z H, et al. Recent advances of electrode materials for sodium ion battery[J]. Electronic Components and Materials, 2020, 39(11): 21-32. | |
| 23 | WHITACRE J F, TEVAR A, SHARMA S. Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device[J]. Electrochemistry Communications, 2010, 12(3): 463-466. |
| 24 | ZHANG Y, LIU L, JAMIL S, et al. Al2O3 coated Na0.44MnO2 as high-voltage cathode for sodium ion batteries[J]. Applied Surface Science, 2019, 494: 1156-1165. |
| 25 | LIU Y C, LIU X B, BU F, et al. Boosting fast and durable sodium-ion storage by tailoring well-shaped Na0.44MnO2 nanowires cathode[J]. Electrochimica Acta, 2019, 313: 122-130. |
| 26 | DELMAS C. Structural classification and properties of the layered oxides[J]. Physica B+C, 1980, 99(1/2/3/4): 81-85. |
| 27 | MA X H, CHEN H L, CEDER G. Electrochemical properties of monoclinic NaMnO2[J]. Journal of the Electrochemical Society, 2011, 158(12): doi: 10.1149/2.035112jes. |
| 28 | SATHIYA M, HEMALATHA K, RAMESHA K, et al. Synthesis, structure, and electrochemical properties of the layered sodium insertion cathode material: NaNi1/3Mn1/3Co1/3O2[J]. Chemistry of Materials, 2012, 24(10): 1846-1853. |
| 29 | YUAN D D, HE W, PEI F, et al. Synthesis and electrochemical behaviors of layered Na0.67[Mn0.65Co0.2Ni0.15]O2 microflakes as a stable cathode material for sodium-ion batteries[J]. Journal of Materials Chemistry A, 2013, 1(12): doi: 10.1039/c3ta01430d. |
| 30 | WANG Y, HU G R, PENG Z D, et al. Influence of Li substitution on the structure and electrochemical performance of P2-type Na0.67Ni0.2Fe0.15Mn0.65O2 cathode materials for sodium ion batteries[J]. Journal of Power Sources, 2018, 396: 639-647. |
| 31 | LU D, YAO Z J, ZHONG Y, et al. Polypyrrole-coated sodium manganate hollow microspheres as a superior cathode for sodium ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(17): 15630-15637. |
| 32 | LU D, YAO Z J, LI Y Q, et al. Sodium-rich manganese oxide porous microcubes with polypyrrole coating as a superior cathode for sodium ion full batteries[J]. Journal of Colloid and Interface Science, 2020, 565: 218-226. |
| 33 | PENG B, SUN Z H, JIAO S H, et al. Electrochemical performance optimization of layered P2-type Na0.67MnO2 through simultaneous Mn-site doping and nanostructure engineering[J]. Batteries & Supercaps, 2020, 3(2): 147-154. |
| 34 | ADAMCZYK E, PRALONG V. Na2Mn3O7: A suitable electrode material for Na-ion batteries?[J]. Chemistry of Materials, 2017, 29(11): 4645-4648. |
| 35 | SHI W J, YAN Y W, CHI C, et al. Fluorine anion doped Na0.44MnO2 with layer-tunnel hybrid structure as advanced cathode for sodium ion batteries[J]. Journal of Power Sources, 2019, 427: 129-137. |
| 36 | CHAE M S, KIM H J, LYOO J, et al. Anomalous sodium storage behavior in Al/F dual-doped P2-type sodium manganese oxide cathode for sodium-ion batteries[J]. Advanced Energy Materials, 2020, 10(43): doi: 10.1002/aenm.202002205. |
| 37 | MANIKANDAN P, RAMASUBRAMONIAN D, SHAIJUMON M M. Layered P2-type Na0.5Ni0.25Mn0.75O2 as a high performance cathode material for sodium-ion batteries[J]. Electrochimica Acta, 2016, 206: 199-206. |
| 38 | LI X L, BAO J, LI Y F, et al. Boosting reversibility of Mn-based tunnel-structured cathode materials for sodium-ion batteries by magnesium substitution[J]. Advanced Science, 2021, 8(9): doi: 10.1002/advs.202004448. |
