Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (3): 898-912.doi: 10.19799/j.cnki.2095-4239.2025.0030
• Emerging Investigator Issue of Energy Storage • Previous Articles Next Articles
Tete HE1( ), Yang LU1(
), Yang LU1( ), Yang LIU2, Bin XU2, Yongle CHEN2, Fangyang LIU1(
), Yang LIU2, Bin XU2, Yongle CHEN2, Fangyang LIU1( )
)
Received:2025-01-07
Revised:2025-02-05
Online:2025-03-28
Published:2025-04-28
Contact:
Yang LU, Fangyang LIU
E-mail:1160794790@qq.com;lu_yang@csu.edu.cn;liufangyang@csu.edu.cn
CLC Number:
Tete HE, Yang LU, Yang LIU, Bin XU, Yongle CHEN, Fangyang LIU. Lithium sulfide: the "cornerstone" material in the era of all-solid-state batteries[J]. Energy Storage Science and Technology, 2025, 14(3): 898-912.
Table 1
Summary of preparation and test conditions of representative sulfide solid-state electrolytes[8-17]"
| 材料化学式 | 制备工艺 | 电导率测试关键参数 | 室温电导率/(mS/cm) |
|---|---|---|---|
| Li9.54Si1.74P1.44S11.7Cl0.3 | 固相球磨-压片-密封烧结 | 制片压强:未提及 阻塞电池:金阻塞电池 频率:0.1 Hz~3 MHz | 25 |
| Li5.5PS4.5Cl1.5 | 固相球磨-压片-密封烧结 | 制片压强:370 MPa 阻塞电池:不锈钢阻塞电池 频率:1 Hz~7 MHz | 9.5 |
| Li7P3S11 | 固相球磨-烧结 | 制片压强:未提及 阻塞电池:气相沉积Au阻塞电池 频率:0.1 Hz~7 MHz | 2 |
| Li3PS4 | 液相反应-密封烧结 | 制片压强:未提及 阻塞电池:涂炭铝箔阻塞电池 频率:1 Hz~1 MHz | 0.16 |
| Li7P3S7.5O3.5 | 固相球磨-烧结 | 制片压强:380 MPa 阻塞电池:不锈钢阻塞电池 频率:1 Hz~35 MHz | 0.46 |
| Li6.8Si0.8As0.2S5I | 固相球磨-压片-密封烧结 | 制片压强:870 MPa 阻塞电池:Swagelok模型电池 频率:1 Hz~8 MHz | 10.4 |
| Li10GeP2S12 | 固相球磨-压片-密封烧结 | 制片压强:未提及 阻塞电池:涂覆金浆,500 ℃烧结 频率范围:0.1 Hz~1 MHz | 12 |
Li9.54[Si0.6Ge0.4]1.74P1.44 S11.1Br0.3O0.6 | 固相球磨-压片-密封烧结 | 制片压强:370MPa 阻塞电池:涂覆金浆,500 ℃烧结 频率范围:0.1 Hz~1 MHz | 32 |
Table 2
The typical quality evaluation indexes of lithium sulfide"
| 指标 | 测试方法 | 测量目标 | 意义 |
|---|---|---|---|
| 粉体颜色(白度) | 目视(白度仪) | 碳、多硫等显色杂质 | 杂质对电解质制备及性能产生显著影响 |
| 物相 | XRD | 无机盐杂质 | 氢氧化锂、氧化锂之类的杂质相在电解质制备中造成杂质相生成,降低电导率 |
| 水含量 | 卡尔-费休水分测试仪 | 硫化锂含水量 | 水分对硫化锂的储存、使用影响较大 |
| 碳含量 | 红外碳硫分析仪 测试电子电导 | 硫化锂含碳量 | 碳含量会造成电解质的电子电导率升高,并可能诱导电解质分解 |
| 溶剂含量 | GC-MS/TGA-DSC | 硫化锂的有机溶剂残留 | 溶剂影响计量比,在后续电解质烧结时碳化会提高电子电导率 |
| 水溶颜色 | 溶于水后的水溶液颜色 | 金属离子、多硫等杂质 | 金属离子或多硫杂质超标会对电解质化学计量比以及晶体结构产生不利影响 |
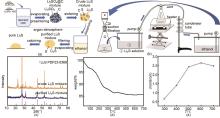
Fig. 11
(a) The preparation processes of crude lithium sulfide by carbothermic reduction method; (b) Purification processes of lithium sulfide crude product; (c) The relative phases of the lithium sulfide product before and after purification; (d) The thermogravimetric curve of purified lithium sulfide; (e) The evolutions of the ionic conductivity of solid-state electrolytes prepared from lithium sulfide dried at different temperatures[21]"
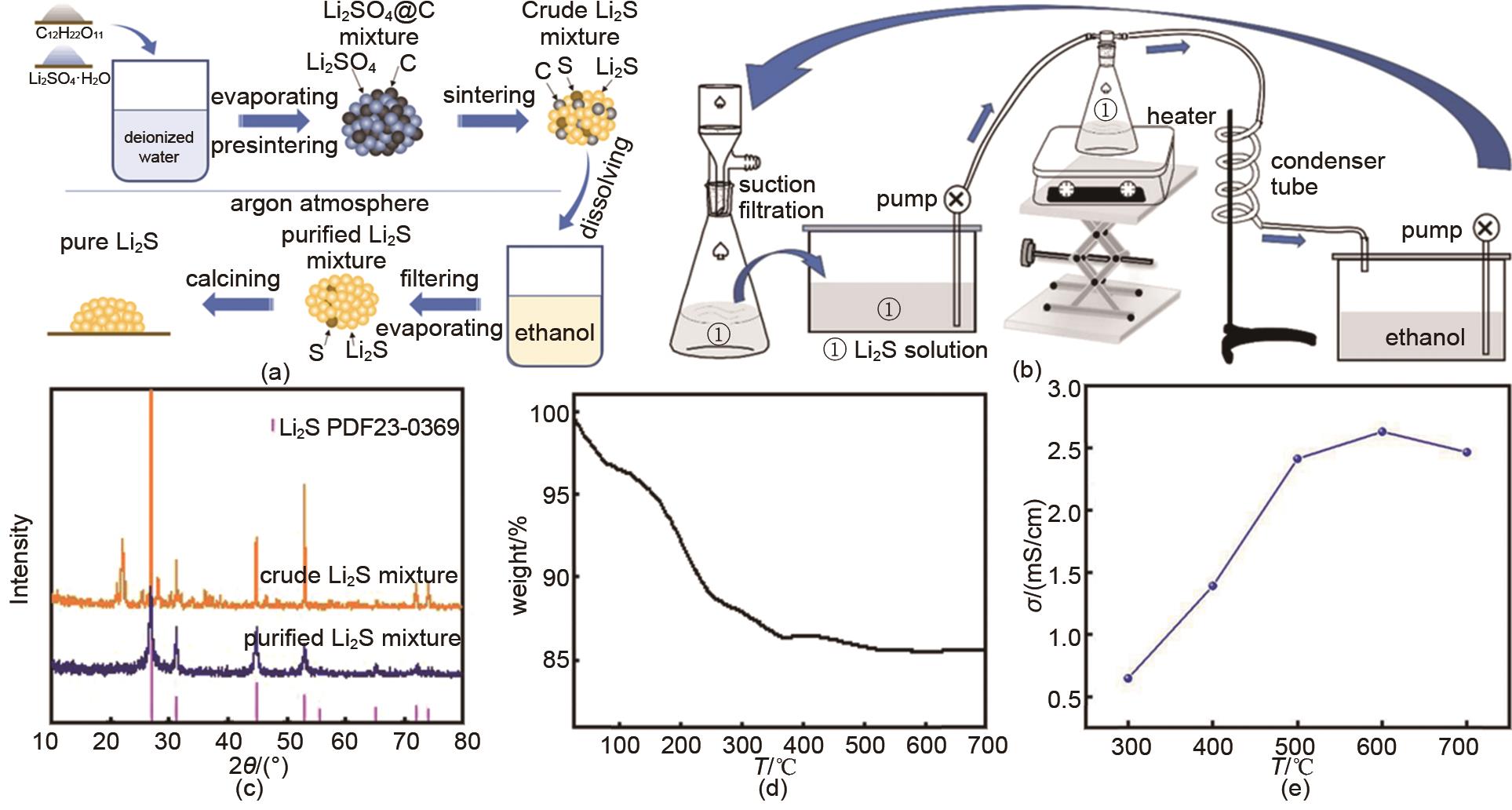
Table 3
Comparison of raw material costs of each process route for lithium sulfide preparation"
