Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (5): 1570-1588.doi: 10.19799/j.cnki.2095-4239.2023.0093
• New Energy Storage Technologies • Previous Articles Next Articles
Kangkang QU( ), Yahua LIU(
), Yahua LIU( ), Die HONG, Zhaoxi SHEN, Xiaozhao HAN, Xu ZHANG
), Die HONG, Zhaoxi SHEN, Xiaozhao HAN, Xu ZHANG
Received:2023-02-22
Online:2023-05-05
Published:2023-05-29
Contact:
Yahua LIU
E-mail:qukangkang1999@163.com;liuyahua@hfut.edu.cn
CLC Number:
Kangkang QU, Yahua LIU, Die HONG, Zhaoxi SHEN, Xiaozhao HAN, Xu ZHANG. Research progress on positive electrolytes for neutral aqueous organic redox flow battery[J]. Energy Storage Science and Technology, 2023, 12(5): 1570-1588.
Table 1
Molecular structure, solubility, potential, theoretical capacitance, diffusion coefficient (D), reaction rate constant (k0), and literature sources of ferrocene-based cathode electrolytes"
| 名称 | 分子结构 | 溶解度/(mol/L) | 电势E1/2 /V | 理论比容量/(Ah/L) | D/(cm2/s) | k0/(cm/s) | 能量效率 EE | 电压效率 VE | 文献出处 |
|---|---|---|---|---|---|---|---|---|---|
| FcNCl | 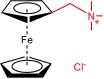 | 4 | 0.61 (vs NHE) | 107.2 | 3.74×10-6 | 3.66×10-5 | 72% (40 mA/cm2) | 72% (40 mA/cm2) | [ |
| FcN2Br2 |  | 3.1 | 0.61 (vs NHE) | 83.1 | 3.64×10-6 | 4.60×10-6 | 70% (40 mA/cm2) | 70% (40 mA/cm2) | [ |
| FC1N112-Br |  | 2.9 | 0.418—0.467 (vs Ag/AgCl) | 77.7 | / | / | / | / | [ |
| BTMAP-Fc | 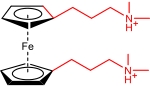 | 1.9 | 0.39(vs SHE) | 50.9 | 6.2×10-10 | 1.40×10-2 | / | / | [ |
| 1,1’FcDS | 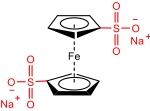 | 0.3 (1 NaNO3, 0.5 EG) | 0.651 (vs Ag/AgCl) | 16.1 | 1.29×10-6 | / | 60% (25 mA/cm2) | / | [ |
| Fc-SO3NH4 |  | 0.22 (1 NaCl) | 0.38 (vs Ag/AgCl) | 5.9 | 3.79×10-8 | / | 63.75% (20 mA/cm2) | 66.99% (20 mA/cm2) | [ |
| HMFc⊂HP-β-CD | 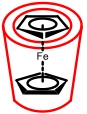 | 0.28 | 0.52 (vs NHE) | 4.23 | 2.22×10-6 | 3.70×10-2 | / | / | [ |

Fig. 2
(a) The cyclic voltammograms of FcNCl (red line), FcN2Br2 (purple line), FcN (black line) and MV (blue line), and the dotted line is the cyclic voltammetry of 0.5 mol/L NaCl aqueous solution; (b) The 0.5 mol/L FcNCl/MV battery cycle test diagram at 60 mA/cm2: the internal illustration is a charge-discharge curves for a representative number of turns[44]"

Table 3
Molecular structure, solubility, potential, theoretical capacitance, diffusion coefficient (D), reaction rate constant (k0), and literature sources of TEMPO-based cathode electrolytes"
| 名称 | 分子结构 | 溶解度/(mol/L) | 电势E1/2/V | 理论电容量/(Ah/L) | D/(cm2/s) | k0/(cm/s) | 能量效率EE | 电压效率VE | 文献出处 |
|---|---|---|---|---|---|---|---|---|---|
| 4-OH-TEMPO | 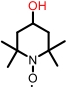 | 2.1 | 0.60 V (vs Ag/AgCl) | 56.3 | 2.95×10-5 | 2.6×10-4 | 62.5% (40 mA/cm2) | 62.1% (40 mA/cm2) | [ |
| 4-SO3K-TEMPO | 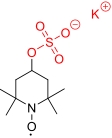 | >1 (2 ZnCl2,1 NH4Cl) | 0.61 V (vs Ag/AgCl) | >26.7 | 2.98×10-6 | 1.91×10-3 | — | 52% (80 mA/cm2) | [ |