| 39 | WANG C C, LIU L J, ZHAO S, et al. Tuning local chemistry of P2 layered-oxide cathode for high energy and long cycles of sodium-ion battery[J]. Nature Communications, 2021, 12: doi: 10.1038/s41467-021-22523-3. |
| 40 | RISTHAUS T, ZHOU D, CAO X, et al. A high-capacity P2 Na2/3Ni1/3Mn2/3O2 cathode material for sodium ion batteries with oxygen activity[J]. Journal of Power Sources, 2018, 395: 16-24. |
| 41 | MORTEMARD DE BOISSE B, CARLIER D, GUIGNARD M, et al. Structural and electrochemical characterizations of P2 and new O3-NaxMn1- yFeyO2Phases prepared by auto-combustion synthesis for Na-ion batteries[J]. Journal of the Electrochemical Society, 2013, 160(4): doi: 10.1149/2.032304jes. |
| 42 | THACKERAY M M, JOHNSON C S, AMINE K, et al. Lithium metal oxide electrodes for lithium cells and batteries: US6680143[P]. 2004-01-20. |
| 43 | YABUUCHI N, HARA R, KUBOTA K, et al. A new electrode material for rechargeable sodium batteries: P2-type Na2/3[Mg0.28Mn0.72]O2 with anomalously high reversible capacity[J]. J Mater Chem A, 2014, 2(40): doi: 10.1039/c4ta04351k. |
| 44 | 郝一帆. 钠离子电池正极材料Na0.67MnO2的掺杂改性[D]. 呼和浩特: 内蒙古大学, 2021. |
| HAO Y F. Doping modification of Na0.67MnO2 cathode material for sodium-ion batteries[D]. Hohhot: Inner Mongolia University, 2021. | |
| 45 | ZHU Y E, QI X G, CHEN X Q, et al. A P2-Na0.67Co0.5Mn0.5O2 cathode material with excellent rate capability and cycling stability for sodium ion batteries[J]. Journal of Materials Chemistry A, 2016, 4(28): doi: 10.1039/c6ta02845d. |
| 46 | WANG P F, YOU Y, YIN Y X, et al. An O3-type NaNi0.5Mn0.5O2cathode for sodium-ion batteries with improved rate performance and cycling stability[J]. Journal of Materials Chemistry A, 2016, 4(45): doi: 10.1039/c6ta07589d. |
| 47 | YANG Y, DANG R, WU K, et al. Semiconductor material ZnO-coated P2-type Na2/3Ni1/3Mn2/3O2 cathode materials for sodium-ion batteries with superior electrochemical performance[J]. The Journal of Physical Chemistry C, 2019, 124(3): doi: 10.1021/acs.jpcc.9b08220. |
| 48 | WANG H, YANG B J, LIAO X Z, et al. Electrochemical properties of P2-Na2/3[Ni1/3Mn2/3]O2 cathode material for sodium ion batteries when cycled in different voltage ranges[J]. Electrochimica Acta, 2013, 113: 200-204. |
| 49 | BILLAUD J, SINGH G, ARMSTRONG A R, et al. Na0.67Mn1- xMgxO2 (0≤x≤0.2): A high capacity cathode for sodium-ion batteries[J]. Energy Environ Sci, 2014, 7(4): 1387-1391. |
| 50 | KWON M S, LIM S G, PARK Y, et al. P2 orthorhombic Na0.7[Mn1- xLix]o2+ y as cathode materials for Na-ion batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(17): doi: 10.1021/acsami.7b00058. |
| 51 | JIANG K Z, ZHANG X P, LI H Y, et al. Suppressed the high-voltage phase transition of P2-type oxide cathode for high-performance sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(16): doi: 10.1021/acsami.9b03326. |
| 52 | QIN M L, YIN C Y, XU W, et al. Facile synthesis of high capacity P2-type Na2/3Fe1/2Mn1/2O2 cathode material for sodium-ion batteries[J]. Transactions of Nonferrous Metals Society of China, 2021, 31(7): 2074-2080. |
