Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (1): 143-156.doi: 10.19799/j.cnki.2095-4239.2023.0713
Previous Articles Next Articles
Su YAN1,2( ), Fangfang ZHONG1(
), Fangfang ZHONG1( ), Junwei LIU2(
), Junwei LIU2( ), Mei DING1, Chuankun JIA1,2
), Mei DING1, Chuankun JIA1,2
Received:2023-10-13
Revised:2023-10-24
Online:2024-01-05
Published:2024-01-22
Contact:
Fangfang ZHONG, Junwei LIU
E-mail:y18570740913@163.com;ffzh@csust.edu.cn;ljw-email@163.com
CLC Number:
Su YAN, Fangfang ZHONG, Junwei LIU, Mei DING, Chuankun JIA. Key materials and advanced characterization of high-energy-density flow battery[J]. Energy Storage Science and Technology, 2024, 13(1): 143-156.
Table 2
Solubility of different active species and energy density of battery"
| 活性物质 | 活性物质浓度 | 能量密度 |
|---|---|---|
| I-/I3- | 6.0 mol/L[ | 43.1 Wh/L |
| ZnI2 | 5.0 mol/L[ | 167 Wh/L |
| (TPyTz)Cl6 | 7.5 mol/L[ | 205 Wh/L |
| Fe(CN)64–/Fe(CN)63– | 1.6 mol/L[ | 41.09 Wh/L |
| Fe(CN)64–/Fe(CN)63– | 1.46 mol/L[ | 73.64 Wh/L |
| KI-KSCN | 6.0 mol/L[ | 221.34 Wh/L |
| AADA | 2.0 mol/L[ | 43.99 Wh/L |
| AQDS(NH4)2 | 1.9 mol/L[ | 12.5 Wh/L |

Fig. 6
(a) Molecular structures of different azobenzene-based derivatives[65]; (b) Solvation energy of different azobenene-based derivatives calculated by DFT[65]; (c) Dissolvability of azobenzene-based derivatives in NaOH solutions with different concentrations[65]; (d) Synthesis of AQSNH4 and AQDS(NH4)2[66]; (e) A comparison of the dissolvability of anthraquinone derivatives[66]"

Table 3
Energy density of different redox-targeted reaction flow batteries"
| 正极 | 负极 | 固体 | 能量密度 |
|---|---|---|---|
| FcBr2/Fc | [Co(Cp)2]/[Co(Cp*)2] | LiFePO4、TiO2 | 500 Wh/L[ |
| VO2+/VO2+ | V2+/V3+ | PBA | 168.75 Wh/L[ |
| [Fe(CN)6]4-/[Fe(CN)6]3- | Zn/Zn2+ | PB | 97.4 Wh/L[ |
| [Fe(CN)6]4-/[Fe(CN)6]3- | Sx2- | PB | 92.8 Wh/L[ |
| NaI | AQDS | Polyimide、NiHCF | 39 Wh/L[ |
| [Fe(CN)6]4-/[Fe(CN)6]3- | DHAQ+DHPS | Ni(OH)2、MH | 128 Wh/L[ |

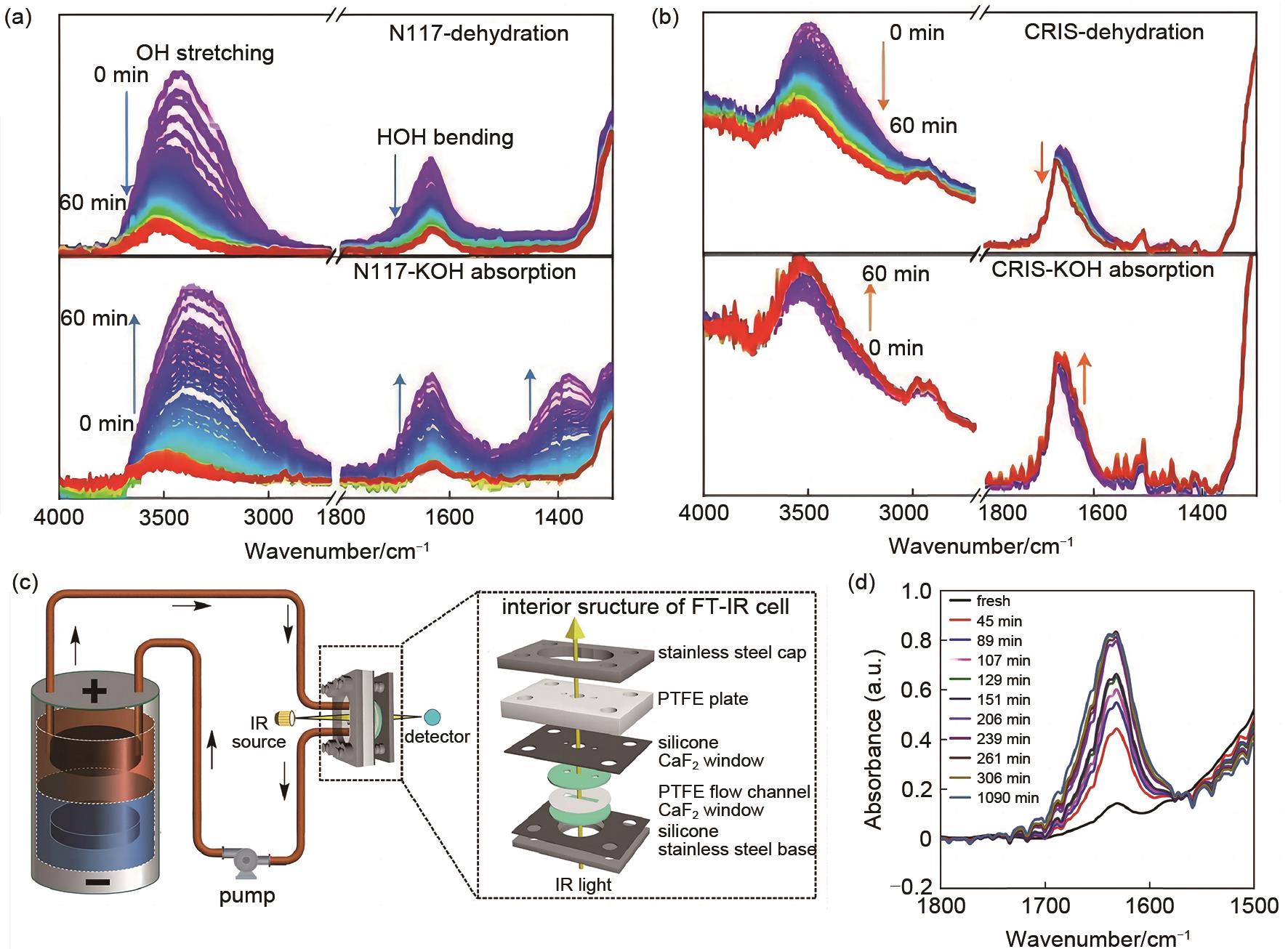
Fig. 11
(a) In situ ATR-FTIR spectra of N117 during dehydration and hydration[87]; (b) In situ ATR-FTIR spectra of CRIS during dehydration and hydration[87]; (c) Schematic of operando FT-IR spectroscopy in the membrane-free flow battery [88]; (d) Evolutive FT-IR spectra of the electrolyte in the membrane-free flow battery[88]"
| 1 | CHU S, CUI Y, LIU N. The path towards sustainable energy[J]. Nature Materials, 2017, 16(1): 16-22. |
| 2 | AGER J W, LAPKIN A A. Chemical storage of renewable energy[J]. Science, 2018, 360(6390): 707-708. |
| 3 | ZANTYE M S, ARORA A, FARUQUE HASAN M M. Renewable-integrated flexible carbon capture: A synergistic path forward to clean energy future[J]. Energy & Environmental Science, 2021, 14(7): 3986-4008. |
| 4 | GÜR T M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage[J]. Energy & Environmental Science, 2018, 11(10): 2696-2767. |
| 5 | DUNN B, KAMATH H, TARASCON J M. Electrical energy storage for the grid: A battery of choices[J]. Science, 2011, 334(6058): 928-935. |
