Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (9): 3389-3401.doi: 10.19799/j.cnki.2095-4239.2025.0167
• Energy Storage Materials and Devices • Previous Articles Next Articles
Yihua QIAN1( ), Yaohong ZHAO1(
), Yaohong ZHAO1( ), Qing WANG1, Peng GUO2, Dating PEI2, Yirou ZENG2(
), Qing WANG1, Peng GUO2, Dating PEI2, Yirou ZENG2( )
)
Received:2025-02-26
Revised:2025-03-06
Online:2025-09-28
Published:2025-09-05
Contact:
Yaohong ZHAO, Yirou ZENG
E-mail:qianyh2001@163.com;zhaoyaohong@naesic.com;zrr7992@163.com
CLC Number:
Yihua QIAN, Yaohong ZHAO, Qing WANG, Peng GUO, Dating PEI, Yirou ZENG. Research progress and prospect of sodium halide solid-state electrolytes[J]. Energy Storage Science and Technology, 2025, 14(9): 3389-3401.
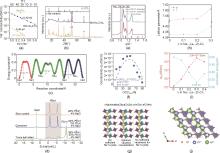
Fig. 2
(a) Arrhenius plots of BM- and HT-Na2ZrCl6[34], (b) XRD spectra of BM- and HT-Na2ZrCl6[34], (c) The energy barrier of Na+ ion migration[34], (d) 23Na NMR spectra of Na2ZrCl6 processed in different ways[19], (e) XRD spectra of Na2.5Zr0.5In0.5Cl6 annealed at different temperatures[21], (f) The relationship of the occupancy of Na 2b sites and the conductivity[21], (g) 2D atomic schematic of the impact of vacancies on Na+ transportation[21], (h) Lattice parameters of Na1-x La1-x Zr x Cl4[22], (i) Ionic conductivities and activation energies of Na1-x La1-x Zr x Cl4[22], (j) The 1D channel along [001] direction[22](1 Å=0.1 nm)"
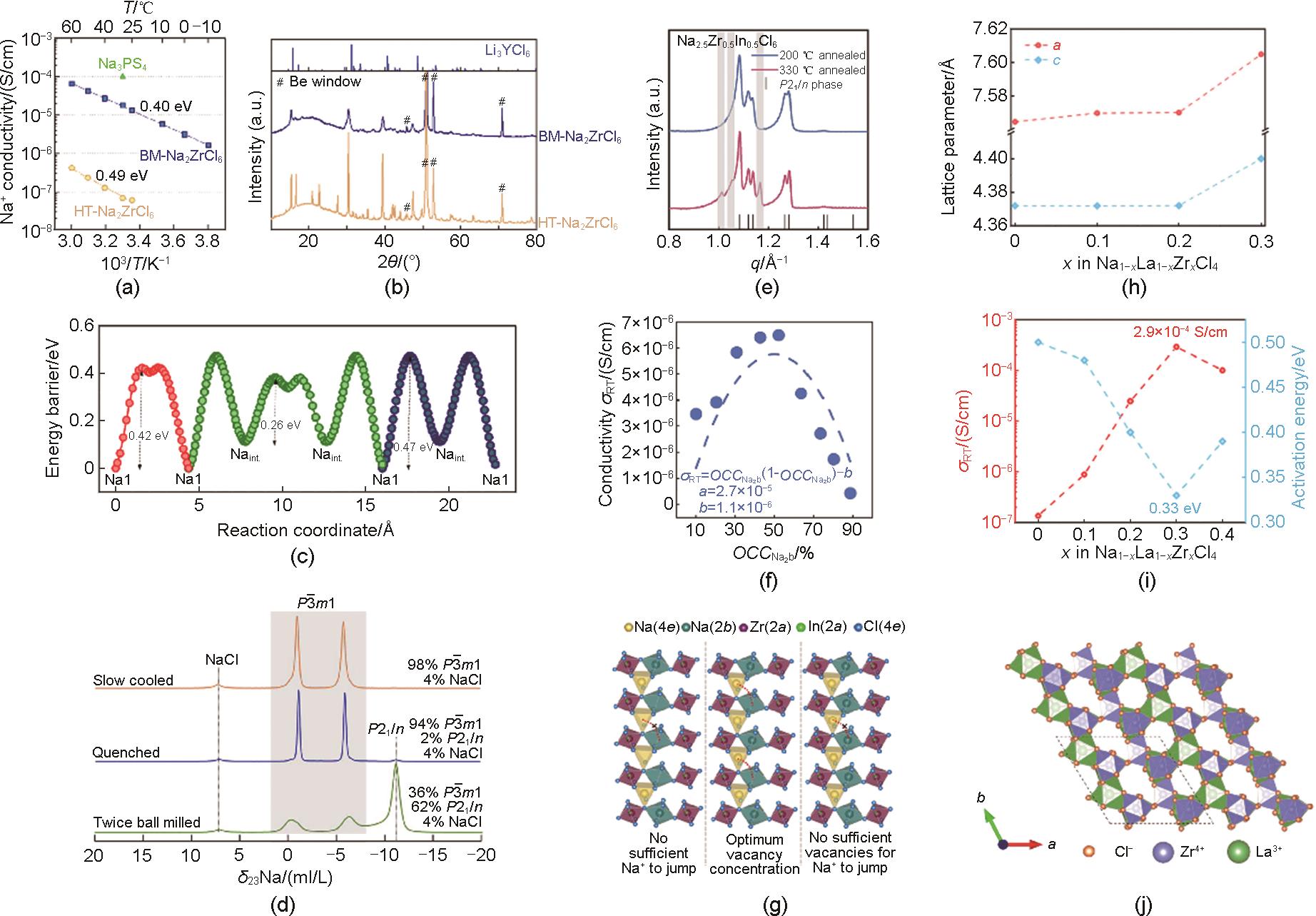

Fig. 3
(a) The stablest structural phases and decomposition energies of Na3MX6[38], (b) The correlation of ionic conductivity and Zr content[20], (c) 23Na ss-NMR spectra of Na3YCl6 processed in different ways[19], (d) Ionic conductivities of NYZC with different Zr content[18], (e) XRD spectra of NTZC with different NaCl content[18], (f) Ionic conductivities and activation energies of NYZC with different NaCl content[18], (g) Arrhenius plots of Na3YI6 in different space group[30], (h) Energy landscape of P31c-Na3YI6 structure[30]"
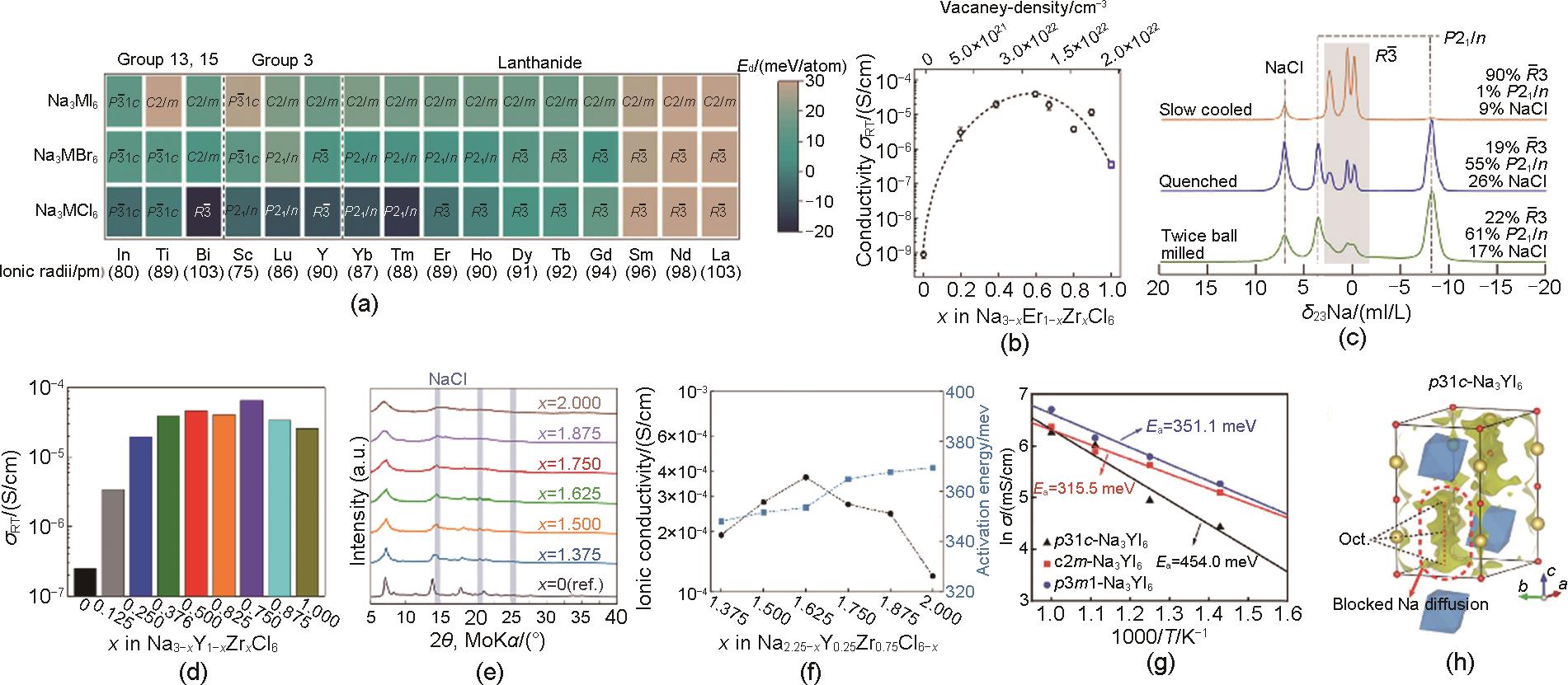

Fig. 4