| 4-COONa-TEMPO | 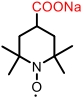 | 2.5 | 0.60 V (vs Ag/AgCl) | 67 | 5.45×10-6 | 2.1×10-2 | 64% (40 mA/cm2) | — | [ |
| TEMPTMA | 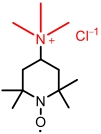 | 3.2 | 0.79 (vs Ag/AgCl) | 85.8 | 4.8×10-6 | 4.2×10-2 | 50% (70 mA/cm2) | — | [ |
| TMAP-TEMPO | 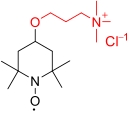 | 4.62 | 0.81 V (vs SHE) | 123.8 | 3.48×10-6 | 1.02×10-2 | 93.41% (10 mA/cm2) | — | [ |
| N2-TEMPO |  | 3.0 | >0.80 V (vs SHE) | 80.4 | 5.15×10-6 | — | 70.3% (40 mA/cm2) | — | [ |
| Ploy(TEMPO) |  | — | 0.70 V (vs Ag/AgCl) | — | 7.0×10-8 | 4.1×10-4 | 75% (40 mA/cm2) | — | [ |
| 1-methyl-imidazolium functio-nalized TEMPO |  | — | 0.71 V (vs Ag/AgCl) | — | — | — | 93.7% (1.25 mA/cm2) | 97.7% (1.25 mA/cm2) | [ |
| (TPABPy)Cl3 |  | >1.5 | 0.967 V (vs SHE) | >37.6 | 2.97×10-6 | 7.50×10-2 | 70.6% (60 mA/cm2) | 71.1% (60 mA/cm2) | [ |
| Pyr-TEMPO | 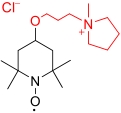 | >3.35 | 0.81 V (vs SHE) | >89.8 | 4.07×10-6 | 1.42×10-2 | 84% (40 mA/cm2) | — | [ |
| PSS-TEMPO | 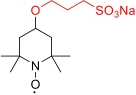 | >2.0 | 0.805 V (vs SHE) | >53.6 | 3.36×10-6 | 5.29×10-3 | 80% (40 mA/cm2) | — | [ |

Fig. 20
(a) Synthetic routes of PSS-TEMPO; (b) the cycle testing results of 0.20 mol/L K3Fe(CN)6/0.15 PSS-TEMPO AORFB at 40 mA/cm2, the internal illustration is the charge-discharge curves of a representative number of turns; (c) CV curves of PSS-TEMPO (red) in 1 mol/L NaCl; (d) 1H NMR spectra of PSS-TEMPO catholyte before and after battery cycling; (e) ESI-MS profile of PSS-TEMPO catholyte after battery cycling[67]"
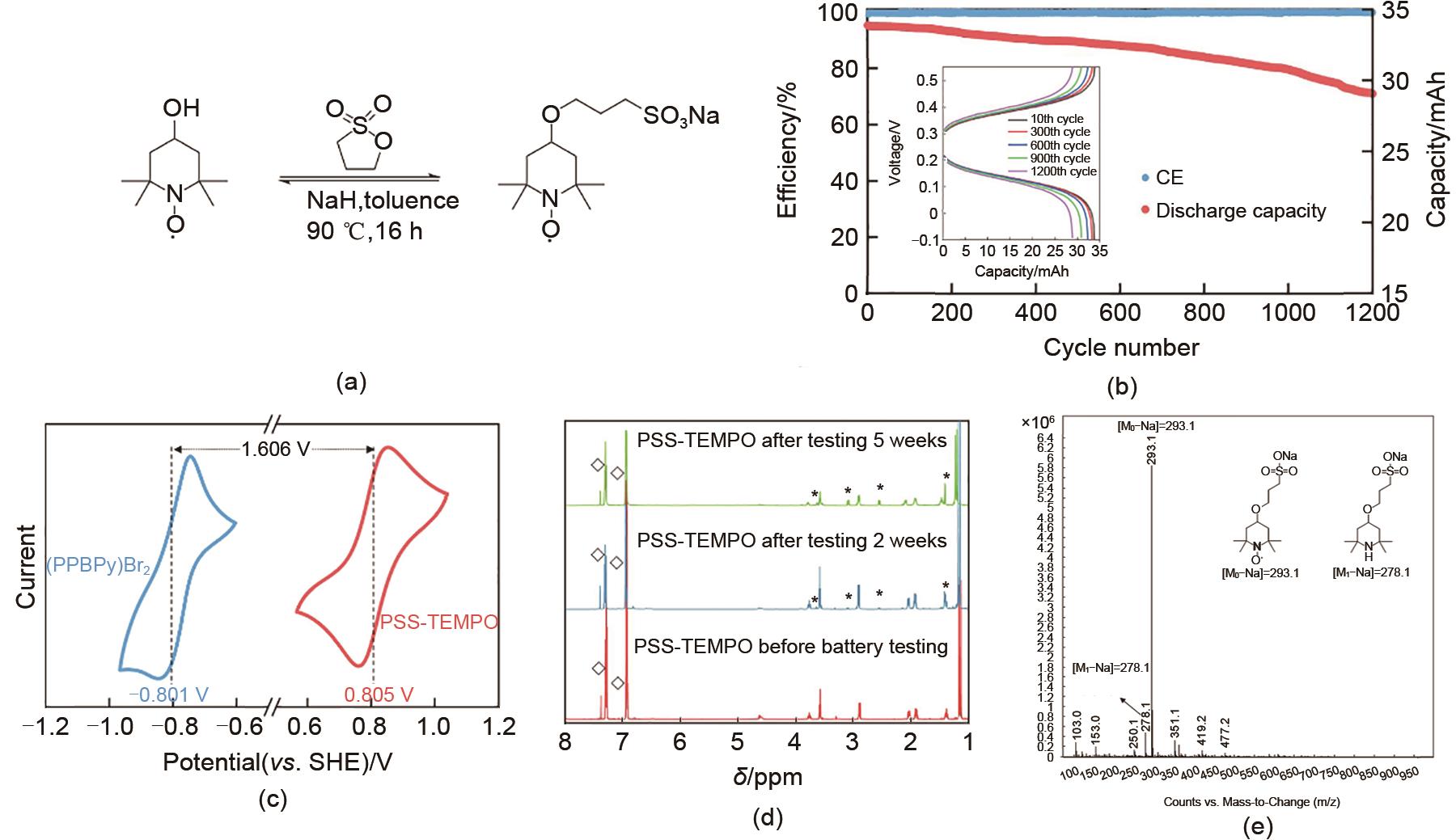
Table 4
Capacity retention rates of several typical TEMPO cathode electrolytes"
| 正极电解质 | 负极电解质 | 正极电解质浓度/(mol/L) | 容量保持率(每次) | 文献 出处 |
|---|---|---|---|---|
| 4-OH-TEMPO | MV | 0.1 | 99.99% | [ |
| 4-SO3K-TEMPO | ZnCl2 | 0.6 | 99.94% | [ |
| TMAP-TEMPO | BTMAP-Vi | 0.1 | 99.993% | [ |
| N2-TEMPO | (NPr)2V | 1.0 | 99.975% | [ |
| (TPABPy)Cl3 | BTMAP-Vi | 1.5 | 99.98% | [ |
| Pyr-TEMPO | [PyrPV]Cl4 | 0.2 | 99.96% | [ |
| PSS-TEMPO | K3Fe(CN)6 | 0.15 | 99.988% | [ |
| 1 | JIN S, SHAO Y Q, GAO X S, et al. Designing interphases for practical aqueous zinc flow batteries with high power density and high areal capacity[J]. Science Advances, 2022, 8(39): doi: 10.1126/sciadv.abq4456. |
| 2 | 2021年全国电力工业统计数据发布[N]. 国家电网报, 2022-01-27(1). |
| 3 | DUNN B, KAMATH H, TARASCON J M. Electrical energy storage for the grid: A battery of choices[J]. Science, 2011, 334(6058): 928-935. |
| 4 | SERVICE R F. Advances in flow batteries promise cheap backup power[J]. Science, 2018, 362(6414): 508-509. |
| 5 | LIN K X, CHEN Q, GERHARDT M R, et al. Alkaline quinone flow battery[J]. Science, 2015, 349(6255): 1529-1532. |
| 6 | SINGH V, KIM S, KANG J, et al. Aqueous organic redox flow batteries[J]. Nano Research, 2019, 12(9): 1988-2001. |
