Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (8): 2681-2690.doi: 10.19799/j.cnki.2095-4239.2022.0284
Ziying CHEN( ), Xiang DING(
), Xiang DING( ), Qingsong TONG(
), Qingsong TONG( ), Junyan LI, Jingyu HUANG
), Junyan LI, Jingyu HUANG
Received:2022-05-25
Revised:2022-06-20
Online:2022-08-05
Published:2022-08-03
Contact:
Xiang DING, Qingsong TONG
E-mail:2607979319@qq.com;dingx@fjnu.edu.cn;qstong_3503@fjnu.edu.cn
CLC Number:
Ziying CHEN, Xiang DING, Qingsong TONG, Junyan LI, Jingyu HUANG. Application progress of doping technology in Mn-based lithium rich oxide cathode materials[J]. Energy Storage Science and Technology, 2022, 11(8): 2681-2690.
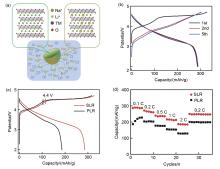
Fig. 2
(a) Schematic illustration of the structure design of gradient surface Na+ doping Li-rich Material; (b) The charge-discharge curves of SLR at 0.1 C; (c), (d) Comparison of magnification and cycle performance between original lithium rich material (PLR) and Na+ gradient doped lithium rich material (SLR) at 0.2 C"
Table 1
Comparison of Li-rich cathode materials with different dopants (1 C=250 mAh/g)"
| 合成方法 | 材料 | 首次放电比容量 | 循环性能 | 倍率性能 | 参考文献 |
|---|---|---|---|---|---|
| 共沉淀 | Na@0.5LiMnO3, 0.5LiNi1/3Co1/3Mn1/3O2 | 286,0.1 C | 223,0.2 C,100圈 | 185,2 C | [ |
| 共沉淀 | Na@Li1.23Mn0.54 Ni0.1Co0.13O2 | 276,0.1 C | 202,1 C,100圈 | 125,5 C | [ |
| 溶胶凝胶 | 1%Na@Li1.2Mn0.54 Ni0.13Co0.13O2 | 227,0.1 C | 85%,10 C,1000圈 | 112,10 C | [ |
| 溶胶凝胶 | 5% Cs@Li1.23Mn0.54 Ni0.1Co0.13O2 | 280,0.1 C | 195,0.5 C,100圈 | 116,10 C | [ |
| 水热 | 5%K@Li2MoO3 | 235,0.064 C | 56%,0.064 C,100圈 | 83,2.56 C | [ |
| 水热 | (900 ℃)K@Li1.2Mn0.54Co0.13Ni0.13O2 | 315,0.08 C | 267,1.6 C,110圈 | — | [ |
| 共沉淀 | K@LiNi0.5Co0.2Mn0.3O2 | 176.5,0.11 C | 148,1.11 C,100圈 | 100,5.54 C | [ |
| 共沉淀 | 2.5%Rb@Li1.2Mn0.54Co0.13Ni0.13O2 | 221.9,0.2 C | 92.78%,0.2 C,100圈 | 117.6,2 C | [ |
| — | 2.5%Mg@Li1.2Mn0.54Co0.13Ni0.13O2 | 250,0.08 C | 195,0.08 C,100圈 | 120,4 C | [ |
| 溶胶凝胶 | 1%Mg@Li1.2Mn0.54Co0.13Ni0.13O2 | 304 | 210,0.1 C,100圈 | — | [ |
| 溶胶凝胶 | 2%Mg@Li1.2Mn0.54Co0.13Ni0.13O2 | 275.8,0.16 C | 254.9,0.16 C,50圈 | 127.5,4 C | [ |
| 溶胶凝胶 | 0.4%Ca@Li1.2Mn0.54Co0.13Ni0.13O2 | 246,0.2 C | 87%,0.2 C,100圈 | 133,4 C | [ |
| 溶剂热 | 3%Al@Li1.5Mn0.675Ni0.1675Co0.1675O2 | 323.7,0.1 C | 200,0.5 C,150圈 | 120,20 C | [ |
| 溶胶凝胶 | 2.5%Ti@Li1.2Mn0.54Co0.13Ni0.13O2 | 320,0.24 C | 229,0.24 C,300圈 | 136,6 C | [ |
| 共沉淀 | 8%Mg@Li1.17Ni0.25-x Mn0.58Mg x O2 | 201 | 95.1%,2 C,100圈 | 104.4,5 C | [ |
| 共沉淀 | 1%Mg@Li1.2Ni0.2Mn0.6O2 | 140.6,0.04 C | 226.5,0.08 C,60圈 | 212.7,0.08 C | [ |
| 共沉淀 | 4%Mg@Li1.2Ni0.12Co0.12Mn0.56O2 | 217.8,0.05 C | 236.9,30圈 | 176.5,1.0 C | [ |
| — | 9%Mg@Li1.2Ni0.16Mn0.56Co0.08O2 | 160,0.1 C | 210,0.1 C,100圈 | 120,4 C | [ |
| 溶胶凝胶 | 4%Al@Li1.14(Ni0.136Co0.136Mn0.544)O2 | 212,0.08 C | 94.65%,100圈 | — | [ |
