Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (6): 1772-1787.doi: 10.19799/j.cnki.2095-4239.2022.0176
Previous Articles Next Articles
OU Yu( ), HOU Wenhui, LIU Kai(
), HOU Wenhui, LIU Kai( )
)
Received:2022-03-30
Revised:2022-04-09
Online:2022-06-05
Published:2022-06-13
Contact:
LIU Kai
E-mail:ouyu200105@163.com;liukai2019@tsinghua.edu.cn
CLC Number:
OU Yu, HOU Wenhui, LIU Kai. Research progress of smart safety electrolytes in lithium-ion batteries[J]. Energy Storage Science and Technology, 2022, 11(6): 1772-1787.

Fig. 1
Three stages for the thermal runaway process. Stage 1: The onset of overheating. The batteries change from a normal to an abnormal state, and the internal temperature starts to increase; Stage 2: Heat accumulation and gas release process. The internal temperature quickly rises, and the battery undergoes exothermal reactions; Stage 3: Combustion and explosion. The flammable electrolyte combusts, leading to firesand even explosions[6]"

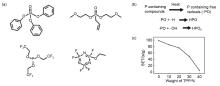
Fig. 2
(a) Typical molecular structures of flame-retardant additives; (b) The mechanism for the flame retardation effects of phosphorus-containing compounds; (c) The self-extinguish time (SET) of the typical carbonate electrolyte can be signifificantly reduced with the addition of triphenyl phosphate[20]"


Fig. 3
(a) Illustration of the migration inhibition of the ionic or electron conduction between electrodes via reversibly thermal-responsive polymers for preventing the thermal runaway of electrochemical storage devices[22]; (b) Illustrative schematic of a thermally-controllable polymer electrolyte for electrochemical energy storage; (c) pH and ionic conductivity response to temperature of 2.2% (weight fraction) solution containing the PNcA at a heating rate of approximately 4.7 ℃/min[23]"


Fig. 4
(a) Photographs of the PEO/IL phase separation[25]; (b) Schematic of the LIB setup, the thermal-response machanism at high temperatures at the electrode surface and the structure of the responsive polymer, PBMA, and ionic liquid, [EMIM][TFSI]; (c) Electrochemical performance of a cell composed of LTO/LFP electrode, 5% PBMA and 0.2 mol/L/0.5 mol/L LiTFSI/[EMIM][TFSI] at 60 ℃ and 150 ℃[26]"


Fig. 5