| 7 | ZHAO E W, LIU T, JÓNSSON E, et al. In situ NMR metrology reveals reaction mechanisms in redox flow batteries[J]. Nature, 2020, 579(7798): 224-228. |
| 8 | PERRY M L. BATTERIES. Expanding the chemical space for redox flow batteries[J]. Science, 2015, 349(6255): 1452. |
| 9 | 夏力行, 刘昊, 刘琳, 等. 有机氧化还原液流电池的研究进展[J]. 电化学, 2018, 24(5): 466-487. |
| XIA L (H /X), LIU H, LIU L, et al. Recent progresses in organic redox flow batteries[J]. Journal of Electrochemistry, 2018, 24(5): 466-487. | |
| 10 | 杨霖霖, 王少鹏, 倪蕾蕾, 等. 新型液流电池研究进展[J]. 上海电气技术, 2015, 8(1): 46-49. |
| YANG L L, WANG S P, NI L L, et al. The progression on the development of new redox flow batteries[J]. Journal, 2015, 8(1): 46-49. | |
| 11 | SÁNCHEZ-DÍEZ E, VENTOSA E, GUARNIERI M, et al. Redox flow batteries: Status and perspective towards sustainable stationary energy storage[J]. Journal of Power Sources, 2021, 481: doi:10.1016/j.jpowsour.2020.228804. |
| 12 | ARCHANA K S, SURESH S, RAGUPATHY P, et al. Investigations on new Fe-Mn redox couple based aqueous redox flow battery[J]. Electrochimica Acta, 2020, 345: doi: 10.1016/j.electacta.2020. 136245. |
| 13 | JEENA C B, ELSA P, MOLY P P, et al. A dendrite free Zn‐Fe hybrid redox flow battery for renewable energy storage[J]. Energy Storage, 2021, 4: doi: 10.1002/est2.275. |
| 14 | SHIN M, NOH C, KWON Y. Stability enhancement for all‐iron aqueous redox flow battery using iron-3-[bis(2‐hydroxyethyl) amino]-2-hydroxy propane sulfonic acid complex and ferrocyanide as redox couple[J]. International Journal of Energy Research, 2021, 46: 6866-6875. |
| 15 | SHOU-GUANG Y, SUN X F, XIAO M, et al. Optimization analysis of the internal structure of flow-assisted zinc-nickel battery driven by a propeller[J]. Advances in Mechanical Engineering, 2019, 11(2): 1-8. |
| 16 | ZHEN Y H, ZHANG C J, YUAN J S, et al. A high-performance all-iron non-aqueous redox flow battery[J]. Journal of Power Sources, 2020, 445: doi: 10.1016/j.jpowsour.2019.227331. |
| 17 | HU J, YUE M, ZHANG H M, et al. A boron nitride nanosheets composite membrane for a long-life zinc-based flow battery[J]. Angewandte Chemie (International Ed in English), 2020, 59(17): 6715-6719. |
| 18 | XU Z Y,WU M C. Toward dendrite-free deposition in zinc-based flow batteries: status and prospects[J]. Batteries, 2022, 8: doi: 10.3390/batteries8090117. |
| 19 | KHOR A, LEUNG P, MOHAMED M R, et al. Review of zinc-based hybrid flow batteries: From fundamentals to applications[J]. Materials Today Energy, 2018, 8: 80-108. |
| 20 | MUNDARAY E, SÁEZ A, SOLLA-GULLÓN J, et al. New insights into the performance of an acid-base electrochemical flow battery[J]. Journal of Power Sources, 2021, 506: doi: 10.1016/j.jpowsour. 2021.230233. |
| 21 | ANDREWS J, SEIF MOHAMMADI S. Towards a 'proton flow battery': Investigation of a reversible PEM fuel cell with integrated metal-hydride hydrogen storage[J]. International Journal of Hydrogen Energy, 2014, 39(4): 1740-1751. |