(a) XRD spectra of BM-, HT100- and HT200-NaAlCl4[29], (b) Arrhenius plots of NaAlCl4, NaAlCl3.9(BH4)0.1 and NaAlCl3.5F0.5[43], (c) Arrhenius plots of NaAlCl4, NaCl-6NaAlCl4 and Al2O3-6NaAlCl4[44], (d) The ionic conductivity of NaTaCl6 at different reaction times[24], (e) Schematic of Na+ ion transport mechanism in NaTaCl6[24], (f) Heterogeneous structure of composite halide[25], (g) Arrhenius plot of xNa2O2-TaCl5[26], (h) Arrhenius plot of xNa2O2-HfCl4[27], (i) Structure of NHOC[27]"
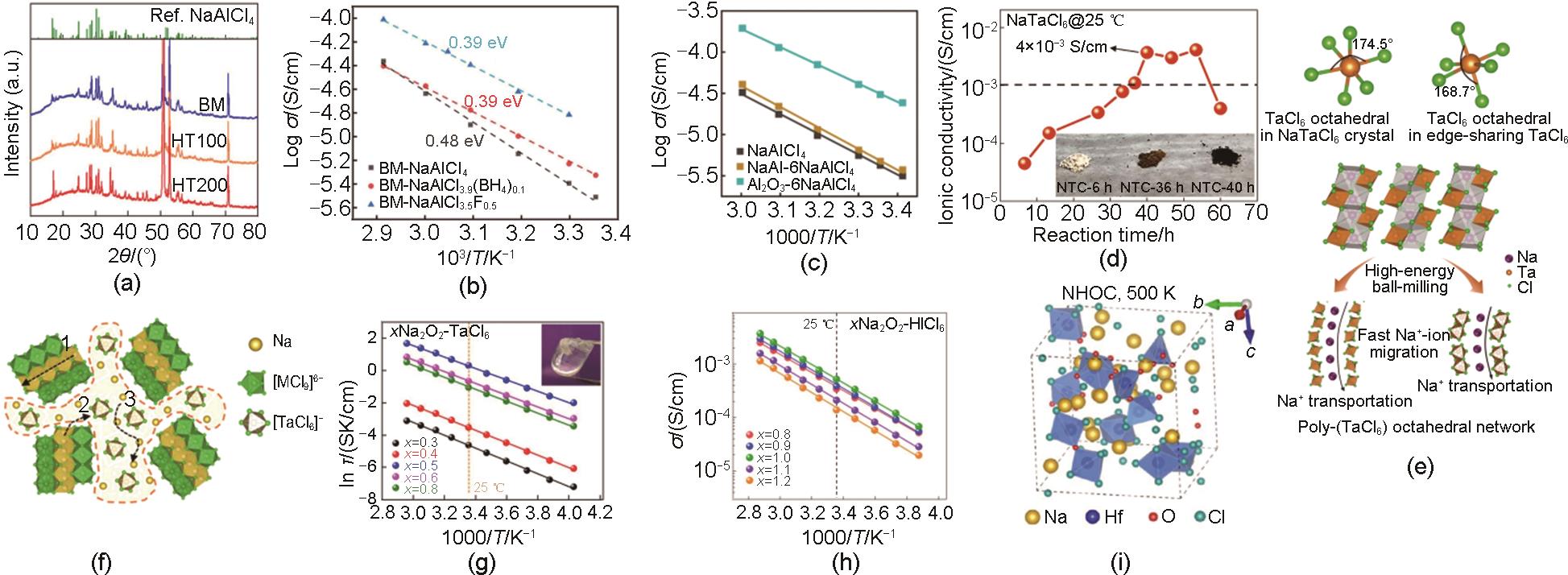

Fig. 5
(a) Electrochemical stability windows of Na3MX6 and Na3PnS4[38], (b) Linear sweep voltammograms of Na3PS4 and NaAlCl4[29], (c) XPS signals of NaAlCl4 before and after 20 cycles[29], (d) Impedance growth for the Na9Sn4|SSE|Sn half cells using NYZC, NPS, and NBH electrolytes[47], (e) XPS spectra for NYZC, NPS, NBH before and after electrochemical deposition, Zr metal, Y metal, Na2S, and B are also added as references[47], (f) Cyclic voltammograms for Na3Sn|Na3PS4|(SE-C) cells for ZrO2-2Na2ZrCl5F, Na2ZrCl5F, and Na2ZrCl6[48]"
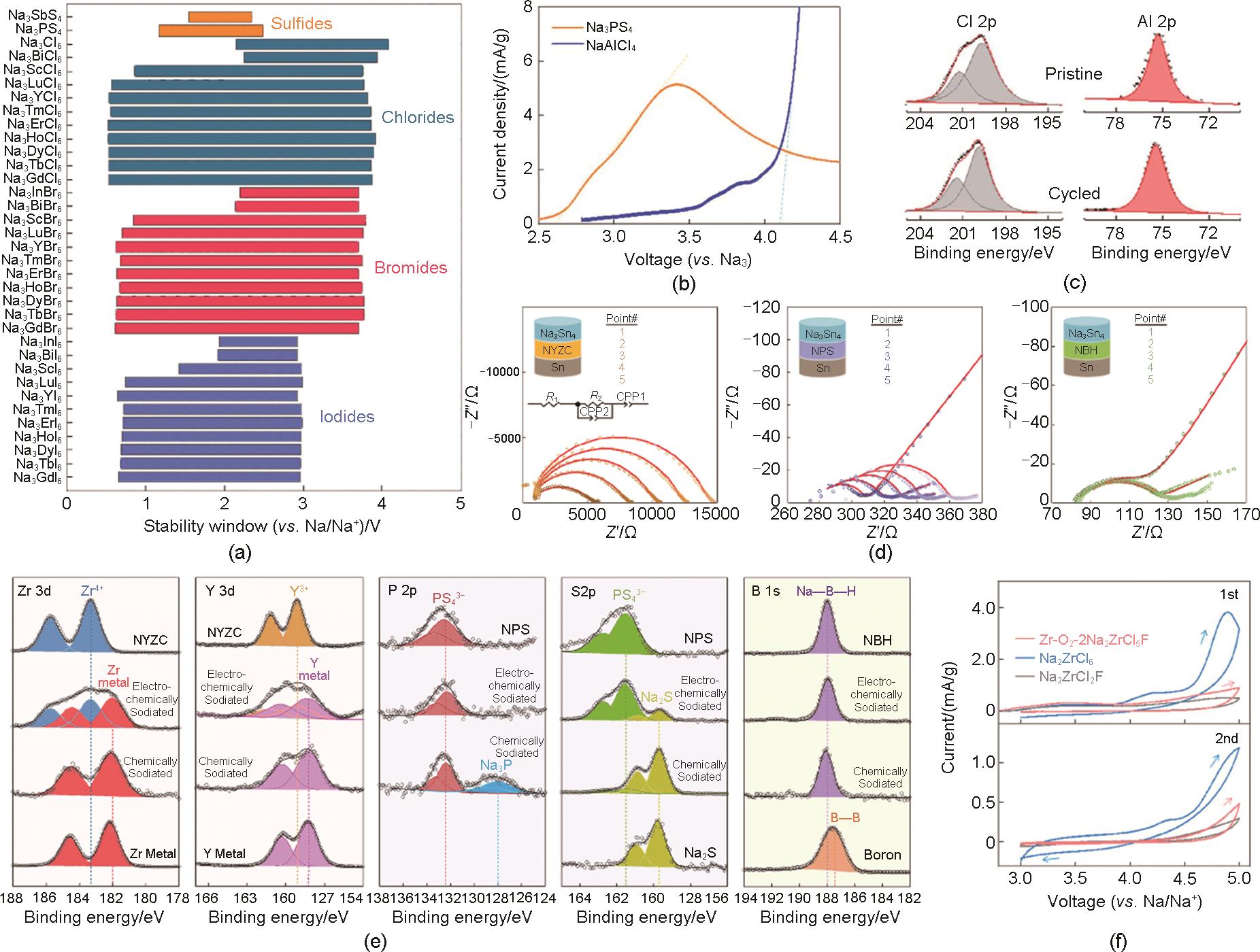

Fig. 6
(a) Charge-discharge curves of Na15Sn4|Na3PS4|NaTaCl6|NVP/NaTaCl6 batteries during cycling under 0.2 C rate[24], (b) The value of ICE in the reported literature[24], (c) Rate performance of Na15Sn4|Na3PS4|NaTaCl6|NVP/NaTaCl6 batteries[24], (d) Long cycling profile of Na15Sn4|Na3PS4|NaTaCl6|NVP/NaTaCl6 batteries under 1 C rate at RT[24], (e) Long cycling profile of Na15Sn4|Na3PS4|NaTaCl6|NVP/NaTaCl6 batteries under 3 C rate at 60 ℃[24]"
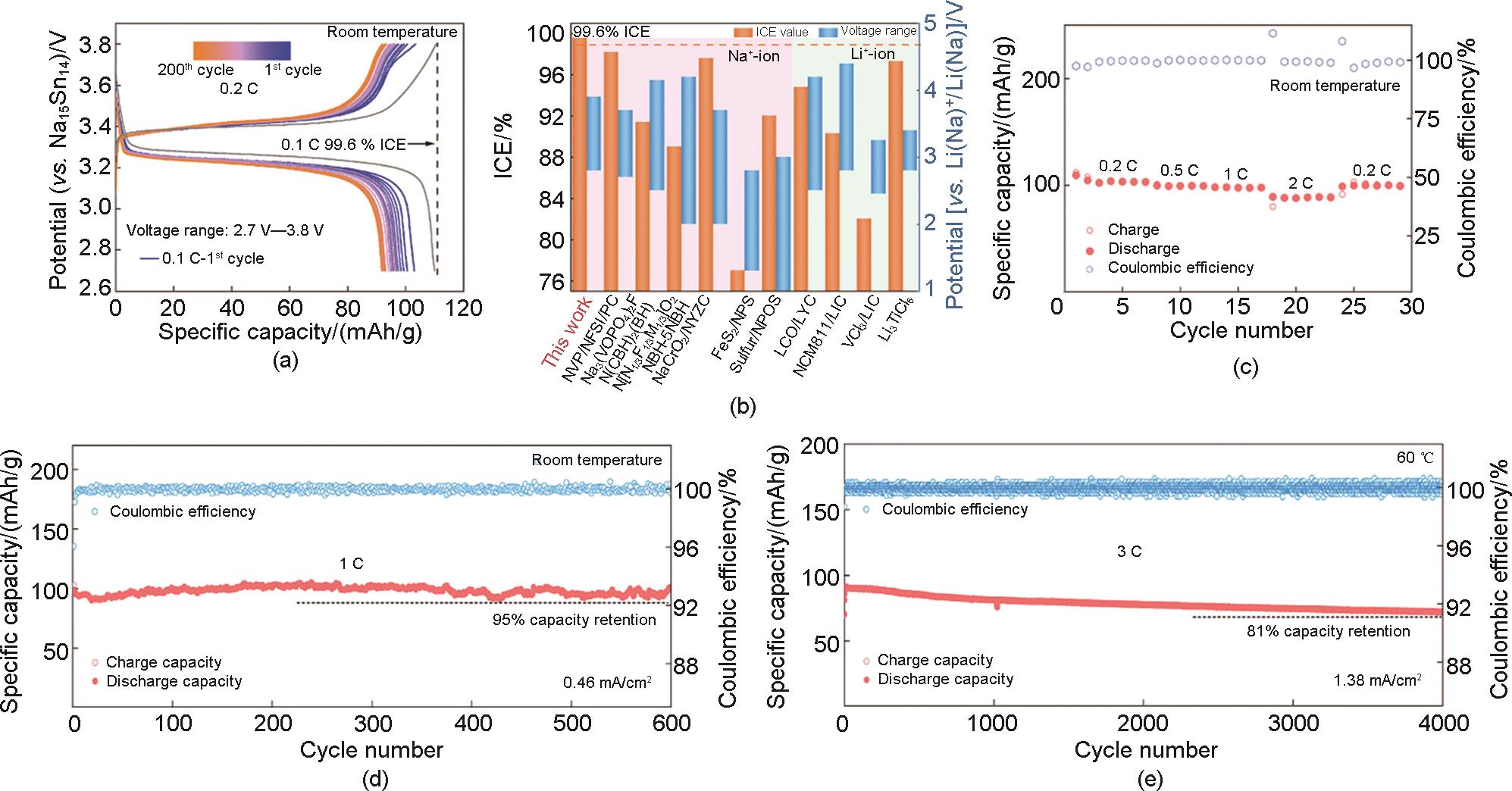
Table 1
Performance of partial sodium halide-based cells"
| 正极电解质 | 正极 | 隔层电解质 | 负极 | 初始库仑效率/% | 比容量/mAh/g | 容量保持率/% | Ref. |
|---|---|---|---|---|---|---|---|
| Na2ZrCl6 | NaCrO2 | Na3PS4 | Na-Sn | 93.1 | 111(0.1C, 30 ℃) | — | [ |
| Na0.7La0.7Zr0.3Cl4 | NaCrO2 | Na3PS4 | Na2Sn | 95.0 | 94(1C) | 88 (0.3C, 70圈) | [ |
| Na2.25Y0.25Zr0.75Cl6 | NaCrO2 | Na3PS4 | Na-Sn | 97.1 | — | 89.3 (40 ℃, 1000圈) | [ |
| NaAlCl4 | NaCrO2 | Na3PS4 | Na3Sn | 94.0 | 112 (60 ℃, 1C) | 82 (60 ℃, 1C, 500圈) | [ |
| NaAlCl4 | NaNCM118 | Na3PS3.85O0.15 | Na3Sn | — | 124(0.1C, 25 ℃) | — | [ |