| 反应类型 | 反应方程式 | 锂源量/t | 硫源量/t | 助剂量/t | 单吨硫化锂的原料成本/(万元/t) |
|---|---|---|---|---|---|
| 碳热还原工艺 | 2C+Li2SO4·H2O=Li2S+2CO2+H2O | 2.78 | 0.52 | 13.61 | |
| 硫化氢中和工艺 | H2S+2LiOH·H2O=Li2S+4H2O | 1.83 | 0.92 | — | 14.61 |
| 水合肼还原工艺 | 2S+4LiOH·H2O+N2H4·H2O =2Li2S+N2(g)+9H2O | 1.83 | 0.70 | 0.68 | 14.75 |
| 复分解工艺 | Na2S·nH2O+2LiCl=Li2S+2NaCl+nH2O | 1.85 | 2.83 | — | 15.01 |
| 锂硫化合工艺 | 2Li+S=Li2S | 0.30 | 0.70 | — | 21.26 |
| 1 | TAN D H S, MENG Y S, JANG J. Scaling up high-energy-density sulfidic solid-state batteries: A lab-to-pilot perspective[J]. Joule, 2022, 6(8): 1755-1769. DOI:10.1016/j.joule.2022.07.002. |
| 2 | ZHANG Q, CAO D X, MA Y, et al. Sulfide-based solid-state electrolytes: Synthesis, stability, and potential for all-solid-state batteries[J]. Advanced Materials, 2019, 31(44): e1901131. DOI:10.1002/adma.201901131. |
| 3 | REN D S, LU L G, HUA R, et al. Challenges and opportunities of practical sulfide-based all-solid-state batteries[J]. eTransportation, 2023, 18: 100272. DOI:10.1016/j.etran.2023.100272. |
| 4 | 吴敬华, 杨菁, 刘高瞻, 等. 固态锂电池十年(2011—2021)回顾与展望[J]. 储能科学与技术, 2022, 11(9): 2713-2745. DOI: 10.19799/j.cnki.2095-4239.2022.0309. |
| WU J H, YANG J, LIU G Z, et al. Review and prospective of solid-state lithium batteries in the past decade(2011—2021)[J]. Energy Storage Science and Technology, 2022, 11(9): 2713-2745. DOI: 10.19799/j.cnki.2095-4239.2022.0309. | |
| 5 | 殷高峰. 发展前景被坚定看好 各方持续投研"下一代电池"技术 [Z]. 证券日报. 2024. |
| YIN G F. The development prospect is firmly optimistic. All parties continue to invest in the "next generation battery" technology [Z]. Securities Daily. 2024. | |
| 6 | 曾朵红, 阮巧燕. 硫化物未来潜力最大,开启电池发展新纪元[R]. 苏州: 东吴证券, 2024. |
| ZENG D H, RUAN Q Y. Sulfide has the greatest potential in the future, opening a new era of battery development[R]. Suzhou: Soochow Securities, 2024. | |
| 7 | KUDU Ö U, FAMPRIKIS T, FLEUTOT B, et al. A review of structural properties and synthesis methods of solid electrolyte materials in the Li2S-P2S5 binary system[J]. Journal of Power Sources, 2018, 407: 31-43. DOI:10.1016/j.jpowsour.2018.10.037. |
| 8 | ADELI P, BAZAK J D, PARK K H, et al. Boosting solid-state diffusivity and conductivity in lithium superionic argyrodites by halide substitution[J]. Angewandte Chemie (International Ed), 2019, 58(26): 8681-8686. DOI:10.1002/anie.201814222. |
| 9 | KAMAYA N, HOMMA K, YAMAKAWA Y, et al. A lithium superionic conductor[J]. Nature Materials, 2011, 10(9): 682-686. DOI:10. 1038/nmat3066. |
| 10 | KATO Y, HORI S, SAITO T, et al. High-power all-solid-state batteries using sulfide superionic conductors[J]. Nature Energy, 2016, 1(4): 16030. DOI:10.1038/nenergy.2016.30. |