| 22 | LIANG Q, CHEN F M, WANG S F, et al. An organic flow desalination battery[J]. Energy Storage Materials, 2019, 20: 203-207. |
| 23 | LIU Y, CHEN Q, SUN P, et al. Organic electrolytes for aqueous organic flow batteries[J]. Materials Today Energy, 2021, 20: doi: 10.1016/j.mtener.2020.100634. |
| 24 | ZHANG C K, LI X F. Perspective on organic flow batteries for large-scale energy storage[J]. Current Opinion in Electrochemistry, 2021, 30: doi:10.1016/j.coelec.2021.100836. |
| 25 | DUAN W, LI B, LU D, et al. Towards an all-vanadium redox flow battery with higher theoretical volumetric capacities by utilizing the VO2+/V3+ couple[J]. Journal of Energy Chemistry, 2018, 27: 1381-1385. |
| 26 | HSIEH W Y, LEU C H, WU C H, et al. Measurement of local current density of all-vanadium redox flow batteries[J]. Journal of Power Sources, 2014, 271: 245-251. |
| 27 | CHEN H, ZHANG X Y, ZHANG S R, et al. A comparative study of iron-vanadium and all-vanadium flow battery for large scale energy storage[J]. Chemical Engineering Journal, 2022, 429: doi: 10.1016/j.cej.2021.132403. |
| 28 | WEI X, NIE Z, LUO Q, et al. Nanoporous polytetrafluoroethylene/silica composite separator as a high-performance all-vanadium redox flow battery membrane[J]. Advanced Energy Materials, 2013, 3: 1215-1220. |
| 29 | 张华民. 全钒液流电池的技术进展、不同储能时长系统的价格分析及展望[J]. 储能科学与技术, 2022, 11(9): 2772-2780. |
| ZHANG H M. Development, cost analysis considering various durations, and advancement of vanadium flow batteries[J]. Energy Storage Science and Technology, 2022, 11(9): 2772-2780. | |
| 30 | WILK A, SZYPULSKA-KOZIARSKA D, WISZNIEWSKA B. The toxicity of vanadium on gastrointestinal, urinary and reproductive system, and its influence on fertility and fetuses malformations[J]. Postepy Higieny i Medycyny Doswiadczalnej (Online), 2017, 71(0): 850-859. |
| 31 | 袁治章, 刘宗浩, 李先锋. 液流电池储能技术研究进展[J]. 储能科学与技术, 2022, 11(9): 2944-2958. |
| YUAN Z Z, LIU Z H, LI X F. Research progress of flow battery technologies[J]. Energy Storage Science and Technology, 2022, 11(9): 2944-2958. | |
| 32 | RAMAR A, WANG F M, FOENG R, et al. Organic redox flow battery: Are organic redox materials suited to aqueous solvents or organic solvents?[J]. Journal of Power Sources, 2023, 558: doi: 10.1038/s41586-021-03399-1. |
| 33 | HUSKINSON B, MARSHAK M P, SUH C, et al. A metal-free organic-inorganic aqueous flow battery[J]. Nature, 2014, 505(7482): 195-198. |
| 34 | WEI X, PAN W, DUAN W, et al. Materials and systems for organic redox flow batteries: status and challenges[J]. ACS Energy Letters, 2017, 2: 2187-2204. |
| 35 | TANG L, LEUNG P, MOHAMED M R, et al. Capital cost evaluation of conventional and emerging redox flow batteries for grid storage applications[J]. Electrochimica Acta, 2023, 437: doi: 10.1016/j.electacta.2022.141460. |
| 36 | NGUYEN T P, EASLEY A D, KANG N R, et al. Polypeptide organic radical batteries[J]. Nature, 2021, 593(7857): 61-66. |