(a) Schematic diagram of a membrane with thermal-triggered flame retardant consisting of a core-shell structure (flame retardant as core, polymer as shell); (b) After thermal triggering, the polymer shell melts and the encapsulated flame retardant is released into the electrolyte, effectively inhibiting the ignition and combustion of the electrolytePhotographs of the PEO/IL phase separation[21]; (c) Schematic illustration of a thermally responsive gel system used as an electrolyte in electrochemical storage devices. Upon heating, the sol–gel transition of the thermoplastic elastomer solution inhibits the motion of ions, thus actively shutting down the device at high temperature; (d) The temperature dependent rheological behavior of a 30% Pluronic solution; (e) Pictures of a Pluronic solution in the sol and gel states[27]"


Fig. 7
(a) The relationship between viscosity and shear rate at different volume fractions; (b) The relationship between shear rate and viscosity and normal force of 37% suspension[35]; (c) Silica nanorods of controllable aspect ratio are synthesized from tetraethyl orthosilicate (TEOS) using sodium citrate, polyvinylpyrrolidone (PVP), ammonium hydroxide, ethanol and pentanol in an emulsion approach[37]"
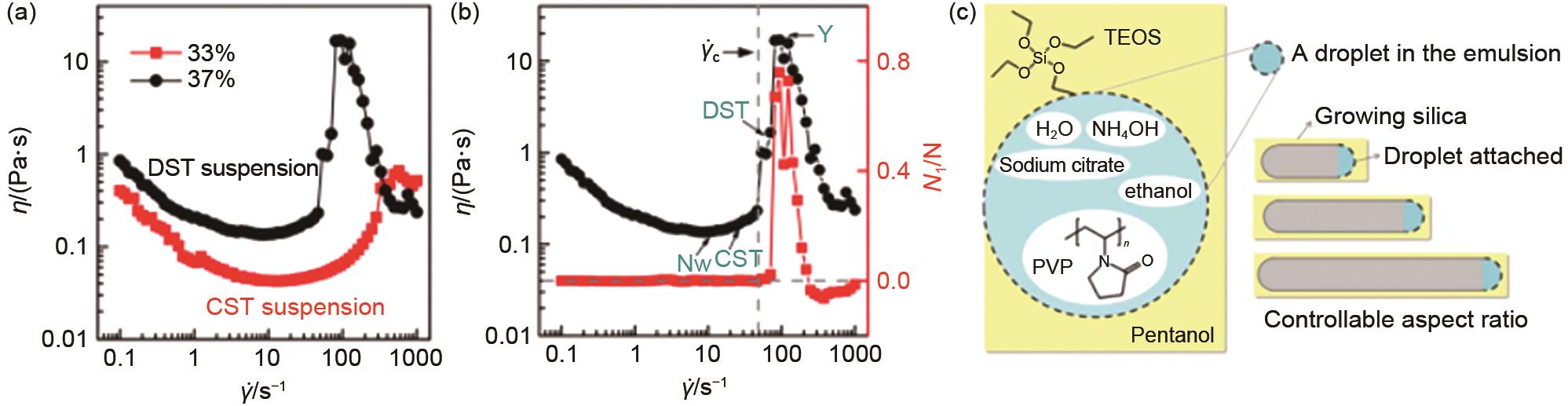
Fig. 8
(a) The distributions of fumed silica particles in the liquid electrolyte at different status; (b) Rheology graph of bare electrolyte and composite electrolytes with 6.3% SiO2, 9.1% SiO2 and 10.7% SiO2; (c) Room temperature variation of ionic conductivity of composite electrolytes (SiO2/LiPF6 in EC/DMC) versus weight fraction of fumed silica. Inset: Ionic conductivity of composite electrolyte with 9.1% SiO2 after impact tests;(d) Discharge curve of LiFePO4 electrode in the STF electrolyte of EC/DMC/LiPF6 with 9.1% SiO2[32];(e) Voltage stability during impact with and without STE[41]"
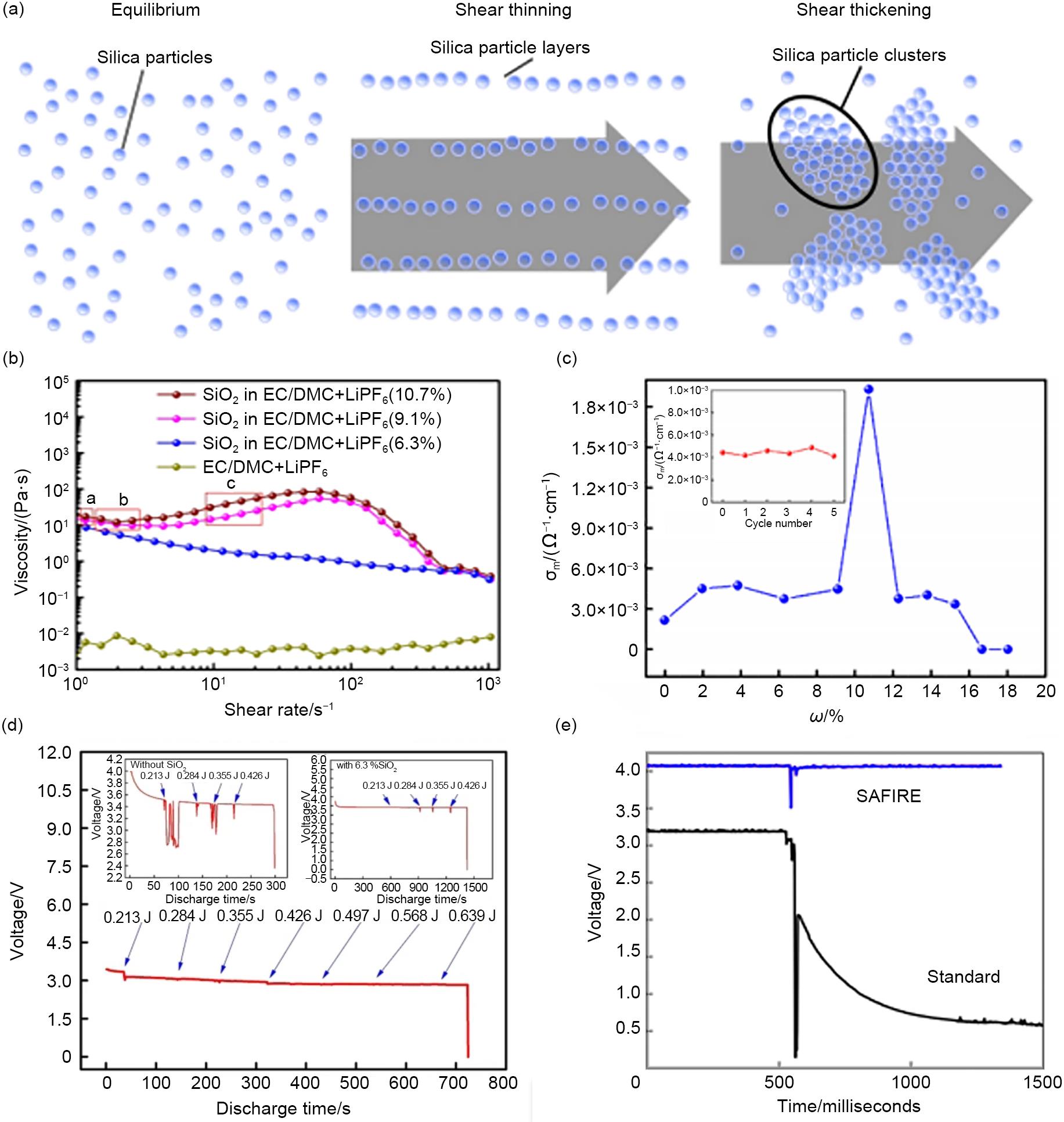

Fig. 9
(a) A General view of aggregation of bare silica nanoparticles in solution, B Scheme depicting attachment of organosilane to silica colloid surfaces (top) and grafting of poly(methyl methacrylate) (PMMA) brushes from colloid surfaces via surface-initiated activators regenerated by electron transfer atom transfer radical polymerization (ARGET-ATRP) (bottom), C Schematic diagram of silica nanoparticles sterically stabilized via surface tethered PMMA brushes[43]; (b) Rheological diagram of electrolyte containing silicon nanorods with different aspect ratios and volume fractions; The discharge capacity (c) and Coulombic efficiency (d) for a full cell composed by NMC| EC/EMC/LiTFSI/33%, AR (5), SiO2 nanorods | graphite and cycled at rates of C/10, 1 C, 2 C, and 1 C[37];(e) Scheme of APTES modifified GFs preparation process[44]"