| 6 | YANG Z G, ZHANG J L, KINTNER-MEYER M C W, et al. Electrochemical energy storage for green grid[J]. Chemical Reviews, 2011, 111(5): 3577-3613. |
| 7 | 陈海生, 刘畅, 徐玉杰, 等. 储能在碳达峰碳中和目标下的战略地位和作用[J]. 储能科学与技术, 2021, 10(5): 1477-1485. |
| CHEN H S, LIU C, XU Y J, et al. The strategic position and role of energy storage under the goal of carbon peak and carbon neutrality[J]. Energy Storage Science and Technology, 2021, 10(5): 1477-1485. | |
| 8 | ZHU Z X, JIANG T L, ALI M, et al. Rechargeable batteries for grid scale energy storage[J]. Chemical Reviews, 2022, 122(22): 16610-16751. |
| 9 | ZANTYE M S, GANDHI A, WANG Y F, et al. Optimal design and integration of decentralized electrochemical energy storage with renewables and fossil plants[J]. Energy & Environmental Science, 2022, 15(10): 4119-4136. |
| 10 | KEBEDE A A, KALOGIANNIS T, VAN MIERLO J, et al. A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration[J]. Renewable and Sustainable Energy Reviews, 2022, 159: 112213. |
| 11 | CHO J, JEONG S, KIM Y. Commercial and research battery technologies for electrical energy storage applications[J]. Progress in Energy and Combustion Science, 2015, 48: 84-101. |
| 12 | 贾传坤, 王庆. 高能量密度液流电池的研究进展[J]. 储能科学与技术, 2015, 4(5): 467-475. |
| JIA C K, WANG Q. The development of high energy density redox flow batteries[J]. Energy Storage Science and Technology, 2015, 4(5): 467-475. | |
| 13 | 袁治章, 刘宗浩, 李先锋. 液流电池储能技术研究进展[J]. 储能科学与技术, 2022, 11(9): 2944-2958. |
| YUAN Z Z, LIU Z H, LI X F. Research progress of flow battery technologies[J]. Energy Storage Science and Technology, 2022, 11(9): 2944-2958. | |
| 14 | ZHANG L Y, FENG R Z, WANG W, et al. Emerging chemistries and molecular designs for flow batteries[J]. Nature Reviews Chemistry, 2022, 6(8): 524-543. |
| 15 | 缪平, 姚祯, LEMMON John, 等. 电池储能技术研究进展及展望[J]. 储能科学与技术, 2020, 9(3): 670-678. |
| MIAO P, YAO Z, JOHN L, et al. Current situations and prospects of energy storage batteries[J]. Energy Storage Science and Technology, 2020, 9(3): 670-678. | |
| 16 | 郑琼, 江丽霞, 徐玉杰, 等. 碳达峰、碳中和背景下储能技术研究进展与发展建议[J]. 中国科学院院刊, 2022, 37(4): 529-540. |
| ZHENG Q, JIANG L X, XU Y J, et al. Research progress and development suggestions of energy storage technology under background of carbon peak and carbon neutrality[J]. Bulletin of Chinese Academy of Sciences, 2022, 37(4): 529-540. | |
| 17 | XIA L, LONG T, LI W Y, et al. Highly stable vanadium redox-flow battery assisted by redox-mediated catalysis[J]. Small, 2020, 16(38): 2003321-2003330. |
| 18 | LONG T, LONG Y, DING M, et al. Large scale preparation of 20 cm×20 cm graphene modified carbon felt for high performance vanadium redox flow battery[J]. Nano Research, 2021, 14(10): 3538-3544. |
| 19 | JIAO M L, LIU T, CHEN C J, et al. Holey three-dimensional wood-based electrode for vanadium flow batteries[J]. Energy Storage Materials, 2020, 27: 327-332. |
| 20 | YE J Y, ZHAO X L, MA Y L, et al. Hybrid membranes dispersed with superhydrophilic TiO2 nanotubes toward ultra-stable and high-performance vanadium redox flow batteries[J]. Advanced Energy Materials, 2020, 10(22): 1904041-1904047. |