| 37 | BEH EUGENE S, DIANA D P, GRACIA REBECCA L, et al. A neutral pH aqueous organic-organometallic redox flow battery with extremely high capacity retention[J]. ACS Energy Letters, 2017, 2(3): 639-644. |
| 38 | JIN M, FELL E M, VINA-LOPEZ L, et al. Near neutral pH redox flow battery with low permeability and long-lifetime phosphonated viologen active species[J]. Advanced Energy Materials, 2020, 10: doi: 10.1002/aenm.202000100. |
| 39 | 陈倩如. 水系有机液流电池正极电解质的制备及性能研究[D]. 合肥: 中国科学技术大学, . |
| CHEN Q R. Preparation and properties of cathode electrolyte for water-based organic flow battery[D]. Hefei: University of Science and Technology of China, . | |
| 40 | COSIMBESCU L, WEI X L, VIJAYAKUMAR M, et al. Anion-tunable properties and electrochemical performance of functionalized ferrocene compounds[J]. Scientific Reports, 2015, 5(1): 1-9. |
| 41 | GOODWIN C A P, GIANSIRACUSA M J, GREER S M, et al. Isolation and electronic structures of derivatized manganocene, ferrocene and cobaltocene anions[J]. Nature Chemistry, 2021, 13(3): 243-248. |
| 42 | MALISCHEWSKI M, ADELHARDT M, SUTTER J, et al. Isolation and structural and electronic characterization of salts of the decamethylferrocene dication[J]. Science, 2016, 353(6300): 678-682. |
| 43 | YAN Q H, ZHI N, YANG L, et al. A highly sensitive uric acid electrochemical biosensor based on a nano-cube cuprous oxide/ferrocene/uricase modified glassy carbon electrode[J]. Scientific Reports, 2020, 10(1): 1-10. |
| 44 | HU B, DEBRULER C, RHODES Z, et al. Long-cycling aqueous organic redox flow battery (AORFB) toward sustainable and safe energy storage[J]. Journal of the American Chemical Society, 2017, 139(3): 1207-1214. |
| 45 | KIM S, KIM D, HWANG G, et al. A bromide-ligand ferrocene derivative redox species with high reversibility and electrochemical stability for aqueous redox flow batteries[J]. Journal of Electroanalytical Chemistry, 2020, 869: doi: 10.1016/j.jelechem. 2020.114131. |
| 46 | ZHAO Z L, ZHANG B S, SCHRAGE B, et al. Investigations into aqueous redox flow batteries based on ferrocene bisulfonate[J]. ACS Applied Energy Materials, 2020, 3: 10270-10277. |
| 47 | YAO Y W, XU H, TIAN Z Z, et al. Simple-synthesized sulfonated ferrocene ammonium for aqueous redox flow batteries[J]. ACS Applied Energy Materials, 2021, 4: 8052-8058. |
| 48 | LI Y Y, XU Z A, LIU Y H, et al. Functioning water-insoluble ferrocenes for aqueous organic flow battery via host-guest inclusion[J]. ChemSusChem, 2021, 14(2): 745-752. |
| 49 | CHEN Q R, LI Y Y, LIU Y H, et al. Designer ferrocene catholyte for aqueous organic flow batteries[J]. ChemSusChem, 2021, 14(5): 1295-1301. |
| 50 | WINSBERG J, HAGEMANN T, JANOSCHKA T, et al. Redox-flow batteries: from metals to organic redox-active materials[J]. Angewandte Chemie International Edition, 2017, 56: 686-711. |
| 51 | 刘静嘉, 张宇婷, 蔡辉宇, 等. TEMPO及其衍生物在有机液流电池体系中的应用[J]. 大学化学, 2017, 32(11): 32-44. |