| 11 | LU P S, XIA Y, SUN G C, et al. Realizing long-cycling all-solid-state Li-In||TiS2 batteries using Li6+ xMxAs1- xS5I (M=Si, Sn) sulfide solid electrolytes[J]. Nature Communications, 2023, 14: 4077. DOI:10.1038/s41467-023-39686-w. |
| 12 | LI Y X, SONG S B, KIM H, et al. A lithium superionic conductor for millimeter-thick battery electrode[J]. Science, 2023, 381(6653): 50-53. DOI:10.1126/science.add7138. |
| 13 | HAYASHI A, HAMA S, MORIMOTO H, et al. Preparation of Li2S-P2S5 amorphous solid electrolytes by mechanical milling[J]. Journal of the American Ceramic Society, 2001, 84(2): 477-479. DOI:10.1111/j.1151-2916.2001.tb00685.x. |
| 14 | TATSUMISAGO M, HAMA S, HAYASHI A, et al. New lithium ion conducting glass-ceramics prepared from mechanochemical Li2S-P2S5 glasses[J]. Solid State Ionics, 2002, 154: 635-640. DOI:10.1016/S0167-2738(02)00509-X. |
| 15 | WANG H, HOOD Z D, XIA Y N, et al. Fabrication of ultrathin solid electrolyte membranes of β-Li3PS4 nanoflakes by evaporation-induced self-assembly for all-solid-state batteries[J]. Journal of Materials Chemistry A, 2016, 4(21): 8091-8096. DOI:10.1039/C6TA02294D. |
| 16 | LIU Z C, FU W J, PAYZANT E A, et al. Anomalous high ionic conductivity of nanoporous β-Li3PS4[J]. Journal of the American Chemical Society, 2013, 135(3): 975-978. DOI:10.1021/ja311 0895. |
| 17 | LI H, LIN Q S, WANG J Z, et al. A cost-effective sulfide solid electrolyte Li7P3S7.5O3.5 with low density and excellent anode compatibility[J]. Angewandte Chemie (International Ed), 2024, 63(37): e202407892. DOI:10.1002/anie.202407892. |
| 18 | SHI H Y, YANG J, XIA L X, et al. Synthesis of lithium sulfide by cold plasma method and its energy storage properties[J]. JOM, 2024, 76(10): 5803-5815. DOI:10.1007/s11837-024-06763-4. |
| 19 | LI X, GAO M X, DU W B, et al. A mechanochemical synthesis of submicron-sized Li2S and a mesoporous Li2S/C hybrid for high performance lithium/sulfur battery cathodes[J]. Journal of Materials Chemistry A, 2017, 5(14): 6471-6482. DOI:10.1039/C7TA00557A. |
| 20 | KARASEVA E V, SHEINA L V, KOLOSNITSYN V S. Synthesis of lithium sulfide by carbothermal reduction of lithium sulfate with petroleum coke[J]. Russian Journal of Applied Chemistry, 2021, 94(1): 1-8. DOI:10.1134/S1070427221010018. |
| 21 | TU F Y, ZHAO Z X, ZHANG X, et al. Low-cost and scalable synthesis of high-purity Li2S for sulfide solid electrolyte[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(47): 15365-15371. DOI:10.1021/acssuschemeng.2c02238. |
| 22 | WU F X, LEE J T, FAN F F, et al. A hierarchical particle-shell architecture for long-term cycle stability of Li2S cathodes[J]. Advanced Materials, 2015, 27(37): 5579-5586. DOI:10.1002/adma.201502289. |