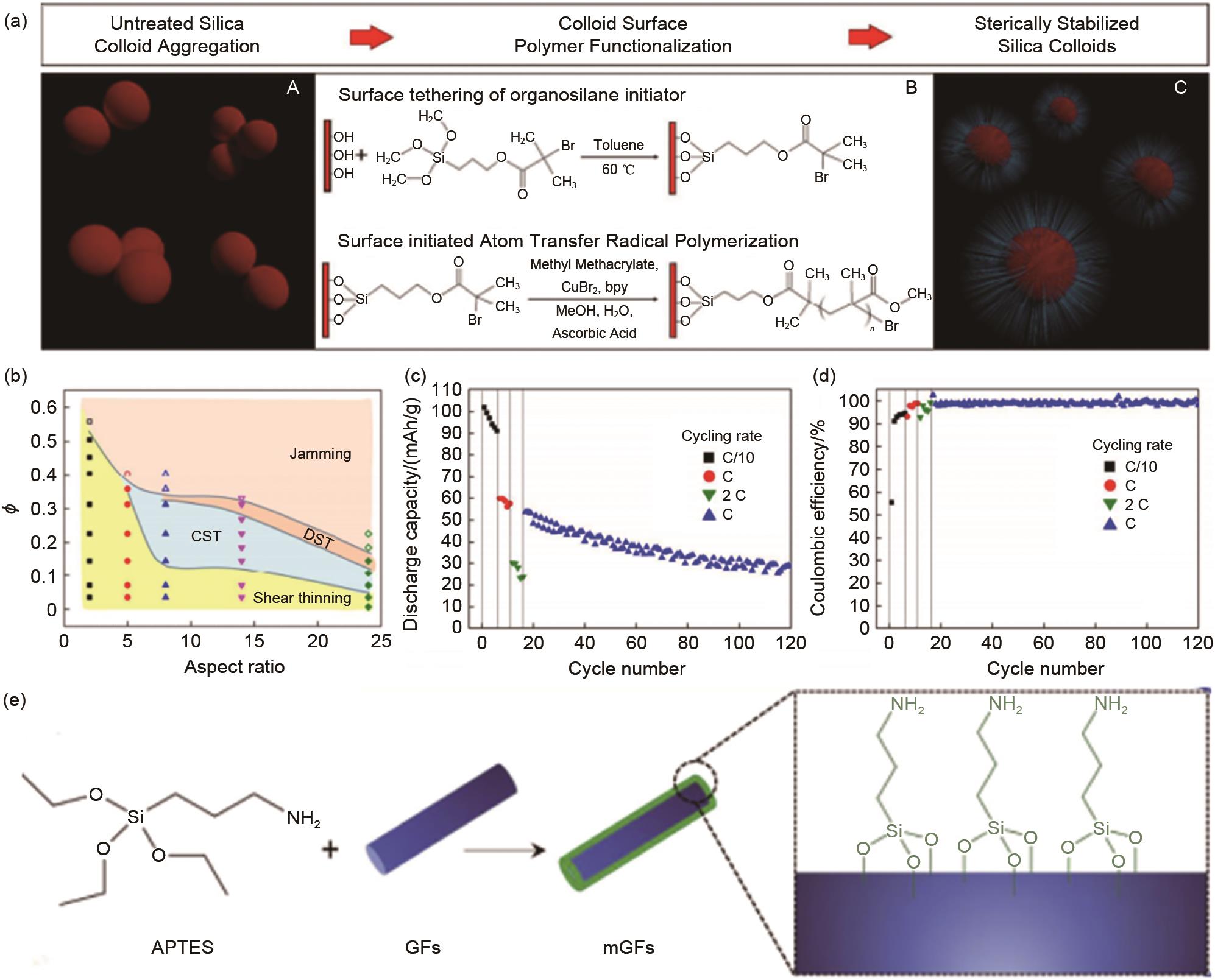
Fig. 12
(a) Schematic illustration of the ferrocene-based redox shuttle mechanism[50]; (b) Charging voltage profile for graphite/C-LFP pouch cells without and with 0.4 mol/L DBBB additive[51]; (c) Three-step synthesis ofN-ethyl3,7-bis(trifluoromethyl)phenothiazine[53]; (d) schematic illustration of the TAC cationelectrostatic-shielding mechanism[54]"

| 1 | ZHANG X X, LI L, FAN E S, et al. Toward sustainable and systematic recycling of spent rechargeable batteries[J]. Chemical Society Reviews, 2018, 47(19): 7239-7302. |
| 2 | ARGYROU M C, CHRISTODOULIDES P, KALOGIROU S A. Energy storage for electricity generation and related processes: Technologies appraisal and grid scale applications[J]. Renewable and Sustainable Energy Reviews, 2018, 94: 804-821. |
| 3 | CHU S, CUI Y, LIU N. The path towards sustainable energy[J]. Nature Materials, 2017, 16(1): 16-22. |
| 4 | LARSSON F, ANDERSSON P, MELLANDER B E. Lithium-ion battery aspects on fires in electrified vehicles on the basis of experimental abuse tests[J]. Batteries, 2016, 2(2): 9. |
| 5 | LOVERIDGE M, REMY G, KOURRA N, et al. Looking deeper into the galaxy (note 7)[J]. Batteries, 2018, 4(1): 3. |
| 6 | LIU K, LIU Y Y, LIN D C, et al. Materials for lithium-ion battery safety[J]. Science Advances, 2018, 4(6): eaas9820. |
| 7 | YUAN S, CHANG C Y, YAN S S, et al. A review of fire-extinguishing agent on suppressing lithium-ion batteries fire[J]. Journal of Energy Chemistry, 2021, 62: 262-280. |
| 8 | DONG H, WANG P C, YAN S S, et al. A thermoresponsive composite separator loaded with paraffin@SiO2 microparticles for safe and stable lithium batteries[J]. Journal of Energy Chemistry, 2021, 62: 423-430. |
| 9 | YAN S S, CHEN X X, ZHOU P, et al. Regulating the growth of lithium dendrite by coating an ultra-thin layer of gold on separator for improving the fast-charging ability of graphite anode[J]. Journal of Energy Chemistry, 2022, 67: 467-473. |
| 10 | CHEN X X, YAN S S, TAN T H, et al. Supramolecular "flame-retardant" electrolyte enables safe and stable cycling of lithium-ion batteries[J]. Energy Storage Materials, 2022, 45: 182-190. |
| 11 | ZHANG W, XIA H R, ZHU Z Q, et al. Decimal solvent-based high-entropy electrolyte enabling the extended survival temperature of lithium-ion batteries to -130 ℃[J]. CCS Chemistry, 2020, 3(4): 1-26. |
| 12 | HU Y M, DUNLAP N, LONG H, et al. Helical covalent polymers with unidirectional ion channels as single lithium-ion conducting electrolytes[J]. CCS Chemistry, 2021, 3(12): 2762-2770. |