| 53 | SOARES C, SILVÁN B, CHOI Y S, et al. Na2.4Al0.4Mn2.6O7 anionic redox cathode material for sodium-ion batteries-a combined experimental and theoretical approach to elucidate its charge storage mechanism[J]. Journal of Materials Chemistry A, 2022, 10(13): 7341-7356. |
| 54 | KONG W J, YANG W Y, DE NING, et al. Tuning anionic/cationic redox chemistry in a P2-type Na0.67Mn0.5Fe0.5O2 cathode material via a synergic strategy[J]. Science China Materials, 2020, 63(9): 1703-1718. |
| 55 | WANG L J, ZHANG X, YANG X H, et al. Co3O4-modified P2-Na2/3Mn0.75Co0.25O2 cathode for Na-ion batteries with high capacity and excellent cyclability[J]. Journal of Alloys and Compounds, 2020, 832: doi: 10.1016/j.jallcom.2020.154960. |
| 56 | QUYEN N Q, NGUYEN T V, THANG H H, et al. Carbon coated NaLi0.2Mn0.8O2 as a superb cathode material for sodium ion batteries[J]. Journal of Alloys and Compounds, 2021, 866: doi: 10.1016/j.jallcom.2021.158950. |
| 57 | VAALMA C, BUCHHOLZ D, PASSERINI S. Beneficial effect of boron in layered sodium-ion cathode materials-The example of Na2/3B0.11Mn0.89O2[J]. Journal of Power Sources, 2017, 364: 33-40. |
| 58 | ZHAO J, XU J, LEE D H, et al. Electrochemical and thermal properties of P2-type Na2/3Fe1/3Mn2/3O2 for Na-ion batteries[J]. Journal of Power Sources, 2014, 264: 235-239. |
| 59 | DE LA LLAVE E, TALAIE E, LEVI E, et al. Improving energy density and structural stability of manganese oxide cathodes for Na-ion batteries by structural lithium substitution[J]. Chemistry of Materials, 2016, 28(24): 9064-9076. |
| 60 | BUCHHOLZ D, VAALMA C, CHAGAS L G, et al. Mg-doping for improved long-term cyclability of layered Na-ion cathode materials-The example of P2-type NaxMg0.11Mn0.89O2[J]. Journal of Power Sources, 2015, 282: 581-585. |
| 61 | WU X H, GUO J H, WANG D W, et al. P2-type Na0.66Ni0.33- xZnxMn0.67O2 as new high-voltage cathode materials for sodium-ion batteries[J]. Journal of Power Sources, 2015, 281: 18-26. |
| 62 | LI Y M, YANG Z Z, XU S Y, et al. Air-stable copper-based P2-Na7/9Cu2/9Fe1/9Mn2/3O2 as a new positive electrode material for sodium-ion batteries[J]. Advanced Science, 2015, 2(6): doi: 10.1002/advs.201500031. |
| 63 | ZHENG L T, OBROVAC M N. Investigation of O3-type Na0.9Ni0.45MnxTi0.55- xO2 (0≤x≤0.55) as positive electrode materials for sodium-ion batteries[J]. Electrochimica Acta, 2017, 233: 284-291. |
| 64 | PARK Y J, CHOI J U, JO J H, et al. A new strategy to build a high-performance P'2-type cathode material through titanium doping for sodium-ion batteries[J]. Advanced Functional Materials, 2019, 29(28): doi: 10.1002/adfm.201901912. |
| 65 | ZHAO J B, ZHANG X, WANG J L, et al. P2-type Na0.59Co0.20Mn0.77Mo0.03O2 cathode with excellent cycle stability for sodium-ion batteries[J]. Journal of Solid State Electrochemistry, 2020, 24(6): 1349-1361. |
| 66 | WANG Q, JIANG K Z, FENG Y Z, et al. P2-type layered Na0.75Ni1/3Ru1/6Mn1/2O2 cathode material with excellent rate performance for sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(35): doi: 10.1021/acsami.0c09082. |
| 67 | KIM D, LEE E, SLATER M, et al. Layered Na[Ni1/3Fe1/3Mn1/3]O2 cathodes for Na-ion battery application[J]. Electrochemistry Communications, 2012, 18: 66-69. |