| 23 | 陈格, 田欢, 徐川, 等. 硫酸锂碳化—还原制备硫化锂[J]. 矿冶, 2021, 30(6): 74-78. |
| CHEN G, TIAN H, XU C, et al. Preparation of lithium sulfide by high temperature pyrolysis of lithium sulfate[J]. Mining and Metallurgy, 2021, 30(6): 74-78. | |
| 24 | FANG L R, ZHANG Q R, HAN A G, et al. Green synthesis of the battery material lithium sulfide via metathetic reactions[J]. Chemical Communications, 2022, 58(36): 5498-5501. DOI:10. 1039/D2CC01077A. |
| 25 | SUN Y J, ZHANG Q R, YANG S J, et al. Making the unfeasible feasible: Synthesis of the battery material lithium sulfide via the metathetic reaction between lithium sulfate and sodium sulfide[J]. Inorganic Chemistry, 2024, 63(1): 485-493. DOI:10.1021/acs.inorgchem.3c03345. |
| 26 | 徐川, 陈格, 田欢, 等. Ev级高纯硫化锂及其制备方法: 中国, CN117069067A [P]. 2023. |
| XU C, CHEN G, TIAN H, et al. Ev grade high purity lithium sulfide and its preparation method: China, CN117069067A [P]. 2023. | |
| 27 | 周复, 杨柳, 陈格, 等. 硫化锂的制备方法: 中国, CN112678781A [P]. 2019. |
| ZHOU F, YANG L, CHEN G, et al. Preparation method of lithium sulfide: China, CN112678781A [P]. 2019. | |
| 28 | LIANG S, XIA Y, LIANG C, et al. A green and facile strategy for the low-temperature and rapid synthesis of Li2S@PC-CNT cathodes with high Li2S content for advanced Li-S batteries[J]. Journal of Materials Chemistry A, 2018, 6(21): 9906-9914. DOI:10.1039/C8TA01342J. |
| 29 | NAN C Y, LIN Z, LIAO H G, et al. Durable carbon-coated Li2(S) core-shell spheres for high performance lithium/sulfur cells[J]. Journal of the American Chemical Society, 2014, 136(12): 4659-4663. DOI:10.1021/ja412943h. |
| 30 | HAN F D, WESTOVER A S, YUE J, et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes[J]. Nature Energy, 2019, 4: 187-196. DOI:10.1038/s41560-018-0312-z. |
| 31 | SHAO B W, HUANG Y L, HAN F D. Electronic conductivity of lithium solid electrolytes[J]. Advanced Energy Materials, 2023, 13(16): 2204098. DOI:10.1002/aenm.202204098. |
| 32 | ZHAO C Z, CHENG X B, ZHANG R, et al. Li2S5-based ternary-salt electrolyte for robust lithium metal anode[J]. Energy Storage Materials, 2016, 3: 77-84. DOI:10.1016/j.ensm.2016.01.007. |
| 33 | YANG S J, HU X H, XU S J, et al. Synthesis of deliquescent lithium sulfide in air[J]. ACS Applied Materials & Interfaces, 2023, 15(34): 40633-40647. DOI:10.1021/acsami.3c08506. |
| 34 | ZHAO Y Z, YANG Y A, WOLDEN C A. Scalable synthesis of size-controlled Li2S nanocrystals for next-generation battery technologies[J]. ACS Applied Energy Materials, 2019, 2(3): 2246-2254. DOI:10.1021/acsaem.9b00032. |
| 35 | FANTAUZZI M, ELSENER B, ATZEI D, et al. Exploiting XPS for the identification of sulfides and polysulfides[J]. RSC Advances, 2015, 5(93): 75953-75963. DOI:10.1039/C5RA14915K. |