| NaTaCl6 | Na3V2(PO4)3 | Na3PS4 | Na15Sn4 | 99.6 | 111(0.1C) | 95 (1C, 600圈) | [ |
| 0.5Na2O2-TaCl5 | Na0.85Mn0.5Ni0.4Fe0.1O2 | Na3PS4 | Na15Sn4 | — | 106.3(0.1C) | 66 (0.1C, 500圈) | [ |
| Na2O2-HfCl4 | Na0.85Mn0.5Ni0.4Fe0.1O2 | Na3PS4 | Na15Sn4 | 99.9 | 125.5(0.1C) | 78 (0.1C, 700圈) | [ |
| [1] | ZHAO C, LIU L, QI X, et al. Solid-State Sodium Batteries [J]. Advanced Energy Materials, 2018, 8(17): 1703012. |
| [2] | JANEK J, ZEIER W G. Challenges in speeding up solid-state battery development[J]. Nature Energy, 2023, 8(3): 230-240. DOI: 10.1038/s41560-023-01208-9. |
| [3] | LEWIS J A, TIPPENS J, CORTES F J Q, et al. Chemo-mechanical challenges in solid-state batteries[J]. Trends in Chemistry, 2019, 1(9): 845-857. DOI: 10.1016/j.trechm.2019.06.013. |
| [4] | ZOU S H, YANG Y, WANG J R, et al. In situ polymerization of solid-state polymer electrolytes for lithium metal batteries: A review[J]. Energy & Environmental Science, 2024, 17(13): 4426-4460. DOI: 10.1039/D4EE00822G. |
| [5] | TUO K Y, SUN C W, LIU S Q. Recent progress in and perspectives on emerging halide superionic conductors for all-solid-state batteries[J]. Electrochemical Energy Reviews, 2023, 6(1): 17. DOI: 10.1007/s41918-023-00179-5. |
| [6] | NIKODIMOS Y, SU W N, HWANG B J. Halide solid-state electrolytes: Stability and application for high voltage all-solid-state Li batteries[J]. Advanced Energy Materials, 2023, 13(3): 2202854. DOI: 10.1002/aenm.202202854. |
| [7] | HATZELL K B. Opportunities for halide solid electrolytes in solid-state batteries[J]. Matter, 2022, 5(8): 2533-2535. DOI: 10.1016/j.matt.2022.06.055. |
| [8] | GINNINGS D C, PHIPPS T E. Temperature-conductance curves of solid salts. iii. halides of lithium[J]. Journal of the American Chemical Society, 1930, 52(4): 1340-1345. DOI: 10.1021/ja01367a006. |
| [9] | ASANO T, SAKAI A, OUCHI S, et al. Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state batteries[J]. Advanced Materials, 2018, 30(44): 1803075. DOI: 10.1002/adma.201803075. |