| 共沉淀 | Cr@Li(Li0.2Mn0.54Ni0.13Co0.13)O2 | 219,0.05 C | 170,0.2 C,133圈 | — | [ |
| 高温固相 | 0.3%Cu@Li1.05FePO4 | 145 | 85%,40圈 | 75,3 C | [ |
| 共沉淀 | Ga@Li1.2Mn0.54Co0.13Ni0.13O2 | 259.8,0.05 C | 229.4,0.1 C,100圈 | 155.7,5 C | [ |
| 共沉淀 | 2.5%Nb@Li1.2Mn0.54Co0.13Ni0.13O2 | 282.6,0.05 C | 83%,1 C,100圈 | 173.1,2 C | [ |
| 共沉淀 | 17.5%Se@Li1.2Mn0.56Ni0.16Co0.08O2 | 265,0.08 C | 93%,0.08 C,100圈 | 178,8 C | [ |
| 高温固相 | Li1.2Mn0.568Ni0.2Sn0.032O2 | 225,0.02 C | 125,0.4 C,100圈 | 123,0.4 C | [ |
| 固相热解 | 2%V@Li1.2Mn0.52Co0.08Ni0.2O2 | 253,0.1 C | 152.2,1 C,50圈 | 99,5 C | [ |
| 热聚合 | 2%Mo@Li1.2Ni0.2Mn0.6O2 | 235,0.1 | 229,0.1 C,204圈 | 110,5 C | [ |
| 共沉淀 | S@Li1.2Mn0.6Ni0.2O2 | 293.3,0.1 C | 213.4,0.1 C,67圈 | 190,2 C | [ |
| 高温固相 | 0.6%B@Li1.2Mn0.54Co0.13Ni0.13O2 | 293.9,0.4 C | 228.55,0.4 C,100圈 | — | [ |
| 高温固相 | 0.5%B@Li1.2Mn0.54Co0.13Ni0.13O2 | — | 211,0.2 C,50圈 | — | [ |
| 溶胶凝胶 | B@Li1.2Mn0.54Co0.13Ni0.13O2 | — | 190,0.2 C,100圈 | — | [ |
| 共沉淀 | 2.5%F@Li1.2Mn0.54Co0.13Ni0.13O2 | 200,0.2 C | 87%,0.2 C,100圈 | — | [ |
| 高温固相 | 2.5%F@Li(Li1/6Ni1/6Co1/6Mn1/2)O2 | 275,0.1 C | 198.75,1 C,40圈 | — | [ |
| 1 | ZHENG J M, XIAO J, ZHANG J G. The roles of oxygen non-stoichiometry on the electrochemical properties of oxide-based cathode materials[J]. Nano Today, 2016, 11(5): 678-694. |
| 2 | NAYAK P K, ERICKSON E M, SCHIPPER F, et al. Review on challenges and recent advances in the electrochemical performance of high capacity Li- and Mn-rich cathode materials for Li-ion batteries[J]. Advanced Energy Materials, 2018, 8(8): doi: 10.1002/aenm.201702397. |
| 3 | 李雨, 赵慧春, 白莹, 等. 高能量密度层状富锂锰基正极材料的改性研究进展[J]. 储能科学与技术, 2018, 7(3): 394-403. |
| LI Y, ZHAO H C, BAI Y, et al. Progress in the modification of lithium-rich manganese-based layered cathode material[J]. Energy Storage Science and Technology, 2018, 7(3): 394-403. | |
| 4 | QING R P, SHI J L, XIAO D D, et al. Enhancing the kinetics of Li-rich cathode materials through the pinning effects of gradient surface Na+ doping[J]. Advanced Energy Materials, 2016, 6(6): doi: 10.1002/aenm.201501914. |
| 5 | LI Q, LI G S, FU C C, et al. K(+)-doped Li(1.2)Mn(0.54)Co(0.13)Ni(0.13)O2: A novel cathode material with an enhanced cycling stability for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2014, 6(13): 10330-10341. |
| 6 | LI N, HE Y S, WANG X P, et al. Incorporation of rubidium cations into Li1.2Mn0.54Co0.13Ni0.13O2 layered oxide cathodes for improved cycling stability[J]. Electrochimica Acta, 2017, 231: 363-370. |
| 7 | SALLARD S, SHEPTYAKOV D, VILLEVIEILLE C. Improved electrochemical performances of Li-rich nickel cobalt manganese oxide by partial substitution of Li+ by Mg2+[J]. Journal of Power Sources, 2017, 359: 27-36. |
| 8 | LAISA C P, RAMESHA R N, RAMESHA K. Enhanced electrochemical performance of lithium rich layered cathode materials by Ca2+ substitution[J]. Electrochimica Acta, 2017, 256: 10-18. |