| 36 | PILYUGINA Y A, MISHINKIN V Y, KUZMINA E V, et al. The sulfide solid electrolyte synthesized via carbothermal reduction of lithium sulfate for solid-state lithium-sulfur batteries[J]. Inorganic Chemistry Communications, 2025, 174: 113926. DOI:10.1016/j.inoche.2025.113926. |
| 37 | YANG H Y, SUN Y J, YANG S J, et al. "One stone two birds" strategy of synthesizing the battery material lithium sulfide: Aluminothermal reduction of lithium sulfate[J]. Inorganic Chemistry, 2023, 62(14): 5576-5585. DOI:10.1021/acs.inorgchem.3c00087. |
| 38 | ZHANG Q R, HAN A G, ZHANG X, et al. Green synthesis for battery materials: A case study of making lithium sulfide via metathetic precipitation[J]. ACS Applied Materials & Interfaces, 2023, 15(1): 1358-1366. DOI:10.1021/acsami.2c19218. |
| 39 | LI Y Y, CHENG J, LI J W, et al. Indium doped sulfide solid electrolyte with tamed lithium dendrite and improved ionic conductivity for all-solid-state battery applications[J]. Journal of Power Sources, 2022, 542: 231794. DOI:10.1016/j.jpowsour. 2022.231794. |
| 40 | ZHOU J B, CHEN P, WANG W, et al. Li7P3S11 electrolyte for all-solid-state lithium-ion batteries: Structure, synthesis, and applications[J]. Chemical Engineering Journal, 2022, 446: 137041. DOI:10.1016/j.cej.2022.137041. |
| 41 | SMITH W H, VASELABADI S A, WOLDEN C A. Argyrodite superionic conductors fabricated from metathesis-derived Li2S[J]. ACS Applied Energy Materials, 2022, 5(4): 4029-4035. DOI:10.1021/acsaem.2c00442. |
| 42 | ZHANG X, YANG H Y, SUN Y J, et al. Lithium sulfide: Magnesothermal synthesis and battery applications[J]. ACS Applied Materials & Interfaces, 2022, 14(36): 41003-41012. DOI:10.1021/acsami.2c11196. |
| 43 | JANG B, WOO J, SONG Y B, et al. Microwave heating enables near-carbonless liquid-phase-derived Li Argyrodites for all-solid-state batteries[J]. Energy Storage Materials, 2024, 65: 103154. DOI:10.1016/j.ensm.2023.103154. |
| 44 | JIANG H Z, HAN Y, WANG H, et al. In-situ generated Li2S-based composite cathodes with high mass and capacity loading for all-solid-state Li-S batteries[J]. Journal of Alloys and Compounds, 2021, 874: 159763. DOI:10.1016/j.jallcom.2021.159763. |
| 45 | YAN H F, WANG H C, WANG D H, et al. In situ generated Li2S-C nanocomposite for high-capacity and long-life all-solid-state lithium sulfur batteries with ultrahigh areal mass loading[J]. Nano Letters, 2019, 19(5): 3280-3287. DOI:10.1021/acs.nanolett.9b00882. |
| 46 | ZHANG K, WANG L J, HU Z, et al. Ultrasmall Li2S nanoparticles anchored in graphene nanosheets for high-energy lithium-ion batteries[J]. Scientific Reports, 2014, 4: 6467. DOI:10.1038/srep 06467. |