| 21 | ULAGANATHAN M, ARAVINDAN V, YAN Q Y, et al. Recent advancements in all-vanadium redox flow batteries[J]. Advanced Materials Interfaces, 2016, 3(1): 1500309-1500330. |
| 22 | THALLER L H. Electrically rechargeable REDOX flow cell: US3996064[P]. 1976-12-07. |
| 23 | XIE C Y, YAN H, SONG Y F, et al. Catalyzing anode Cr2+/Cr3+ redox chemistry with bimetallic electrocatalyst for high-performance iron-chromium flow batteries[J]. Journal of Power Sources, 2023, 564: 232860. |
| 24 | WANG S L, XU Z Y, WU X L, et al. Analyses and optimization of electrolyte concentration on the electrochemical performance of iron-chromium flow battery[J]. Applied Energy, 2020, 271: 115252. |
| 25 | SKYLLAS-KAZACOS M, RYCHCIK M, ROBINS R G, et al. New all-vanadium redox flow cell[J]. Journal of the Electrochemical Society, 1986, 133(5): 1057-1058. |
| 26 | WANG G X, ZOU H T, ZHU X B, et al. Recent progress in zinc-based redox flow batteries: A review[J]. Journal of Physics D: Applied Physics, 2022, 55(16): 163001. |
| 27 | WEI X L, XIA G G, KIRBY B, et al. An aqueous redox flow battery based on neutral alkali metal ferri/ferrocyanide and polysulfide electrolytes[J]. Journal of the Electrochemical Society, 2015, 163(1): A5150-A5153. |
| 28 | XU Z C, FAN Q, LI Y, et al. Review of zinc dendrite formation in zinc bromine redox flow battery[J]. Renewable and Sustainable Energy Reviews, 2020, 127: 109838. |
| 29 | DING M, FU H, LOU X C, et al. A stable and energy-dense polysulfide/permanganate flow battery[J]. ACS Nano, 2023, 17(16): 16252-16263. |
| 30 | CHENG J, ZHANG L, YANG Y S, et al. Preliminary study of single flow zinc-nickel battery[J]. Electrochemistry Communications, 2007, 9(11): 2639-2642. |
| 31 | JIN S, SHAO Y Q, GAO X S, et al. Designing interphases for practical aqueous zinc flow batteries with high power density and high areal capacity[J]. Science Advances, 2022, 8(39): eabq4456. |
| 32 | LIN D, RAO D W, CHIOVOLONI S, et al. Prototypical study of double-layered cathodes for aqueous rechargeable static Zn-I2 batteries[J]. Nano Letters, 2021, 21(9): 4129-4135. |
| 33 | YANG J, SONG Y X, LIU Q H, et al. High-capacity zinc-iodine flow batteries enabled by a polymer-polyiodide complex cathode[J]. Journal of Materials Chemistry A, 2021, 9(29): 16093-16098. |
| 34 | XIE C X, LI T Y, DENG C Z, et al. A highly reversible neutral zinc/manganese battery for stationary energy storage[J]. Energy & Environmental Science, 2020, 13(1): 135-143. |
| 35 | ULAGANATHAN M, SURESH S, MARIYAPPAN K, et al. New zinc-vanadium (Zn-V) hybrid redox flow battery: High-voltage and energy-efficient advanced energy storage system[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(6): 6053-6060. |
| 36 | ZHI L P, LI T Y, LIU X Q, et al. Functional complexed zincate ions enable dendrite-free long cycle alkaline zinc-based flow batteries[J]. Nano Energy, 2022, 102: 107697. |