| 9 | FENG X, GAO Y R, BEN L B, et al. Enhanced electrochemical performance of Ti-doped Li1.2Mn0.54Co0.13Ni0.13O2 for lithium-ion batteries[J]. Journal of Power Sources, 2016, 317: 74-80. |
| 10 | YI T F, LI Y M, YANG S Y, et al. Improved cycling stability and fast charge-discharge performance of cobalt-free lithium-rich oxides by magnesium-doping[J]. ACS Applied Materials & Interfaces, 2016, 8(47): 32349-32359. |
| 11 | SONG B H, ZHOU C F, WANG H L, et al. Advances in sustain stable voltage of Cr-doped Li-rich layered cathodes for lithium ion batteries[J]. Journal of the Electrochemical Society, 2014, 161(10): A1723-A1730. |
| 12 | AN J, SHI L Y, CHEN G R, et al. Insights into the stable layered structure of a Li-rich cathode material for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2017, 5(37): 19738-19744. |
| 13 | SUN Z H, XU L Q, DONG C Q, et al. Enhanced cycling stability of boron-doped lithium-rich layered oxide cathode materials by suppressing transition metal migration[J]. Journal of Materials Chemistry A, 2019, 7(7): 3375-3383. |
| 14 | LI L, SONG B H, CHANG Y L, et al. Retarded phase transition by fluorine doping in Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathode material[J]. Journal of Power Sources, 2015, 283: 162-170. |
| 15 | MING L, ZHANG B, CAO Y, et al. Effect of Nb and F co-doping on Li1.2Mn0.54Ni0.13Co0.13O2 cathode material for high-performance lithium-ion batteries[J]. Frontiers in Chemistry, 2018, 6: 76. |
| 16 | LIU D M, FAN X J, LI Z H, et al. A cation/anion co-doped Li1.12Na0.08Ni0.2Mn0.6O1.95F0.05 cathode for lithium ion batteries[J]. Nano Energy, 2019, 58: 786-796. |
| 17 | LIM S N, SEO J Y, JUNG D S, et al. The crystal structure and electrochemical performance of Li1.167Mn0.548Ni0.18Co0.105O2 composite cathodes doped and co-doped with Mg and F[J]. Journal of Electroanalytical Chemistry, 2015, 740: 88-94. |
| 18 | XUE Z C, QI X Y, LI L Y, et al. Sodium doping to enhance electrochemical performance of overlithiated oxide cathode materials for Li-ion batteries via Li/Na ion-exchange method[J]. ACS Applied Materials & Interfaces, 2018, 10(32): 27141-27149. |
| 19 | DING X, LI Y X, WANG S, et al. Towards improved structural stability and electrochemical properties of a Li-rich material by a strategy of double gradient surface modification[J]. Nano Energy, 2019, 61: 411-419. |
| 20 | DING X, LI Y X, DENG M M, et al. Cesium doping to improve the electrochemical performance of layered Li1.2Ni0.13Co0.13Mn0.54O2 cathode material[J]. Journal of Alloys and Compounds, 2019, 791: 100-108. |
| 21 | YU S S, PENG C, LI Z H, et al. K-doped Li-rich molybdenum-based oxide with improved electrochemical properties for lithium-ion batteries[J]. Arabian Journal for Science and Engineering, 2017, 42(10): 4291-4298. |