| 47 | YE F M, NOH H, LEE J H, et al. Li2S/carbon nanocomposite strips from a low-temperature conversion of Li2SO4 as high-performance lithium-sulfur cathodes[J]. Journal of Materials Chemistry A, 2018, 6(15): 6617-6624. DOI:10.1039/C8TA00515J. |
| 48 | TAN G Q, XU R, XING Z Y, et al. Burning lithium in CS2 for high-performing compact Li2S-graphene nanocapsules for Li-S batteries[J]. Nature Energy, 2017, 2(7): 17090. DOI:10.1038/nenergy.2017.90. |
| 49 | DRESSEL C B, JHA H, EBERLE A M, et al. Electrochemical performance of lithium-sulfur batteries based on a sulfur cathode obtained by H2S gas treatment of a lithium salt[J]. Journal of Power Sources, 2016, 307: 844-848. DOI:10.1016/j.jpowsour.2015.12.140. |
| 50 | LI X M, WOLDEN C A, BAN C M, et al. Facile synthesis of lithium sulfide nanocrystals for use in advanced rechargeable batteries[J]. ACS Applied Materials & Interfaces, 2015, 7(51): 28444-28451. DOI:10.1021/acsami.5b09367. |
| 51 | YANG S J, WAN F M, HAN A G, et al. Environmentally friendly, non-glove box, closed-system and continuously massive production of lithium sulfide for battery applications[J]. Journal of Cleaner Production, 2023, 382: 135221. DOI:10.1016/j.jclepro. 2022.135221. |
| 52 | 李良彬, 叶明, 潘志芳, 等. 一种利用电池级金属锂制备高纯硫化锂的方法: 中国, CN116040587A [P]. 2023. |
| LI L B, YE M, PAN Z F, et al. A method for preparing high purity lithium sulfide from battery grade lithium metal: China, CN116040587A [P]. 2023. | |
| 53 | 李良彬, 廖萃, 潘志芳, 等. 一种利用金属锂制备硫化锂的方法: CN112607712A[P]. 2021-04-06. |
| 54 | YUAN K, YUAN L X, CHEN J, et al. Methods and cost estimation for the synthesis of nanosized lithium sulfide[J]. Small Structures, 2021, 2(3): 2000059. DOI:10.1002/sstr.202000059. |
| 55 | 于姗, 段元刚, 张怡欣, 等. 分步法分解硫化氢制氢和硫黄催化剂研究进展[J]. 化工进展, 2023, 42(7): 3780-3790. DOI: 10.16085/j.issn.1000-6613.2022-1633. |
| YU S, DUAN Y G, ZHANG Y X, et al. Research progress of catalysts for two-step hydrogen sulfide decomposition to produce hydrogen and sulfur[J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3780-3790. DOI: 10.16085/j.issn.1000-6613.2022-1633. | |
| 56 | YU P, ZHAI Y M, BAO R Y, et al. Rapid, repeatable, highly sensitive and semi-quantitative colorimetric detection of elemental sulfur with a colored clathrate[J]. Sensors and Actuators B: Chemical, 2019, 299: 126948. DOI:10.1016/j.snb. 2019.126948. |
| 57 | SUN Y J, ZHANG X, XU S J, et al. A green method of synthesizing battery-grade lithium sulfide: Hydrogen reduction of lithium sulfate[J]. ACS Sustainable Chemistry & Engineering, 2024, 12(7): 2813-2824. DOI:10.1021/acssuschemeng.3c07872. |