| [10] | SCHLEM R, MUY S, PRINZ N, et al. Mechanochemical synthesis: A tool to tune cation site disorder and ionic transport properties of Li3MCl6(M=Y, Er) superionic conductors[J]. Advanced Energy Materials, 2020, 10(6): 1903719. DOI: 10.1002/aenm.2019 03719. |
| [11] | HELM B, SCHLEM R, WANKMILLER B, et al. Exploring aliovalent substitutions in the lithium halide superionic conductor Li3- xIn1- xZrxCl6(0≤x≤0.5)[J]. Chemistry of Materials, 2021, 33(12): 4773-4782. DOI: 10.1021/acs.chemmater.1c01348. |
| [12] | SHI X M, ZENG Z C, ZHANG H T, et al. Gram-scale synthesis of nanosized Li3HoBr6 solid electrolyte for all-solid-state Li-Se battery[J]. Small Methods, 2021, 5(11): 2101002. DOI: 10.1002/smtd.202101002. |
| [13] | LI X N, LIANG J W, CHEN N, et al. Water-mediated synthesis of a superionic halide solid electrolyte[J]. Angewandte Chemie International Edition, 2019, 58(46): 16427-16432. DOI: 10.1002/anie.201909805. |
| [14] | DELMAS C. Sodium and sodium-ion batteries: 50 years of research[J]. Advanced Energy Materials, 2018, 8(17): 1703137. DOI: 10.1002/aenm.201703137. |
| [15] | BENIERE M, CHEMLA M, BENIERE F. Vacancy pairs and correlation effects in kCl and NaCl single crystals[J]. Journal of Physics and Chemistry of Solids, 1976, 37(5): 525-538. DOI: 10.1016/0022-3697(76)90080-9. |
| [16] | D. J M Y V I P I I-C U-I. Determination of conduction parameters of sodium halides [J]. |
| [17] | BÉNIÈRE F, REDDY K V. Enhanced ionic transport in NaCl-Al2O3 heterogeneous electrolytes[J]. Journal of Physics and Chemistry of Solids, 1999, 60(6): 839-847. DOI: 10.1016/S0022-3697(98)00331-X. |
| [18] | WU E A, BANERJEE S, TANG H M, et al. A stable cathode-solid electrolyte composite for high-voltage, long-cycle-life solid-state sodium-ion batteries[J]. Nature Communications, 2021, 12: 1256. DOI: 10.1038/s41467-021-21488-7. |
| [19] | SEBTI E, QI J, RICHARDSON P M, et al. Synthetic control of structure and conduction properties in Na-Y-Zr-Cl solid electrolytes[J]. Journal of Materials Chemistry A, 2022, 10(40): 21565-21578. DOI: 10.1039/D2TA05823E. |
| [20] | SCHLEM R, BANIK A, ECKARDT M, et al. Na3- xEr1- xZrxCl6—a halide-based fast sodium-ion conductor with vacancy-driven ionic transport[J]. ACS Applied Energy Materials, 2020, 3(10): 10164-10173. DOI: 10.1021/acsaem.0c01870. |