| 22 | YANG Z G, GUO X D, XIANG W, et al. K-doped layered LiNi0.5Co0.2Mn0.3O2 cathode material: Towards the superior rate capability and cycling performance[J]. Journal of Alloys and Compounds, 2017, 699: 358-365. |
| 23 | JIN X, XU Q J, LIU H M, et al. Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery[J]. Electrochimica Acta, 2014, 136: 19-26. |
| 24 | XU H J, DENG S N, CHEN G H. Improved electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 by Mg doping for lithium ion battery cathode material[J]. J Mater Chem A, 2014, 2(36): 15015-15021. |
| 25 | YAN W C, XIE Y, JIANG J C, et al. Enhanced rate performance of Al-doped Li-rich layered cathode material via nucleation and post-solvothermal method[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(4): 4625-4632. |
| 26 | WANG D, HUANG Y, HUO Z Q, et al. Synthesize and electrochemical characterization of Mg-doped Li-rich layered Li[Li0.2Ni0.2Mn0.6]O2 cathode material[J]. Electrochimica Acta, 2013, 107: 461-466. |
| 27 | XIANG Y H, LI J, WU X W, et al. Synthesis and electrochemical characterization of Mg-doped Li-rich Mn-based cathode material[J]. Ceramics International, 2016, 42(7): 8833-8838. |
| 28 | NAYAK P K, GRINBLAT J, LEVI E, et al. Understanding the influence of Mg doping for the stabilization of capacity and higher discharge voltage of Li-and Mn-rich cathodes for Li-ion batteries[J]. Physical Chemistry Chemical Physics, 2017, 19(8): 6142-6152. |
| 29 | GUO H C, XIA Y G, ZHAO H, et al. Stabilization effects of Al doping for enhanced cycling performances of Li-rich layered oxides[J]. Ceramics International, 2017, 43(16): 13845-13852. |
| 30 | LEE S B, CHO S H, HEO J B, et al. Copper-substituted, lithium rich iron phosphate as cathode material for lithium secondary batteries[J]. Journal of Alloys and Compounds, 2009, 488(1): 380-385. |
| 31 | YU T H, LI J L, XU G F, et al. Improved cycle performance of Li[Li0.2Mn0.54Co0.13Ni0.13]O2 by Ga doping for lithium ion battery cathode material[J]. Solid State Ionics, 2017, 301: 64-71. |
| 32 | HU X, GUO H J, PENG W J, et al. Effects of Nb doping on the performance of 0.5Li2MnO3 ·0.5LiNi1/3Co1/3Mn1/3O2 cathode material for lithium-ion batteries[J]. Journal of Electroanalytical Chemistry, 2018, 822: 57-65. |
| 33 | MA Q X, LI R H, ZHENG R J, et al. Improving rate capability and decelerating voltage decay of Li-rich layered oxide cathodes via selenium doping to stabilize oxygen[J]. Journal of Power Sources, 2016, 331: 112-121. |
| 34 | WANG Y Q, YANG Z Z, QIAN Y M, et al. New insights into improving rate performance of lithium-rich cathode material[J]. Advanced Materials, 2015, 27(26): 3915-3920. |
| 35 | LU C, YANG S Q, WU H, et al. Enhanced electrochemical performance of Li-rich Li1.2Mn0.52Co0.08Ni0.2O2 cathode materials for Li-ion batteries by vanadium doping[J]. Electrochimica Acta, 2016, 209: 448-455. |