| 13 | WANG W J, ZHU X H, FU L. Touch ablation of lithium dendrites via liquid metal for high-rate and long-lived batteries[J]. CCS Chemistry, 2021, 3(1): 686-695. |
| 14 | GUO K R, LI S Q, CHEN G, et al. One-pot synthesis of polyester-based linear and graft copolymers for solid polymer electrolytes[J]. CCS Chemistry, 2021: 3485-3500. |
| 15 | STUART M A C, HUCK W T S, GENZER J, et al. Emerging applications of stimuli-responsive polymer materials[J]. Nature Materials, 2010, 9(2): 101-113. |
| 16 | YAN X Z, WANG F, ZHENG B, et al. Stimuli-responsive supramolecular polymeric materials[J]. Chemical Society Reviews, 2012, 41(18): 6042-6065. |
| 17 | HYUNG Y E, VISSERS D R, AMINE K. Flame-retardant additives for lithium-ion batteries[J]. Journal of Power Sources, 2003, 119/120/121: 383-387. |
| 18 | PIRES J, CASTETS A, TIMPERMAN L, et al. Tris(2,2,2-trifluoroethyl) phosphite as an electrolyte additive for high-voltage lithium-ion batteries using lithium-rich layered oxide cathode[J]. Journal of Power Sources, 2015, 296: 413-425. |
| 19 | XIA L, XIA Y G, LIU Z P. A novel fluorocyclophosphazene as bifunctional additive for safer lithium-ion batteries[J]. Journal of Power Sources, 2015, 278: 190-196. |
| 20 | YUAN M Q, LIU K. Rational design on separators and liquid electrolytes for safer lithium-ion batteries[J]. Journal of Energy Chemistry, 2020, 43: 58-70. |
| 21 | LIU K, LIU W, QIU Y C, et al. Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries[J]. Science Advances, 2017, 3(1): doi: 10.1126/sciadv.1601978. |
| 22 | YANG H, LEOW W R, CHEN X D. Thermal-responsive polymers for enhancing safety of electrochemical storage devices[J]. Advanced Materials, 2018, 30(13): doi: 10.1002/adma.201704347. |
| 23 | KELLY J C, PEPIN M, HUBER D L, et al. Reversible control of electrochemical properties using thermally-responsive polymer electrolytes[J]. Advanced Materials, 2012, 24(7): 886-889. |
| 24 | WEN L, LIANG J, CHEN J, et al. Smart materials and design toward safe and durable lithium ion batteries[J]. Small Methods, 2019, 3(11): doi: 10.1002/smtd.201900323. |
| 25 | KELLY J C, GUPTA R, ROBERTS M E. Responsive electrolytes that inhibit electrochemical energy conversion at elevated temperatures[J]. Journal of Materials Chemistry A, 2015, 3(7): 4026-4034. |
| 26 | KELLY J C, DEGROOD N L, ROBERTS M E. Li-ion battery shut-off at high temperature caused by polymer phase separation in responsive electrolytes[J]. Chemical Communications (Cambridge, England), 2015, 51(25): 5448-5451. |