| 37 | LU W J, LI T Y, YUAN C G, et al. Advanced porous composite membrane with ability to regulate zinc deposition enables dendrite-free and high-areal capacity zinc-based flow battery[J]. Energy Storage Materials, 2022, 47: 415-423. |
| 38 | HUSKINSON B, MARSHAK M P, SUH C, et al. A metal-free organic-inorganic aqueous flow battery[J]. Nature, 2014, 505(7482): 195-198. |
| 39 | DEBRULER C, HU B, MOSS J, et al. Designer two-electron storage viologen anolyte materials for neutral aqueous organic redox flow batteries[J]. Chem, 2017, 3(6): 961-978. |
| 40 | HOLLAS A, WEI X L, MURUGESAN V, et al. A biomimetic high-capacity phenazine-based anolyte for aqueous organic redox flow batteries[J]. Nature Energy, 2018, 3(6): 508-514. |
| 41 | ZHANG C K, NIU Z H, PENG S S, et al. Redox flow batteries: Phenothiazine-based organic catholyte for high-capacity and long-life aqueous redox flow batteries[J]. Advanced Materials, 2019, 31(24): 1970175. |
| 42 | LIU T B, WEI X L, NIE Z M, et al. A total organic aqueous redox flow battery employing a low cost and sustainable methyl viologen anolyte and 4-HO-TEMPO catholyte[J]. Advanced Energy Materials, 2016, 6(3): 1501449. |
| 43 | LI Z J, WENG G M, ZOU Q L, et al. A high-energy and low-cost polysulfide/iodide redox flow battery[J]. Nano Energy, 2016, 30: 283-292. |
| 44 | 钟芳芳, 颜云皓, 龚晶, 等. 水系有机液流电池中分子体系研究进展[J]. 长沙理工大学学报(自然科学版), 2023, 20(3): 52-68. |
| ZHONG F F, YAN Y H, GONG J, et al. Advances of redox-active molecules in aqueous organic redox flow batteries[J]. Journal of Changsha University of Science & Technology (Natural Science), 2023, 20(3): 52-68. | |
| 45 | FENG R Z, ZHANG X, WANG W, et al. Reversible ketone hydrogenation and dehy-drogenation for aqueous organic redox flow batteries[J]. Science, 2021, 372(6544): 836-840. |
| 46 | LOU X C, FU H, XU J, et al. Cost-effective membrane and advanced electrode for stable polysulfide-ferricyanide flow battery[J]. Energy Material Advances, 2022, 9865618. |
| 47 | YUAN Z Z, LIANG L X, DAI Q, et al. Low-cost hydrocarbon membrane enables commercial-scale flow batteries for long-duration energy storage[J]. Joule, 2022, 6(4): 884-905. |
| 48 | CHU F M, SU M H, XIAO G Z, et al. Analysis of electrode configuration effects on mass transfer and organic redox flow battery performance[J]. Industrial & Engineering Chemistry Research, 2022, 61(7): 2915-2925. |
| 49 | JIANG Y Q, CHENG G, LI Y H, et al. Promoting vanadium redox flow battery performance by ultra-uniform ZrO2@C from metal-organic framework[J]. Chemical Engineering Journal, 2021, 415: 129014. |
| 50 | CHEN B X, HUANG H A, LIN J D, et al. Doping engineering of M-N-C electrocatalyst based membrane-electrode assembly for high-performance aqueous polysulfides redox flow batteries[J]. Advanced Science, 2023, 10(16): 2206949. |
| 51 | XU Y E, XIE C X, LI T Y, et al. A high energy density bromine-based flow battery with two-electron transfer[J]. ACS Energy Letters, 2022, 7(3): 1034-1039. |
| 52 | ZOU Y P, LIU T T, DU Q J, et al. A four-electron Zn-I2 aqueous battery enabled by reversible I-/I2/I+ conversion[J]. Nature Communications, 2021, 12: 170. |