| 68 | OH S M, MYUNG S T, YOOM C S, et al. Advanced Na[Ni0.25Fe0.5Mn0.25]O2/C-Fe3O4 sodium-ion batteries using EMS electrolyte for energy storage[J]. Nano letters, 2014, 14(3): 1620-1626. |
| 69 | YUAN D D, HU X H, QIAN J F, et al. P2-type Na0.67Mn0.65Fe0.2Ni0.15O2 cathode material with high-capacity for sodium-ion battery[J]. Electrochimica Acta, 2014, 116: 300-305. |
| 70 | HWANG J Y, OH S M, MYUNG S T, et al. Radially aligned hierarchical columnar structure as a cathode material for high energy density sodium-ion batteries[J]. Nature Communications, 2015, 6: 6865. |
| 71 | NGUYEN N A, KIM K, CHOI K H, et al. Effect of calcination temperature on a P-type Na0.6Mn0.65Ni0.25Co0.10O2Cathode material for sodium-ion batteries[J]. Journal of the Electrochemical Society, 2016, 164(1): doi: 10.1149/2.0511701jes. |
| 72 | SUN X, JI X Y, XU H Y, et al. Sodium insertion cathode material Na0.67[Ni0.4Co0.2Mn0.4]O2 with excellent electrochemical properties[J]. Electrochimica Acta, 2016, 208: 142-147. |
| 73 | ZHOU D M, HUANG W X, ZHAO F L. P2-type Na0.67Fe0.3Mn0.3Co0.4O2 cathodes for high-performance sodium-ion batteries[J]. Solid State Ionics, 2018, 322: 18-23. |
| 74 | TANG J T, WANG Y Z, WANG L J, et al. Excellent cycle stability of P2Na0.67Co0.25Mn0.705V0.045O2@ NaV6O15 composite cathode for sodium ion battery[J]. Materials Chemistry and Physics, 2019, 224: 1-9. |
| 75 | LI S, XIAO Y, ZHU Y F, et al. A Li-substituted hydrostable layered oxide cathode material with oriented stacking nanoplate structure for high-performance sodium-ion battery[J]. Chemical Engineering Journal, 2021, 412: doi: 10.1016/j.cej.2021.128719. |
| 76 | KONG W J, WANG H B, ZHAI Y W, et al. Enhancing the rate capability and cycling stability of Na0.67Mn0.7Fe0.2Co0.1O2 through a synergy of Zr4+ doping and ZrO2 coating[J]. The Journal of Physical Chemistry C, 2018, 122(45): 25909-25916. |
| 77 | TO N V, NGUYEN K V, NGUYEN H S, et al. P2-type layered structure Na1.0Li0.2Mn0.7Ti0.1O2 as a superb electrochemical performance cathode material for sodium-ion batteries[J]. Journal of Electroanalytical Chemistry, 2021, 880: doi: 10.1016/j.jelechem. 2020.114834. |
| 78 | LI X L, BAO J, SHADIKE Z, et al. Stabilizing transition metal vacancy induced oxygen redox by Co2+/Co3+ redox and sodium-site doping for layered cathode materials[J]. Angewandte Chemie International Edition, 2021, 60(40): doi: 10.1002/anie.202108933. |
| 79 | TANG K, HUANG Y, XIE X, et al. The effects of dual modification on structure and performance of P2-type layered oxide cathode for sodium-ion batteries[J]. Chemical Engineering Journal, 2020, 384: doi: 10.1016/j.cej.2019.123234. |
| 80 | BAO S, LUO S H, WANG Z Y, et al. Novel P2-type concentration-gradient Na0.67Ni0.167Co0.167Mn0.67O2 modified by Mn-rich surface as cathode material for sodium ion batteries[J]. Journal of Power Sources, 2018, 396: 404-411. |
| 81 | HAN M H, GONZALO E, SHARMA N, et al. High-performance P2-phase Na2/3Mn0.8Fe0.1Ti0.1O2 cathode material for ambient-temperature sodium-ion batteries[J]. Chemistry of Materials, 2016, 28(1): 106-116. |
| 82 | LI Z Y, GAO R, SUN L M, et al. Designing an advanced P2-Na0.67Mn0.65Ni0.2Co0.15O2 layered cathode material for Na-ion batteries[J]. Journal of Materials Chemistry A, 2015, 3(31): doi: 10.1039/c5ta02450a. |