| 36 | ZANG Y, DING C X, WANG X C, et al. Molybdenum-doped lithium-rich layered-structured cathode material Li1.2Ni0.2Mn0.6O2 with high specific capacity and improved rate performance[J]. Electrochimica Acta, 2015, 168: 234-239. |
| 37 | LIU J T, WANG S B, DING Z P, et al. The effect of boron doping on structure and electrochemical performance of lithium-rich layered oxide materials[J]. ACS Applied Materials & Interfaces, 2016, 8(28): 18008-18017. |
| 38 | PAN L C, XIA Y G, QIU B, et al. Structure and electrochemistry of B doped Li(Li0.2Ni0.13Co0.13Mn0.54)1- xBxO2 as cathode materials for lithium-ion batteries[J]. Journal of Power Sources, 2016, 327: 273-280. |
| 39 | SONG J H, KAPYLOU A, CHOI H S, et al. Suppression of irreversible capacity loss in Li-rich layered oxide by fluorine doping[J]. Journal of Power Sources, 2016, 313: 65-72. |
| 40 | LIU Y, DE NING, ZHENG L R, et al. Improving the electrochemical performances of Li-rich Li1.20Ni0.13Co0.13Mn0.54O2 through a cooperative doping of Na+ and PO4 3- with Na3PO4[J]. Journal of Power Sources, 2018, 375: 1-10. |
| 41 | CHEN G R, AN J, MENG Y M, et al. Cation and anion Co-doping synergy to improve structural stability of Li- and Mn-rich layered cathode materials for lithium-ion batteries[J]. Nano Energy, 2019, 57: 157-165. |
| 42 | LIU Q, SU X, LEI D, et al. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping[J]. Nature Energy, 2018, 3(11): 936-943. |
| 43 | PENG Z D, MU K C, CAO Y B, et al. Enhanced electrochemical performance of layered Li-rich cathode materials for lithium ion batteries via aluminum and boron dual-doping[J]. Ceramics International, 2019, 45(4): 4184-4192. |
| 44 | LIANG Y W, LI S Y, XIE J, et al. Synthesis and electrochemical characterization of Mg-Al co-doped Li-rich Mn-based cathode materials[J]. New Journal of Chemistry, 2019, 43(30): 12004-12012. |
| 45 | LIU Y Y, LI R R, LI J L, et al. A high-performance Ce and Sn co-doped cathode material with enhanced cycle performance and suppressed voltage decay for lithium ion batteries[J]. Ceramics International, 2019, 45(16): 20780-20787. |
| [1] | Yawen ZHAO, Yu HUANG, Yanru ZHANG. Analysis of safety test standard of rail transit power lithium-ion battery [J]. Energy Storage Science and Technology, 2022, 11(8): 2505-2518. |
| [2] | Tao SUN, Tengteng SHEN, Xin LIU, Dongsheng REN, Jinhai LIU, Yuejiu ZHENG, Luyan WANG, Languang LU, Minggao OUYANG. Application of titration gas chromatography technology in the quantitative detection of lithium plating in Li-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(8): 2564-2573. |
| [3] | Yong XIAO, Jun XU. Risk assessment of battery safe operation in energy storage power station based on combination weighting and TOPSIS [J]. Energy Storage Science and Technology, 2022, 11(8): 2574-2584. |