| 53 | GE G X, ZHANG C K, LI X F. Multi-electron transfer electrode materials for high-energy-density flow batteries[J]. Next Energy, 2023, 1(3): 100043. |
| 54 | HUANG J H, HU S Z, LIANG Z X, et al. Radical stabilization of a tripyridinium-triazine molecule enables reversible storage of multiple electrons[J]. Angewandte Chemie International Edition, 2021, 60(38): 20921-20925. |
| 55 | TANG G G, LIU Y H, LI Y Y, et al. Designing robust two-electron storage extended bipyridinium anolytes for pH-neutral aqueous organic redox flow batteries[J]. JACS Au, 2022, 2(5): 1214-1222. |
| 56 | HU S Z, LI T Y, HUANG M B, et al. Phenylene-bridged bispyridinium with high capacity and stability for aqueous flow batteries[J]. Advanced Materials, 2021, 33(7): 2005839. |
| 57 | ZHANG X R, LIU X, ZHANG H, et al. Robust chalcogenophene viologens as anolytes for long-life aqueous organic redox flow batteries with high battery voltage[J]. ACS Applied Materials & Interfaces, 2022, 14(43): 48727-48733. |
| 58 | ZHEN Y H, ZHANG C J, YUAN J S, et al. Anthraquinone-based electroactive ionic species as stable multi-redox anode active materials for high-performance nonaqueous redox flow batteries[J]. Journal of Materials Chemistry A, 2021, 9(38): 22056-22063. |
| 59 | REMICK R J, ANG P G P. Electrically rechargeable anionically active reduction-oxidation electrical storage-supply system: US4485154[P]. 1984-11-27. |
| 60 | LI B, NIE Z M, VIJAYAKUMAR M, et al. Ambipolar zinc-polyiodide electrolyte for a high-energy density aqueous redox flow battery[J]. Nature Communications, 2015, 6: 6303. |
| 61 | XIE C X, LIU Y, LU W J, et al. Highly stable zinc-iodine single flow batteries with super high energy density for stationary energy storage[J]. Energy & Environmental Science, 2019, 12(6): 1834-1839. |
| 62 | LONG Y, XU Z Z, WANG G X, et al. A neutral polysulfide/ferricyanide redox flow battery[J]. iScience, 2021, 24(10): 103157. |
| 63 | WANG G X, ZOU H T, XU Z Z, et al. Unlocking the solubility limit of ferrocyanide for high energy density redox flow batteries[J]. Materials Today Energy, 2022, 28: 101061. |
| 64 | LU B, YANG M H, DING M, et al. Catholyte engineering to release the capacity of iodide for high-energy-density iodine-based redox flow batteries[J]. SusMat, 2023, 3(4): 522-532. |
| 65 | ZU X H, ZHANG L Y, QIAN Y M, et al. Molecular engineering of azobenzene-based anolytes towards high-capacity aqueous redox flow batteries[J]. Angewandte Chemie International Edition, 2020, 59(49): 22163-22170. |
| 66 | HU B, LUO J, HU M W, et al. A pH-neutral, metal-free aqueous organic redox flow battery employing an ammonium anthraquinone anolyte[J]. Angewandte Chemie, 2019, 131(46): 16782-16789. |
| 67 | WANG C X, YU B, LIU Y Z, et al. N-alkyl-carboxylate-functionalized anthraquinone for long-cycling aqueous redox flow batteries[J]. Energy Storage Materials, 2021, 36: 417-426. |