| LIU J J, ZHANG Y T, CAI H Y, et al. TEMPO and lts derivatives in organic redox-flow batteries[J]. University Chemistry, 2017, 32(11): 32-44. | |
| 52 | HU X Q, QI X T, CHEN J R, et al. Catalytic N-radical cascade reaction of hydrazones by oxidative deprotonation electron transfer and TEMPO mediation[J]. Nature Communications, 2016, 7(1): 1-12. |
| 53 | YOKOI T, OTANI T, ISHII K. In vivo fluorescence bioimaging of ascorbic acid in mice: Development of an efficient probe consisting of phthalocyanine, TEMPO, and albumin[J]. Scientific Reports, 2018, 8(1): 1-9. |
| 54 | JI L L, DAI Y Y, YAN S M, et al. A fast electrochromic polymer based on TEMPO substituted polytriphenylamine[J]. Scientific Reports, 2016, 6(1): 1-9. |
| 55 | JIE X M, SHANG Y P, CHEN Z N, et al. Differentiation between enamines and tautomerizable imines in the oxidation reaction with TEMPO[J]. Nature Communications, 2018, 9(1): 1-10. |
| 56 | NUTTING J E, RAFIEE M, STAHL S S. Tetramethylpiperidine N-oxyl (TEMPO), phthalimide N-oxyl (PINO), and related N-oxyl species: Electrochemical properties and their use in electrocatalytic reactions[J]. Chemical Reviews, 2018, 118(9): 4834-4885. |
| 57 | LIU T, WEI X L, NIE Z, et al. A total organic aqueous redox flow battery employing a low cost and sustainable methyl viologen anolyte and 4-HO-TEMPO catholyte[J]. Advanced Energy Materials, 2016, 6: doi: 10.1002/aenm.201501449. |
| 58 | JAN W, CHRISTIAN S, ALMUT S, et al. Aqueous 2, 2, 6, 6-tetramethylpiperidine-N-oxyl catholytes for a high-capacity and high current density oxygen-insensitive hybrid-flow battery[J]. ACS Energy Letters, 2017, 2(2): 411-416. |
| 59 | LIU B, TANG C W, JIANG H R, et al. Carboxyl-functionalized TEMPO catholyte enabling high-cycling-stability and high-energy-density aqueous organic redox flow batteries[J]. ACS Sustainable Chemistry & Engineering, 2021, 9: 6258-6265. |
| 60 | JANOSCHKA T, MARTIN N, HAGER M D, et al. An aqueous redox-flow battery with high capacity and power: The TEMPTMA/MV system[J]. Angewandte Chemie (International Ed in English), 2016, 55(46): 14427-14430. |
| 61 | LIU Y H, GOULET M A, TONG L C, et al. A long-lifetime all-organic aqueous flow battery utilizing TMAP-TEMPO radical[J]. Chem, 2019, 5: 1861-1870. |
| 62 | HU B, HU M W, LUO J, et al. A stable, low permeable TEMPO catholyte for aqueous total organic redox flow batteries [J]. Advanced Energy Materials, 2021, 12: doi: 10.1002/aenm.202102577. |
| 63 | JANOSCHKA T, MARTIN N, MARTIN U, et al. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials[J]. Nature, 2016, 534(7607): S9-S10. |
| 64 | CHANG Z J, HENKENSMEIER D, CHEN R Y. Shifting redox potential of nitroxyl radical by introducing an imidazolium substituent and its use in aqueous flow batteries[J]. Journal of Power Sources, 2019, 418: 11-16. |
| 65 | HU S Z, WANG L W, YUAN X Z, et al. Viologen-decorated TEMPO for neutral aqueous organic redox flow batteries[J]. Energy Material Advances, 2021, 2021: 1-8. |