| [21] | ZHAO T, SOBOLEV A N, SCHLEM R, et al. Synthesis-controlled cation solubility in solid sodium ion conductors Na2+ xZr1- xInxCl6[J]. Acs Applied Energy Materials, 2023, 6(8): 4334-4341. DOI: 10. 1021/acsaem.3c00277. |
| [22] | FU C Y, LI Y F, XU W J, et al. LaCl3-based sodium halide solid electrolytes with high ionic conductivity for all-solid-state batteries[J]. Nature Communications, 2024, 15: 4315. DOI: 10.1038/s41 467-024-48712-4. |
| [23] | RIDLEY P, NGUYEN L H B, SEBTI E, et al. Amorphous and nanocrystalline halide solid electrolytes with enhanced sodium-ion conductivity[J]. Matter, 2024, 7(2): 485-499. DOI: 10.1016/j.matt.2023.12.028. |
| [24] | HU Y, FU J M, XU J B, et al. Superionic amorphous NaTaCl6 halide electrolyte for highly reversible all-solid-state Na-ion batteries[J]. Matter, 2024, 7(3): 1018-1034. DOI: 10.1016/j.matt. 2023.12.017. |
| [25] | FU J M, WANG S, WU D J, et al. Halide heterogeneous structure boosting ionic diffusion and high-voltage stability of sodium superionic conductors[J]. Advanced Materials, 2024, 36(3): 2308012. DOI: 10.1002/adma.202308012. |
| [26] | LIN X T, ZHAO Y, WANG C H, et al. A dual anion chemistry-based superionic glass enabling long-cycling all-solid-state sodium-ion batteries[J]. Angewandte Chemie International Edition, 2024, 63(2): e202314181. DOI: 10.1002/anie.202314181. |
| [27] | LIN X T, ZHANG S M, YANG M H, et al. A family of dual-anion-based sodium superionic conductors for all-solid-state sodium-ion batteries[J]. Nature Materials, 2024, 24(1): 83-91. DOI: 10. 1038/s41563-024-02011-x. |
| [28] | ZHAO T, SAMANTA B, DE IRUJO-LABALDE X M, et al. Sodium metal oxyhalides NaMOCl4(M=Nb, Ta) with high ionic conductivities[J]. ACS Materials Letters, 2024, 6(8): 3683-3689. DOI: 10.1021/acsmaterialslett.4c01145. |
| [29] | PARK J, SON J P, KO W, et al. NaAlCl4: New halide solid electrolyte for 3 V stable cost-effective all-solid-state Na-ion batteries[J]. ACS Energy Letters, 2022, 7(10): 3293-3301. DOI: 10.1021/acsenergylett.2c01514. |