| 83 | WANG Q C, SHADIKE Z, LI X L, et al. Tuning sodium occupancy sites in P2-layered cathode material for enhancing electrochemical performance[J]. Advanced Energy Materials, 2021, 11(13): doi: 10.1002/aenm.202003455. |
| 84 | LI J K, RISTHAUS T, WANG J, et al. The effect of Sn substitution on the structure and oxygen activity of Na0.67Ni0.33Mn0.67O2 cathode materials for sodium ion batteries[J]. Journal of Power Sources, 2020, 449: doi: 10.1016/j.jpowsour.2019.227554. |
| 85 | MA X H, LI L L, CHENG L, et al. P2-type Na0.8(Li0.33Mn0.67- xTix)O2 doped by Ti as cathode materials for high performance sodium-ion batteries[J]. Journal of Alloys and Compounds, 2020, 815: doi: 10.1016/j.jallcom.2019.152402. |
| 86 | DENG J Q, LUO W B, LU X, et al. High energy density sodium-ion battery with industrially feasible and air-stable O3-type layered oxide cathode[J]. Advanced Energy Materials, 2018, 8(5): doi: 10.1016/j.jallcom.2019.152402. |
| 87 | XIAO J, ZHANG F, TANG K K, et al. Rational design of a P2-type spherical layered oxide cathode for high-performance sodium-ion batteries[J]. ACS Central Science, 2019, 5(12): 1937-1945. |
| 88 | CHU S Y, CHEN Y B, WANG J, et al. A cobalt and nickel co-modified layered P2-Na2/3Mn1/2Fe1/2O2 with excellent cycle stability for high-energy density sodium-ion batteries[J]. Journal of Alloys and Compounds, 2019, 775: 383-392. |
| 89 | QIU J X, CHEN B R, HOU H Y, et al. Improving na+ diffusion and performance of P2-type layered Na0.6Li0.07Mn0.66Co0.17Ni0.17O2 by expanding the interplanar spacing[J]. ACS Applied Materials & Interfaces, 2020, 12(43): doi: 10.1021/acsami.0c14931. |
| 90 | SUN X, JIN Y, ZHANG C Y, et al. Na[Ni0.4Fe0.2Mn0.4– xTix]O2: a cathode of high capacity and superior cyclability for Na-ion batteries[J]. J Mater Chem A, 2014, 2(41): doi: 10.1039/c4ta03828b. |
| 91 | TIWARI B, BHATTACHARYA I. Layered P2-type novel Na0.7Ni0.3Mn0.59Co0.1Cu0.01O2 cathode material for high-capacity & stable rechargeable sodium ion battery[J]. Electrochimica Acta, 2018, 270: 363-368. |
| 92 | CHEN T, GUO J, ZHUO Y, et al. An inactive metal supported oxide cathode material with high rate capability for sodium ion batteries[J]. Energy Storage Materials, 2019, 20: 263-268. |
| 93 | GUO S H, LIU P, YU H J, et al. A layered P2- and O3-type composite as a high-energy cathode for rechargeable sodium-ion batteries[J]. Angewandte Chemie International Edition, 2015, 54(20): 5894-5899. |
| 94 | LI X, WU D, ZHOU Y N, et al. O3-type Na(Mn0.25Fe0.25Co0.25Ni0.25)O2: A quaternary layered cathode compound for rechargeable Na ion batteries[J]. Electrochemistry Communications, 2014, 49: 51-54. |
| 95 | XU J, LIU H D, MENG Y S, et al. Exploring Li substituted O3- structured layered oxides NaLixNi1/3- xMn1/3+ xCo1/3- xO2 (x = 0.07, 0.13, and 0.2) as promising cathode materials for rechargeable Na batteries[J]. Electrochemistry Communications, 2015, 60: 13-16. |
| 96 | YU T Y, HWANG J Y, BAE I T, et al. High-performance Ti-doped O3-type Na[Tix(Ni0.6Co0.2Mn0.2)1- x]O2 cathodes for practical sodium-ion batteries[J]. Journal of Power Sources, 2019, 422: 1-8. |
| 97 | WALCZAK K, PLEWA A, GHICA C, et al. NaMn0.2Fe0.2Co0.2Ni0.2Ti0.2O2 high-entropy layered oxide-experimental and theoretical evidence of high electrochemical performance in sodium batteries[J]. Energy Storage Materials, 2022, 47: 500-514. |