| 66 | PAN M G, GAO L Z, LIANG J C, et al. Reversible redox chemistry in pyrrolidinium‐based TEMPO radical and extended viologen for high-voltage and long-life aqueous redox flow batteries[J]. Advanced Energy Materials, 2022, 12: doi: 10.1002/aenm.202103478. |
| 67 | GAO M Q, SALLA M, SONG Y X, et al. High-power near-neutral aqueous all organic redox flow battery enabled with a pair of anionic redox species[J]. Angewandte Chemie (International Ed in English), 2022, 61(41): doi: 10.1002/anie.202208223. |
| 68 | LUO J, HU B, HU M W, et al. Status and prospects of organic redox flow batteries toward sustainable energy storage[J]. ACS Energy Letters, 2019, 4(9): 2220-2240. |
| 69 | WINSBERG J, STOLZE C, MUENCH S, et al. TEMPO/phenazine combi-molecule: a redox-active material for symmetric aqueous redox-flow batteries[J]. ACS Energy Letters, 2016, 1: 976-980. |
| 70 | JANOSCHKA T, FRIEBE C, HAGER M D, et al. An approach toward replacing vanadium: A single organic molecule for the anode and cathode of an aqueous redox-flow battery[J]. ChemistryOpen, 2017, 6(2): 216-220. |
| 71 | ZHU Y Z, YANG F, NIU Z H, et al. Enhanced cyclability of organic redox flow batteries enabled by an artificial bipolar molecule in neutral aqueous electrolyte[J]. Journal of Power Sources, 2019, 417: 83-89. |
| 72 | HEILAND N, CIDARÉR C, ROHR C, et al. Design and evaluation of a boron dipyrrin electrophore for redox flow batteries[J]. ChemSusChem, 2017, 10(21): 4215-4222. |
| 73 | HAGEMANN T, WINSBERG J, HÄUPLER B, et al. A bipolar nitronyl nitroxide small molecule for an all-organic symmetric redox-flow battery[J]. NPG Asia Materials, 2017, 9(1): e340. |
| 74 | TAYLOR P, MILLER J L, BUTLER P. Energy storage opportunities analysis phase ii final report a study for the doe energy storage systems program[N]. Sandia National Laboratories, 2002-05-01. |
| 75 | 朱兆武, 张旭堃, 苏慧, 等. 全钒液流电池提高电解液浓度的研究与应用现状[J]. 储能科学与技术, 2022, 11(11): 3439-3446. |
| ZHU Z W, ZHANG X K, SU H, et al. Research and application of increasing electrolyte concentration in all vanadium redox flow battery[J]. Energy Storage Science and Technology, 2022, 11(11): 3439-3446. |
| [1] | Lei LEI, Peng GAO, Nana FENG, Kunpeng CAI, Hai ZHANG, Yang ZHANG. The influences of multifactors in the synthesis progress on the characteristics of lithium lanthanum zirconate solid electrolytes [J]. Energy Storage Science and Technology, 2023, 12(5): 1625-1635. |
| [2] | Jintao LI, Yue MU, Jing WANG, Jingyi QIU, Hai MING. Investigation of the structural evolution and interface behavior in cathode materials for Li-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(5): 1636-1654. |
| [3] | Xuanchen WANG, Da WANG, Zhaomeng LIU, Xuanwen GAO, Wenbin LUO. Research progress and prospect of potassium ion battery electrolyte [J]. Energy Storage Science and Technology, 2023, 12(5): 1409-1426. |