| 27 | SHI Y, HA H, AL-SUDANI A, et al. Thermoplastic elastomer-enabled smart electrolyte for thermoresponsive self-protection of electrochemical energy storage devices[J]. Advanced Materials, 2016, 28(36): 7921-7928. |
| 28 | SHU K W, WANG C Y, LI W H, et al. Electrolytes with reversible switch between liquid and solid phases[J]. Current Opinion in Electrochemistry, 2020, 21: 297-302. |
| 29 | FAMPRIKIS T, CANEPA P, DAWSON J A, et al. Fundamentals of inorganic solid-state electrolytes for batteries[J]. Nature Materials, 2019, 18(12): 1278-1291. |
| 30 | MACFARLANE D R, FORSYTH M, HOWLETT P C, et al. Ionic liquids and their solid-state analogues as materials for energy generation and storage[J]. Nature Reviews Materials, 2016, 1: 15005. |
| 31 | WAGNER N J, BRADY J F. Shear thickening in colloidal dispersions[J]. Physics Today, 2009, 62(10): 27-32. |
| 32 | DING J, TIAN T F, MENG Q, et al. Smart multifunctional fluids for lithium ion batteries: Enhanced rate performance and intrinsic mechanical protection[J]. Scientific Reports, 2013, 3: 2485. |
| 33 | CHEN Z Q, CHAO Y F, LI W H, et al. Abuse-tolerant electrolytes for lithium-ion batteries[J]. Advanced Science, 2021, 8(11): doi: 10.1002/advs.202003694. |
| 34 | PFAFFENHUBER C, GÖBEL M, POPOVIC J, et al. Soggy-sand electrolytes: Status and perspectives[J]. Physical Chemistry Chemical Physics: PCCP, 2013, 15(42): 18318-18335. |
| 35 | JIANG W F, XUAN S H, GONG X L. The role of shear in the transition from continuous shear thickening to discontinuous shear thickening[J]. Applied Physics Letters, 2015, 106(15): doi: 10.1063/1.4918344. |
| 36 | FERNANDEZ N, MANI R, RINALDI D, et al. Microscopic mechanism for shear thickening of non-Brownian suspensions[J]. Physical Review Letters, 2013, 111(10): doi: 10.1103/physRevLett.111.108301. |
| 37 | YE Y L, XIAO H, REAVES K, et al. Effect of nanorod aspect ratio on shear thickening electrolytes for safety-enhanced batteries[J]. ACS Applied Nano Materials, 2018, 1(6): 2774-2784. |
| 38 | BHATTACHARYYA A, MAIER J. Second phase effects on the conductivity of non-aqueous salt solutions: "soggy sand electrolytes"[J]. Advanced Materials, 2004, 16(9/10): 811-814. |
| 39 | MAIER J. Ionic conduction in space charge regions[J]. Progress in Solid State Chemistry, 1995, 23(3): 171-263. |
| 40 | DAS S K, MANDAL S S, BHATTACHARYYA A J. Ionic conductivity, mechanical strength and Li-ion battery performance of mono-functional and bi-functional ("Janus") "soggy sand" electrolytes[J]. Energy & Environmental Science, 2011, 4(4): 1391. |