| 98 | CHENG Z W, ZHAO B, GUO Y J, et al. Mitigating the large-volume phase transition of P2-type cathodes by synergetic effect of multiple ions for improved sodium-ion batteries[J]. Advanced Energy Materials, 2022, 12(14): doi: 10.1002/aenm.202103461. |
| 99 | WANG Q C, MENG J K, YUE X Y, et al. Tuning P2-structured cathode material by Na-site Mg substitution for Na-ion batteries[J]. Journal of the American Chemical Society, 2019, 141(2): 840-848. |
| 100 | LI Z Y, MA X B, SUN K, et al. Na2/3Li1/9[Ni2/9Li1/9Mn2/3]O2: A high-performance solid-solution reaction layered oxide cathode material for sodium-ion batteries[J]. ACS Applied Energy Materials, 2022, 5(1): 1126-1135. |
| 101 | SHEN Q Y, LIU Y C, ZHAO X D, et al. Transition-metal vacancy manufacturing and sodium-site doping enable a high-performance layered oxide cathode through cationic and anionic redox chemistry[J]. Advanced Functional Materials, 2021, 31(51): doi: 10.1002/adfm.202106923. |
| 102 | KATAOKA R, MUKAI T, YOSHIZAWA A, et al. Development of high capacity cathode material for sodium ion batteries Na0.95Li0.15(Ni0.15Mn0.55Co0.1)O2[J]. Journal of the Electrochemical Society, 2013, 160(6): doi: 10.1149/2.125306jes. |
| 103 | YU T Y, KIM J, HWANG J Y, et al. High-energy O3-Na1–2 xCax[Ni0.5Mn0.5]O2 cathodes for long-life sodium-ion batteries[J]. Journal of Materials Chemistry A, 2020, 8(27): doi: 10.1039/d0ta04847j. |
| 104 | CHEN H, WU Z G, ZHONG Y J, et al. Boosting the reactivity of Ni2+/Ni3+ redox couple via fluorine doping of high performance Na0.6Mn0.95Ni0.05O2- xFx cathode[J]. Electrochimica Acta, 2019, 308: 64-73. |
| 105 | LIU K, TAN S S, MOON J, et al. Insights into the enhanced cycle and rate performances of the F-substituted P2-type oxide cathodes for sodium-ion batteries[J]. Advanced Energy Materials, 2020, 10(19): doi: 10.1002/aenm.202000135. |
| 106 | ZHOU P F, ZHANG J, CHE Z N, et al. Insights into the enhanced structure stability and electrochemical performance of Ti4+/F- co-doped P2-Na0.67Ni0.33Mn0.67O2 cathodes for sodium ion batteries at high voltage[J]. Journal of Energy Chemistry, 2022, 67: 655-662. |
| 107 | CUI X L, WANG S M, YE X S, et al. Insights into the improved cycle and rate performance by ex-situ F and in situ Mg dual doping of layered oxide cathodes for sodium-ion batteries[J]. Energy Storage Materials, 2022, 45: 1153-1164. |
| 108 | KANG W P, MA P, LIU Z N, et al. Tunable electrochemical activity of P2-Na0.6Mn0.7Ni0.3O2- xFx microspheres as high-rate cathodes for high-performance sodium ion batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(13): doi: 10.1021/acsami.1c02216. |
| 109 | LIU W, REN Q C, YANG M, et al. Na0.44MnO2 coated with In2O3 as a high-voltage cathode for sodium-ion batteries[J]. Journal of Alloys and Compounds, 2022, 896: doi: 10.1016/j.jallcom.2021.163087. |
| [1] | Mengyang ZU, Meng ZHANG, Zikun LI, Ling HUANG. Cycle performance and degradation mechanism of Ni-Rich NCA, NCM, and NCMA [J]. Energy Storage Science and Technology, 2023, 12(1): 51-60. |
| [2] | Shaocong WANG, Wei LI, Ruiqin HUANG, Yifei GUO, Zheng LIU. Progress of the Jahn-Teller effect suppression method for manganese-based sodium-ion battery cathode materials [J]. Energy Storage Science and Technology, 2023, 12(1): 139-149. |