| [30] | HUANG H, WU H H, CHI C, et al. Phase-structure-dependent Na ion transport in yttrium-iodide sodium superionic conductor Na3YI6[J]. Journal of Materials Chemistry A, 2021, 9(46): 26256-26265. DOI: 10.1039/D1TA08086E. |
| [31] | LIAN Y X, WU M S, XU B, et al. Phase-structure design for sodium chloride solid electrolytes with outstanding performance: A first-principles approach[J]. Journal of Materials Chemistry A, 2023, 11(4): 1906-1919. DOI: 10.1039/D2TA07603A. |
| [32] | BOGACZ A, BROS J, GAUNE-ESCARD M, et al. New fast-ion conductors from uranium halides-the UCl6/Na2UBr6 structures[J]. Journal of Physics C: Solid State Physics, 1980, 13(29): 5273. DOI: 10.1088/0022-3719/13/29/008. |
| [33] | FOUQUE Y, GAUNE-ESCARD M, SZCZEPANIAK W, et al. Synthèse, mesures des conductibilités électriques et des entropies de changements d'état pour le composé Na2UBr6[J]. Journal de Chimie Physique, 1978, 75: 360-366. DOI: 10.1051/jcp/1978750360. |
| [34] | KWAK H, LYOO J, PARK J, et al. Na2ZrCl6 enabling highly stable 3 V all-solid-state Na-ion batteries[J]. Energy Storage Materials, 2021, 37: 47-54. DOI: 10.1016/j.ensm.2021.01.026. |
| [35] | LISSNER F, KRÄMER K, SCHLEID T, et al. Die chloride Na3 xM2- xCl6(M=La, Sm) und NaM2Cl6 (M=Nd, Sm): Derivate des UCl3-typs. synthese, kristallstruktur und röntgenabsorptionsspektroskopie (XANES)[J]. Zeitschrift Für Anorganische und Allgemeine Chemie, 1994, 620(3): 444-450. DOI: 10.1002/zaac.199462 00307. |
| [36] | LUTZ H D, WUSSOW K, KUSKE P. Ionic conductivity, structural, IR and Raman Spectroscopic data of olivine, Sr2PbO4, and Na2CuF4 type lithium and sodium chlorides Li2ZnCl4 and Na2MCl4(M=Mg, Ti, Cr, Mn, Co, Zn, Cd) [J]. 1987, 42(11): 1379-86. |
| [37] | KANNO R, TAKEDA Y, MURATA K, et al. Crystal structure of double chlorides, Na2MCl4(M=Mg, Cr, Cd): Correlation with ionic conductivity[J]. Solid State Ionics, 1990, 39(3/4): 233-244. DOI: 10.1016/0167-2738(90)90402-D. |
| [38] | YU S, KIM K, WOOD B C, et al. Structural design strategies for superionic sodium halide solid electrolytes[J]. Journal of Materials Chemistry A, 2022, 10(45): 24301-24309. DOI: 10.1039/D2TA05158C. |