| 41 | VEITH G M, ARMSTRONG B L, WANG H, et al. Shear thickening electrolytes for high impact resistant batteries[J]. ACS Energy Letters, 2017, 2(9): 2084-2088. |
| 42 | KOBAYASHI M, JUILLERAT F, GALLETTO P, et al. Aggregation and charging of colloidal silica particles: Effect of particle size[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2005, 21(13): 5761-5769. |
| 43 | SHEN B H, ARMSTRONG B L, DOUCET M, et al. Shear thickening electrolyte built from sterically stabilized colloidal particles[J]. ACS Applied Materials & Interfaces, 2018, 10(11): 9424-9434. |
| 44 | LIU K W, CHENG C F, ZHOU L Y, et al. A shear thickening fluid based impact resistant electrolyte for safe Li-ion batteries[J]. Journal of Power Sources, 2019, 423: 297-304. |
| 45 | DE VICENTE J, KLINGENBERG D J, HIDALGO-ALVAREZ R. Magnetorheological fluids: A review[J]. Soft Matter, 2011, 7(8): 3701. |
| 46 | FENG X N, OUYANG M G, LIU X, et al. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review[J]. Energy Storage Materials, 2018, 10: 246-267. |
| 47 | WANG Q S, JIANG L H, YU Y, et al. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect[J]. Nano Energy, 2019, 55: 93-114. |
| 48 | HAREGEWOIN A M, WOTANGO A S, HWANG B J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives[J]. Energy Environ Sci, 2016, 9(6): 1955-1988. |
| 49 | BEHL W K, CHIN D T. Electrochemical overcharge protection of rechargeable lithium batteries: II. effect of lithium iodide-iodine additives on the behavior of lithium electrode in solutions[J]. Journal of the Electrochemical Society, 1988, 135(1): 21-25. |
| 50 | GÉLINAS B, BIBIENNE T, DOLLÉ M, et al. Electrochemistry and transport properties of electrolytes modified with ferrocene redox-active ionic liquid additives[J]. Canadian Journal of Chemistry, 2020, 98(9): 554-563. |
| 51 | LEONET O, COLMENARES L C, KVASHA A, et al. Improving the safety of lithium-ion battery via a redox shuttle additive 2,5-di-tert-butyl-1,4-bis(2-methoxyethoxy)benzene (DBBB)[J]. ACS Applied Materials & Interfaces, 2018, 10(11): 9216-9219. |
| 52 | ZHANG J J, SHKROB I A, ASSARY R S, et al. An extremely durable redox shuttle additive for overcharge protection of lithium-ion batteries[J]. Materials Today Energy, 2019, 13: 308-311. |
| 53 | ERGUN S L, CASSELMAN M D, KAUR A P, et al. Improved synthesis of N-ethyl-3,7-bis(trifluoromethyl)phenothiazine[J]. New Journal of Chemistry, 2020, 44(26): 11349-11355. |