| [3] | Kaiqiang GUO, Haiying CHE, Haoran ZHANG, Jianping LIAO, Huang ZHOU, Yunlong ZHANG, Hangda CHEN, Zhan SHEN, Haimei LIU, Zifeng MA. Preparation and characterization of B2O3-coated NaNi1/3Fe1/3Mn1/3O2 cathode materials for sodium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2980-2988. |
| [4] | ZHANG Yan, WANG Hai, LIU Zhaomeng, ZHANG Deliu, WANG Jiadong, LI Jianzhong, GAO Xuanwen, LUO Wenbin. Research progress of nickel-rich ternary cathode material ncm for lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1693-1705. |
| [5] | ZHAO Yifei, YANG Zhendong, LI Feng, XIE Zhaojun, ZHOU Zhen. Nitrogen-doped carbon-coated Na3V2 (PO4 ) 2F3 cathode materials for sodium-ion batteries: Preparation and electrochemical performance [J]. Energy Storage Science and Technology, 2022, 11(6): 1883-1891. |
| [6] | WU Yida, ZHANG Yi, ZHAN Yuanjie, GUO Yaqi, ZHANG liao, LIU Xingjiang, YU Hailong, ZHAO Wenwu, HUANG Xuejie. The effect of B2O3 modification on the electrochemical properties of LiCoO2 cathode [J]. Energy Storage Science and Technology, 2022, 11(6): 1687-1692. |
| [7] | Chang SUN, Zerong DENG, Ningbo JIANG, Lulu ZHANG, Hui FANG, Xuelin YANG. Recent research progress of sodium vanadium fluorophosphate as cathode material for sodium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(4): 1184-1200. |
| [8] | Xiaohan FENG, Jie SUN, Jianhao HE, Yihua WEI, Chenggang ZHOU, Ruimin SUN. Research progress in LiFePO4 cathode material modification [J]. Energy Storage Science and Technology, 2022, 11(2): 467-486. |
| [9] | Fanju MENG, Xi ZHANG, Zhijun QIAO, Bin YANG, Miao YU, Yuzuo WANG, Dianbo RUAN. Study on the effects of carbon coating on lithium-storage kinetics for soft carbon [J]. Energy Storage Science and Technology, 2022, 11(11): 3548-3557. |
| [10] | Shouli WEI, Xichao LI, Xiuliang CHANG, Bing CHEN, Zhuo XU, Tao ZHANG, Lili ZHENG, Zuoqiang DAI. Review of development of bipolar plate materials for solid oxide fuel cell [J]. Energy Storage Science and Technology, 2021, 10(6): 1943-1951. |
| [11] | Qiang CHEN, Min LI, Jingfa LI. Application of Prussian blue analogs and their derivatives in potassium ion batteries [J]. Energy Storage Science and Technology, 2021, 10(3): 1002-1015. |
| [12] | Yongli HENG, Zhenyi GU, Jinzhi GUO, Xinglong WU. Na3V2(PO4)3@C cathode material for aqueous zinc-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(3): 938-944. |
| [13] | Min'an YANG, Ning CHEN, Bo WANG, Qian ZHANG, Jingpei CHEN, Hailei ZHAO, Fushen LI. Gene law about cycle stability of cathode material for lithium-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(2): 462-469. |
| [14] | Weihua LIANG, Dayong WU, Junguo SHU. Theoretical analysis and numerical simulation of comma roll coating flow field [J]. Energy Storage Science and Technology, 2021, 10(2): 565-576. |
| [15] | Zuhao ZHANG, Xiaokai DING, Dong LUO, Jiaxiang CUI, Huixian XIE, Chenyu LIU, Zhan LIN. Challenges and solutions of lithium-rich manganese-based layered oxide cathode materials [J]. Energy Storage Science and Technology, 2021, 10(2): 408-424. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