| [39] | PARK D, KIM K, CHUN G H, et al. Materials design of sodium chloride solid electrolytes Na3MCl6 for all-solid-state sodium-ion batteries[J]. Journal of Materials Chemistry A, 2021, 9(40): 23037-23045. DOI: 10.1039/D1TA07050A. |
| [40] | YAMADA K, KUMANO K, OKUDA T. Conduction path of the sodium ion in Na3InCl6 studied by X-ray diffraction and 23Na and 115In NMR[J]. Solid State Ionics, 2005, 176(7/8): 823-829. DOI: 10.1016/j.ssi.2004.10.016. |
| [41] | QIE Y, WANG S, FU S J, et al. Yttrium-sodium halides as promising solid-state electrolytes with high ionic conductivity and stability for Na-ion batteries[J]. The Journal of Physical Chemistry Letters, 2020, 11(9): 3376-3383. DOI: 10.1021/acs.jpclett.0c00010. |
| [42] | NIU X Y, DOU X Y, FU C Y, et al. Sodium halide solid state electrolyte of Na3YBr6 with low activation energy[J]. RSC Advances, 2024, 14(21): 14716-14721. DOI: 10.1039/D4RA026 63B. |
| [43] | XU X L, LI Y X, WANG X, et al. Effect of lattice fluoride and borohydride on the electrochemical performances of NaAlCl4 solid electrolyte[J]. Journal of Solid State Electrochemistry, 2024, 28(9): 3501-3507. DOI: 10.1007/s10008-024-05838-1. |
| [44] | RUOFF E, KMIEC S, MANTHIRAM A. Enhanced interfacial conduction in low-cost NaAlCl4 composite solid electrolyte for solid-state sodium batteries[J]. Advanced Energy Materials, 2024, 14(37): 2402091. DOI: 10.1002/aenm.202402091. |
| [45] | MIYAZAKI R, NAKAYAMA M, HIHARA T. Experimental study on Na+ conductivity in NaAlBr4 and atomic-scale investigation of Na+ conduction[J]. Journal of Solid State Electrochemistry, 2025, 29(2): 585-593. DOI: 10.1007/s10008-024-06086-z. |
| [46] | DAI T, WU S Y, LU Y X, et al. Inorganic glass electrolytes with polymer-like viscoelasticity[J]. Nature Energy, 2023, 8(11): 1221-1228. DOI: 10.1038/s41560-023-01356-y. |
| [47] | DEYSHER G, CHEN Y T, SAYAHPOUR B, et al. Evaluating electrolyte-anode interface stability in sodium all-solid-state batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(42): 47706-47715. DOI: 10.1021/acsami.2c12759. |