| 68 | WU W D, WANG A P, LUO J A, et al. A highly stable, capacity dense carboxylate viologen anolyte towards long-duration energy storage[J]. Angewandte Chemie International Edition, 2023, 62(7): e202216662. |
| 69 | WANG C X, LI X A, YU B, et al. Molecular design of fused-ring phenazine derivatives for long-cycling alkaline redox flow batteries[J]. ACS Energy Letters, 2020, 5(2): 411-417. |
| 70 | PAN M G, LU Y, LU S Y, et al. The dual role of bridging phenylene in an extended bipyridine system for high-voltage and stable two-electron storage in redox flow batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(37): 44174-44183. |
| 71 | DUDUTA M, HO B, WOOD V C, et al. Semi-solid lithium rechargeable flow battery[J]. Advanced Energy Materials, 2011, 1(4): 511-516. |
| 72 | CHEN H N, LU Y C. A high-energy-density multiple redox semi-solid-liquid flow battery[J]. Advanced Energy Materials, 2016, 6(8): 1502183. |
| 73 | YANG Z F, ZHANG Q, LI W B, et al. A semi-solid zinc powder-based slurry anode for advanced aqueous zinc-ion batteries[J]. Angewandte Chemie International Edition, 2023, 62(3): e202215306. |
| 74 | WANG Q, ZAKEERUDDIN S M, WANG D Y, et al. Redox targeting of insulating electrode materials: A new approach to high-energy-density batteries[J]. Angewandte Chemie International Edition, 2006, 45(48): 8197-8200. |
| 75 | HUANG Q Z, LI H, GRÄTZEL M, et al. Reversible chemical delithiation/lithiation of LiFePO4: Towards a redox flow lithium-ion battery[J]. Physical Chemistry Chemical Physics, 2013, 15(6): 1793-1797. |
| 76 | JIA C K, PAN F, ZHU Y G, et al. High-energy density nonaqueous all redox flow lithium battery enabled with a polymeric membrane[J]. Science Advances, 2015, 1(10): e1500886. |
| 77 | CHENG Y H, WANG X, HUANG S P, et al. Redox targeting-based vanadium redox-flow battery[J]. ACS Energy Letters, 2019, 4(12): 3028-3035. |
| 78 | CHEN Y, ZHOU M Y, XIA Y H, et al. A stable and high-capacity redox targeting-based electrolyte for aqueous flow batteries[J]. Joule, 2019, 3(9): 2255-2267. |
| 79 | YAN S, HUANG S P, XU H, et al. Redox targeting-based neutral aqueous flow battery with high energy density and low cost[J]. ChemSusChem, 2023, 16(19): e202300710. |
| 80 | ZHOU M Y, CHEN Y, SALLA M, et al. Single-molecule redox-targeting reactions for a pH-neutral aqueous organic redox flow battery[J]. Angewandte Chemie International Edition, 2020, 59(34): 14286-14291. |
| 81 | PÁEZ T, ZHANG F F, MUÑOZ M Á, et al. The redox-mediated nickel-metal hydride flow battery[J]. Advanced Energy Materials, 2022, 12(1): 2102866. |
| 82 | MIELE E, DOSE W M, MANYAKIN I, et al. Hollow-core optical fibre sensors for operando Raman spectroscopy investigation of Li-ion battery liquid electrolytes[J]. Nature Communications, 2022, 13: 1651. |
| 83 | LI J W, LUO N J, KANG L Q, et al. Hydrogen-bond reinforced superstructural manganese oxide As the cathode for ultra-stable aqueous zinc ion batteries[J]. Advanced Energy Materials, 2022, 12(44): 2201840. |
| 84 | YANG L, HAO Y H, LIN J D, et al. POM anolyte for all-anion redox flow batteries with high capacity retention and coulombic efficiency at mild pH[J]. Advanced Materials, 2022, 34(7): e2107425. |