| [4] | Weibin HUANG, Biao ZHANG, Jincheng FAN, Wei YANG, Hanbo ZOU, Shengzhou CHEN. Preparation and modification of ZIF-8 composite PEO based solid electrolyte [J]. Energy Storage Science and Technology, 2023, 12(4): 1083-1092. |
| [5] | Kaiyuan XUE, Yan WANG, Junwei LANG, Tian HE, Zuoqiang DAI, Zongmin ZHENG. The progress in applications of dicationic ionic liquids in the energy storage and conversion system [J]. Energy Storage Science and Technology, 2023, 12(3): 808-821. |
| [6] | Xin SHEN, Rui ZHANG, Chenzi ZHAO, Peng WU, Yutong ZHANG, Jundong ZHANG, Lizhen FAN, Quanbing LIU, Aibing CHEN, Qiang ZHANG. Recent advances in mechano-electrochemistry in lithium metal batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2781-2797. |
| [7] | Jinghua WU, Jing YANG, Gaozhan LIU, Zhiyan WANG, Zhihua ZHANG, Hailong YU, Xiayin YAO, Xuejie HUANG. Review and prospective of solid-state lithium batteries in the past decade (2011—2021) [J]. Energy Storage Science and Technology, 2022, 11(9): 2713-2745. |
| [8] | Pengbo ZHAI, Dongmei CHANG, Zhijie BI, Ning ZHAO, Xiangxin GUO. Research progress on key interfacial issues in lithium lanthanum zirconium oxide-based solid-state [J]. Energy Storage Science and Technology, 2022, 11(9): 2847-2865. |
| [9] | ZHOU Weidong, HUANG Qiu, XIE Xiaoxin, CHEN Kejun, LI Wei, QIU Jieshan. Research progress of polymer electrolyte for solid state lithium batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1788-1805. |
| [10] | XIE Chenglu, HUANG Xiankun, KANG Lixia, LIU Yongzhong. Electrocatalytic performances of Ru nanoparticles supported on carbon nanotubes by colloidal solution for synthetic ammonia [J]. Energy Storage Science and Technology, 2022, 11(6): 1947-1956. |
| [11] | Xingxing WANG, Ziyu SONG, Hao WU, Wenfang FENG, Zhibin ZHOU, Heng ZHANG. Advances in conducting lithium salts for solid polymer electrolytes [J]. Energy Storage Science and Technology, 2022, 11(4): 1226-1235. |
| [12] | Zhiqiang ZHAO, Hengjun LIU, Xixiang XU, Yuanyuan PAN, Qinghao LI, Hongsen LI, Han HU, Qiang LI. Magnetometry technique in energy storage science [J]. Energy Storage Science and Technology, 2022, 11(3): 818-833. |
| [13] | Xiubo ZHANG, Chang YU, Jinhe YU, Yingbin LIU, Yuanyang XIE, Jianjian WANG, Shuqin LAN, Jieshan QIU. Recent progress of polymer electrolytes for supercapacitors under extreme environments [J]. Energy Storage Science and Technology, 2022, 11(12): 3808-3818. |
| [14] | Yue SU, Xuhua LIU, Fanglei ZENG, Yurong REN, Bencai LIN. Preparation and properties of polyvinylidene fluoride/polyvinylidene fluoride sulfonate lithium/lithium salt composite solid electrolyte [J]. Energy Storage Science and Technology, 2021, 10(6): 2069-2076. |
| [15] | Shiying ZHAN, Dongxu YU, Nan CHEN, Fei DU. Advances of aqueous batteries with non-metallic cation charge carriers [J]. Energy Storage Science and Technology, 2021, 10(6): 2144-2155. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