| [4] | Bozheng LIU, Liuyang CAO, Tao ZENG, Yaxia YIN, Yuguo GUO. Effect of the binding force on the safety of LiFePO4 cells [J]. Energy Storage Science and Technology, 2022, 11(8): 2556-2563. |
| [5] | Yong MA, Xiaohan LI, Lei SUN, Dongliang GUO, Jinggang YANG, Jianjun LIU, Peng XIAO, Guangjun QIAN. Parameter design of lithium-ion batteries based on a three-dimensional electrochemical thermal coupling lithium precipitation model [J]. Energy Storage Science and Technology, 2022, 11(8): 2600-2611. |
| [6] | ping ZHUO, Yanli ZHU, Chuang QI, Congjie WANG, Fei GAO. Combustion and explosion characteristics of lithium-ion battery pack under overcharge [J]. Energy Storage Science and Technology, 2022, 11(8): 2471-2479. |
| [7] | Congjia ZHANG, Minda SHI, Chen XU, Zhenyu HUANG, Song CI. Intrinsic safety mechanism and case analysis of energy storage systems based on dynamically reconfigurable battery network [J]. Energy Storage Science and Technology, 2022, 11(8): 2442-2451. |
| [8] | Chengshan XU, Borui LU, Mengqi ZHANG, Huaibin WANG, Changyong JIN, Minggao OUYANG, Xuning FENG. Study on thermal runaway gas evolution in the lithium-ion battery energy storage cabin [J]. Energy Storage Science and Technology, 2022, 11(8): 2418-2431. |
| [9] | Kai DING, Jian ZHENG, Wei LI, Zengrui HUANG, Yi WANG, Yimin QIAN, Zixuan ZHENG, Qi XIE. Hierarchical voltage sag mitigation scheme based on user-side energy storage systems and its economic analysis [J]. Energy Storage Science and Technology, 2022, (): 1-11. |
| [10] | Jie CHEN, Weilun CHEN, Xu ZHANG, Yanwei ZHOU, Wuxing ZHANG. Research progress of pre-sodiation technologies in sodium-ion batteries [J]. Energy Storage Science and Technology, 2022, (): 1-10. |
| [11] | Haoyi XIAO, Xiaoxia HE, Jiajia LIANG, Chunli LI. A Lithium Battery Life Prediction Method Based on Mode Decomposition and Machine Learning [J]. Energy Storage Science and Technology, 2022, (): 1-12. |
| [12] | Wei KONG, Jingtao JIN, Xipo LU, Yang SUN. Study on cooling performance of lithium ion batteries with symmetrical serpentine channel [J]. Energy Storage Science and Technology, 2022, 11(7): 2258-2265. |
| [13] | Yingwei PEI, Hong ZHANG, Xinghui WANG. Recent advances in the electrolytes of rechargeable zinc-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(7): 2075-2082. |
| [14] | LIU Hangxin, CHEN Xiantao, SUN Qiang, ZHAO Chenxi. Cycle performance characteristics of soft pack lithium-ion batteries under vacuum environment [J]. Energy Storage Science and Technology, 2022, 11(6): 1806-1815. |
| [15] | XIN Yaoda, LI Na, YANG Le, SONG Weili, SUN Lei, CHEN Haosen, FANG Daining. Integrated sensing technology for lithium ion battery [J]. Energy Storage Science and Technology, 2022, 11(6): 1834-1846. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