| 58 | WU Z Z, HAN C, WANG J S, et al. Low-cost synthesis of high-purity Li2S for sulfide solid state electrolytes enabled by polyvinyl alcohol[J]. Journal of Central South University, 2024, 31(12): 4449-4459. DOI:10.1007/s11771-024-5824-z. |
| 59 | 雷振, 陈格, 徐川, 等. 氮化锂对金属锂制备硫化锂改性研究[J]. 矿冶工程, 2024, 44(4): 81-83. |
| LEI Z, CHEN G, XU C, et al. Modification of lithium sulfide prepared with lithium metal by lithium nitride[J]. Mining and Metallurgical Engineering, 2024, 44(4): 81-83. | |
| 60 | 雷振, 徐川, 陈格, 等. 基于静电喷雾的纳米级硫化锂及其制备方法: 中国, CN117246980A [P]. 2023. |
| LEI Z, XU C, CHEN G, et al. Preparation of nano-scale lithium sulfide based on electrostatic spray: China, CN117246980A [P]. 2023. | |
| 61 | 刘芳洋, 何特特, 张宗良, 等. 一种以含锂矿石为原料制备硫化锂的方法: 中国, CN202411103832.6 [P]. 2024. |
| LIU F Y, HE T T, ZHANG Z L, et al. A method for preparation of lithium sulfide from lithium containing ore: China, CN202411103832.6 [P]. 2024. | |
| 62 | 刘芳洋, 李莉璇, 何特特, 等. 一种以废旧锂离子电池为原料制备硫化锂的方法: CN119018856A[P]. 2024-11-26. |
| 63 | 凌忠钱. 多孔介质内超绝热燃烧及硫化氢高温裂解制氢的试验研究和数值模拟 [D]. 杭州: 浙江大学, 2008. |
| LING Z Q. Experimental Study and numerical simulation of superadiabatic combustion and pyrolysis of hydrogen sulfide in porous media for hydrogen production [D].Hangzhou: Zhejiang University, 2008. | |
| 64 | 凌忠钱, 周昊, 钱欣平, 等. 硫化氢高温裂解制氢的动力学研究[J]. 热能动力工程, 2008, 23(5): 547-550, 559. |
| LING Z Q, ZHOU H, QIAN X P, et al. Kinetics study of hydrogen preparation from a pyrolysis of hydrogen sulfide[J]. Journal of Engineering for Thermal Energy and Power, 2008, 23(5): 547-550, 559. |
| [1] | Shaojia DANG, Liqiang SUN, Shenyou WANG, Wentao TIAN, Pengfei HU. A dual-layer power optimization strategy for multi-energy storage power station considering system economic efficiency and state of charge balance [J]. Energy Storage Science and Technology, 2025, 14(3): 1247-1257. |
| [2] | Liqiang SUN, Shaojia DANG, Gang LIU, Shenyou WANG, Pengfei HU. A frequency-modulation power optimization method for energy storage power stations considering the transition state of charge-discharge and power constraints [J]. Energy Storage Science and Technology, 2025, 14(3): 1286-1298. |
| [3] | Shihao HOU, Bo ZHAO, Li ZHANG. Optimization study of a double-layer pumped storage model based on a step penalty mechanism for carbon emissions and new energy abandonment [J]. Energy Storage Science and Technology, 2024, 13(7): 2414-2424. |
| [4] | Qili LIN, Zhen CHEN, Xiaohu WANG, Hongxun QI, Wei WANG. Economic analysis of large-scale hydrogen energy storage based on the “electric-hydrogen-electric” process [J]. Energy Storage Science and Technology, 2024, 13(6): 2068-2077. |