| 54 | JI W X, HUANG H, ZHENG D, et al. A redox-active organic cation for safer metallic lithium-based batteries[J]. Energy Storage Materials, 2020, 32: 185-190. |
| 55 | ZHANG J J, SHKROB I A, ASSARY R S, et al. Dual overcharge protection and solid electrolyte interphase-improving action in Li-ion cells containing a bis-annulated dialkoxyarene electrolyte additive[J]. Journal of Power Sources, 2018, 378: 264-267. |
| 56 | GUPTA D, CAI C, KOENIG G M. Comparative analysis of chemical redox between redox shuttles and a lithium-ion cathode material via electrochemical analysis of redox shuttle conversion[J]. Journal of the Electrochemical Society, 2021, 168(5): 050546. |
| 57 | KISE M, YOSHIOKA S, HAMANO K, et al. Alternating Current impedance behavior and overcharge tolerance of lithium-ion batteries using positive temperature coefficient cathodes[J]. Journal of the Electrochemical Society, 2006, 153(6): A1004. |
| 58 | LIU H M, SAIKIA D, WU H C, et al. Towards an understanding of the role of hyper-branched oligomers coated on cathodes, in the safety mechanism of lithium-ion batteries[J]. RSC Advance, 2014, 4 (99): 56147-56155. |
| 59 | CHENG C L, WAN C C, WANG Y Y, et al. Thermal shutdown behavior of PVdF-HFP based polymer electrolytes comprising heat sensitive cross-linkable oligomers[J]. Journal of Power Sources, 2005, 144(1): 238-243. |
| 60 | WU H, ZHUO D, KONG D S, et al. Improving battery safety by early detection of internal shorting with a bifunctional separator[J]. Nature Communications, 2014, 5: 5193. |
| [1] | Xianxi LIU, Anliang SUN, Chuan TIAN. Research on liquid cooling and heat dissipation of lithium-ion battery pack based on bionic wings vein channel cold plate [J]. Energy Storage Science and Technology, 2022, 11(7): 2266-2273. |
| [2] | Jianxiang DENG, Jinliang ZHAO, Chengde HUANG. High energy density lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(7): 2092-2102. |
| [3] | HAN Junwei, XIAO Jing, TAO Ying, KONG Debin, LV Wei, YANG Quanhong. Compact energy storage: Methodology with graphenes and the applications [J]. Energy Storage Science and Technology, 2022, 11(6): 1865-1873. |
| [4] | DING Yi, YANG Yan, CHEN Kai, ZENG Tao, HUANG Yunhui. Intelligent fire protection of lithium-ion battery and its research method [J]. Energy Storage Science and Technology, 2022, 11(6): 1822-1833. |