| 85 | YANG Y Q, LIANG S Q, LU B G, et al. Eutectic electrolyte based on N-methylacetamide for highly reversible zinc-iodine battery[J]. Energy & Environmental Science, 2022, 15(3): 1192-1200. |
| 86 | SHAN J J, LI M W, ALLARD L F, et al. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts[J]. Nature, 2017, 551(7682): 605-608. |
| 87 | LI Z J, LU Y C. Polysulfide-based redox flow batteries with long life and low levelized cost enabled by charge-reinforced ion-selective membranes[J]. Nature Energy, 2021, 6(5): 517-528. |
| 88 | WANG X, LASHGARI A, CHAI J C, et al. A membrane-free, aqueous/nonaqueous hybrid redox flow battery[J]. Energy Storage Materials, 2022, 45: 1100-1108. |
| 89 | ZHAO E W, LIU T, JÓNSSON E, et al. In situ NMR metrology reveals reaction mechanisms in redox flow batteries[J]. Nature, 2020, 579(7798): 224-228. |
| 90 | JING Y, ZHAO E W, GOULET M A, et al. In situ electrochemical recomposition of decomposed redox-active species in aqueous organic flow batteries[J]. Nature Chemistry, 2022, 14(10): 1103-1109. |
| 91 | 苗建军. 面向新能源的全钒液流电池储能电站优化设计[J]. 分布式能源, 2023, 8(2): 44-51. |
| MIAO J J. Optimal design of vanadium redox flow battery energy storage station for new energy[J]. Distributed Energy, 2023, 8(2): 44-51. | |
| 92 | 杜念慈. 兆瓦级液流电池储能系统的均衡技术研究[J]. 科技资讯, 2023, 21(17): 73-78, 199. |
| DU N C. Research on the balancing technology of the megawatt-level flow battery energy storage system[J]. Science & Technology Information, 2023, 21(17): 73-78, 199. | |
| 93 | 廖涛, 张建飞, 赵志磊, 等. 独立储能电站经济效益方案分析[J]. 现代工业经济和信息化, 2023, 13(7): 214-216. |
| LIAO T, ZHANG J F, ZHAO Z L, et al. Analysis on economic benefit scheme of independent energy storage power statio[J]. Modern Industrial Economy and Informationization, 2023, 13(7): 214-216. | |
| 94 | 杜预则, 董海鹰. 基于主从博弈的储能电站协同源荷消纳新能源调控策略[J/OL]. 综合智慧能源:1-9[2023-9-20]. http://kns.cnki.net/kcms/detail/41.1461.tk.20230511.0909.004.html. |
| Du Y Z, Dong H Y. Research on the source-load-storage collaborative scheduling strategy for new energy accommodation based on Stackelberg game[J/OL]. Integrated Intelligent Energy:1-9[2023-9-20].http://kns.cnki.net/kcms/detail/41.1461.tk.20230511. 0909.004.html. |
| [1] | Ran XU, Baodong WANG, Shaoliang WANG, Qi ZHANG, Lei ZHANG, Ziyang FENG. Research progress on heteroatom-doped electrodes used in all vanadium redox flow batteries [J]. Energy Storage Science and Technology, 2024, 13(6): 1849-1860. |
| [2] | Jianhang YANG, Wenting FENG, Junwei HAN, Xinru WEI, Chenyu MA, Changming MAO, Linjie ZHI, Debin KONG. Recent advances in rechargeable Li/Na-Cl2 batteries: From material construction to performance evaluation [J]. Energy Storage Science and Technology, 2024, 13(6): 1824-1834. |
| [3] | Yuanyuan JIANG, Fangfang TU, Fangping ZHANG, Yinglai WANG, Jiawen CAI, Donghui YANG, Yanhong LI, Jiayuan XIANG, Xinhui XIA, Jipeng FU. Study on technology and mechanism of prelithiation for high-performance lithium iron phosphate battery [J]. Energy Storage Science and Technology, 2024, 13(5): 1435-1442. |