| [5] | Tianchen ZHAO, Gong ZHANG, Yunfei ZHANG, Shihao HOU, Tingting WANG. Technical and economic research on the capacity of supply assurance for pumped-storage systems under the target of “dual carbon” [J]. Energy Storage Science and Technology, 2024, 13(3): 1059-1073. |
| [6] | Yun WANG, Fei MENG, Chao ZHANG, Tao LI, Bo TIAN, Jiangpeng LI, Haidong CHEN, Zhihua ZHANG. Effect of ammonia decomposition hydrogen production and energy storage system capacity on performance of power system [J]. Energy Storage Science and Technology, 2024, 13(2): 589-597. |
| [7] | Jian CHANG, Hang SONG, Yuzhen KANG, Tao LU, Zhiwei TANG. Application of high-temperature composite phase change heat storage in urban clean energy transformation [J]. Energy Storage Science and Technology, 2023, 12(11): 3471-3478. |
| [8] | Qili LIN, Hongxun QI, Jingjing HUANG, Bingcheng ZHANG, Zhen CHEN, Zhenkun XIAO. Levelized cost of combined hydrogen production by water electrolysis with alkaline-proton exchange membrane [J]. Energy Storage Science and Technology, 2023, 12(11): 3572-3580. |
| [9] | Hao YIN, Zhiwei TANG, Hao WANG, Yi JIN, Yulong DING. Investigation on a time-sharing heating system using a high-density composite phase change heat storage material-an electric boiler [J]. Energy Storage Science and Technology, 2022, 11(9): 3003-3010. |
| [10] | Huamin ZHANG. Development, cost analysis considering various durations, and advancement of vanadium flow batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2772-2780. |
| [11] | Yu SHI, Zhong ZHANG, Jingying YANG, Wei QIAN, Hao LI, Xiang ZHAO, Xintong YANG. Opportunity cost modelling and market strategy of energy storage participating in the AGC market [J]. Energy Storage Science and Technology, 2022, 11(7): 2366-2373. |
| [12] | Guojing LIU, Bingjie LI, Xiaoyan HU, Fen YUE, Jiqiang XU. Australia policy mechanisms and business models for energy storage and their applications to china [J]. Energy Storage Science and Technology, 2022, 11(7): 2332-2343. |
| [13] | ZHANG Ping, KANG Libin, WANG Mingju, ZHAO Guang, LUO Zhenhua, TANG Kun, LU Yaxiang, HU Yongsheng. Technology feasibility and economic analysis of Na-ion battery energy storage [J]. Energy Storage Science and Technology, 2022, 11(6): 1892-1901. |
| [14] | Liang FANG, Kai ZHANG, Limin ZHOU. Recent advances and prospects of electrolyte for aluminum ion batteries [J]. Energy Storage Science and Technology, 2022, 11(4): 1236-1245. |
| [15] | Xiong LI, Peiqiang LI. Analysis of economics and economic boundaries of large-scale application of power batteries in cascade utilization [J]. Energy Storage Science and Technology, 2022, 11(2): 717-725. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