| [5] | Lei LI, Zhao LI, Dan JI, Huichang NIU. Overcharge induced thermal runaway behaviors of pouch-type lithium-ion batteries with LFP and NCM cathodes: the differences and reasons [J]. Energy Storage Science and Technology, 2022, 11(5): 1419-1427. |
| [6] | Biao MA, Chunjing LIN, Lei LIU, Xiaole MA, Tianyi MA, Shiqiang LIU. Venting characteristics and flammability limit of thermal runaway gas of lithium ion battery [J]. Energy Storage Science and Technology, 2022, 11(5): 1592-1600. |
| [7] | Ce ZHANG, Siwu LI, Jia XIE. Research progress on the prelithiation technology of alloy-type anodes [J]. Energy Storage Science and Technology, 2022, 11(5): 1383-1400. |
| [8] | Qiaomin KE, Jian GUO, Yiwei WANG, Wenjiong CAO, Man CHEN, Fangming JIANG. The effect of liquid-cooled thermal management on thermal runaway of power battery [J]. Energy Storage Science and Technology, 2022, 11(5): 1634-1640. |
| [9] | Yuanxia DONG, Hengyun ZHANG, Jiajun ZHU, Xiaobin XU, Shunliang ZHU. Numerical simulation study on thermal runaway propagation mitigation structure of automotive battery module [J]. Energy Storage Science and Technology, 2022, 11(5): 1608-1616. |
| [10] | Nan LIN, Ulrike KREWER, Jochen ZAUSCH, Konrad STEINER, Haibo LIN, Shouhua FENG. Development and application of multiphysics models for electrochemical energy storage and conversion systems [J]. Energy Storage Science and Technology, 2022, 11(4): 1149-1164. |
| [11] | Hongzhang ZHU, Chuanping WU, Tiannian ZHOU, Jie DENG. Thermal runaway characteristics of LiFePO4 and ternary lithium batteries with external overheating [J]. Energy Storage Science and Technology, 2022, 11(1): 201-210. |
| [12] | Jun WANG, Zhuangzhuang JIA, Peng QIN, Zheng HUANG, Jingyun WU, Wen QI, Qingsong WANG. Simulation of thermal runaway gas diffusion in LiFePO4 battery module [J]. Energy Storage Science and Technology, 2022, 11(1): 185-192. |
| [13] | Zhihui GUO, Xiaodan CUI, Linshuang ZHAO, Jiawei CHEN. Fire and gas explosion hazards of high-nickel lithium-ion battery [J]. Energy Storage Science and Technology, 2022, 11(1): 193-200. |
| [14] | Xinlong ZHU, Junyi WANG, Jiashuang PAN, Chuanzhi KANG, Yitao ZOU, Kaijie YANG, Hong SHI. Present situation and development of thermal management system for battery energy storage system [J]. Energy Storage Science and Technology, 2022, 11(1): 107-118. |
| [15] | Xiuliang CHANG, Lili ZHENG, Shouli WEI, Tao ZHANG, Bing CHEN, Zhuo XU, Zuoqiang DAI. Progress in thermal runaway simulation of lithium-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(6): 2191-2199. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