| [4] | Wenshuo DAI, Qianyuan GUO, Xiangnan CHEN, Huamin ZHANG, Xiangkun MA. Research progress of bipolar plate materials for vanadium flow battery [J]. Energy Storage Science and Technology, 2024, 13(4): 1310-1325. |
| [5] | Xiaoping ZHANG, Yuanjia RONG, Qianyan WANG, Menglin GAO, Yaling LIAO, Minsheng WU, Xinxin ZHUANG, Zhongyu HUANG, Meijun WAN, Weirong CHEN. Advancements in insitu characterization techniques for lithium-oxygen batteries [J]. Energy Storage Science and Technology, 2024, 13(4): 1225-1238. |
| [6] | Xupeng XU, Xuming XU, Hongyan CHEN, LIANGYaru, Weixin LEI, Zengsheng MA, Guoxin CHEN, Peiling KE. Applications of in situ characterization techniques in the study of lithium-sulfur battery mechanisms [J]. Energy Storage Science and Technology, 2024, 13(4): 1239-1252. |
| [7] | Aifang ZHANG, Bangda WEI, Zhuohao LI, Yang YANG, Tianqiang YANG, Jun YAO, Jie ZHANG, Fei LIU, Haomiao LI, Kangli WANG, Kai JIANG. Research progress on modeling and SOC online estimation of vanadium redox-flow batteries [J]. Energy Storage Science and Technology, 2024, 13(3): 1036-1049. |
| [8] | Ang LI, Xiaomeng LI, Jinghao LI, Jinyi ZHANG. A stack model of the redox flow battery analysis and computing program [J]. Energy Storage Science and Technology, 2024, 13(3): 879-892. |
| [9] | Xueli ZHANG, Weiqing SUN, Junhua ZHENG. Study on the influence of polyurethane-type solid-solid phase change energy storage materials on the temperature control effect of asphalt [J]. Energy Storage Science and Technology, 2024, 13(3): 841-843. |
| [10] | Miao LI, Yongli YU, Jianyang WU, Min LEI, Henghui ZHOU. Design of high-energy-density LiFePO4 cathode materials [J]. Energy Storage Science and Technology, 2023, 12(7): 2045-2058. |
| [11] | Kangkang QU, Yahua LIU, Die HONG, Zhaoxi SHEN, Xiaozhao HAN, Xu ZHANG. Research progress on positive electrolytes for neutral aqueous organic redox flow battery [J]. Energy Storage Science and Technology, 2023, 12(5): 1570-1588. |
| [12] | Jipeng YANG, Qiang YE. Effects of electrodeposition of bismuth in an operating iron-chromium redox flow battery base on a strategy of slow release of Bi3+ across the membrane [J]. Energy Storage Science and Technology, 2023, 12(4): 1075-1082. |
| [13] | Xiaoyu GUO, Hao YU, Xin ZHENG, Yujia LIU, Yuanjie ZUO, Miaomiao ZHANG. Optimal configuration of liquid flow battery energy storage in photovoltaic system [J]. Energy Storage Science and Technology, 2023, 12(4): 1158-1167. |
| [14] | Xin ZHENG, Hao YU, Xiaoyu GUO, Ying ZHOU, Yuanjie ZUO, Yujia LIU. Design optimization of integrated energy system using liquid flow battery and heating and cooling storage energy system [J]. Energy Storage Science and Technology, 2023, 12(3): 870-877. |
| [15] | Huamin ZHANG. Development, cost analysis considering various durations, and advancement of vanadium flow batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2772-2780. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