| [48] | PARK J, HAN D, SON J P, et al. Extending the electrochemical window of Na+ halide nanocomposite solid electrolytes for 5 V-class all-solid-state Na-ion batteries[J]. ACS Energy Letters, 2024, 9(5): 2222-2230. DOI: 10.1021/acsenergylett.4c00490. |
| [1] | Tete HE, Yang LU, Yang LIU, Bin XU, Yongle CHEN, Fangyang LIU. Lithium sulfide: the "cornerstone" material in the era of all-solid-state batteries [J]. Energy Storage Science and Technology, 2025, 14(3): 898-912. |
| [2] | Qin WANG, Yangang ZHANG, Junfei LIANG, Hua WANG. Challenges and strategies for interface failures in silicon-based solid-state batteries [J]. Energy Storage Science and Technology, 2025, 14(2): 570-582. |
| [3] | Yuhang LI, Zhuo HAN, Xufei AN, Danfeng ZHANG, Guorui ZHENG, Ming LIU, Yanbing HE. Progress of ion transport in solid-state battery research based on solid state nuclear magnetic resonance [J]. Energy Storage Science and Technology, 2024, 13(1): 178-192. |
| [4] | Zhengguang ZHAO, Zhenying CHEN, Guangqun ZHAI, Xi ZHANG, Xiaodong ZHUANG. Preparation of Sc/O-doped sulfide electrolyte for all-solid-state batteries [J]. Energy Storage Science and Technology, 2023, 12(8): 2412-2423. |
| [5] | Xunchang JIANG, Minhui LIAO, Yang ZHOU, Daxiang YANG, Qiang WANG. Design and performance of nanofiber membrane-based elastic solid electrolyte [J]. Energy Storage Science and Technology, 2023, 12(11): 3307-3317. |
| [6] | Chaochao WEI, Chuang YU, Zhongkai WU, Linfeng PENG, Shijie CHENG, Jia XIE. Research progress of Li3PS4 solid electrolyte [J]. Energy Storage Science and Technology, 2022, 11(5): 1368-1382. |
| [7] | Suli LI, Peng WU, Yirong XIAO, Peiwen YU, Yuede PAN, Wen YANG. Application of poly(ethylene glycol) methyl ether acrylate in all-solid-state batteries [J]. Energy Storage Science and Technology, 2022, 11(12): 3768-3775. |
| [8] | Saisai ZHANG, Hailei ZHAO. Electrode/electrolyte interfaces in Li7La3Zr2O12 garnet-based solid-state lithium metal battery: Challenges and progress [J]. Energy Storage Science and Technology, 2021, 10(3): 863-871. |
| [9] | Yanfang ZHAI, Guanming YANG, Wangshu HOU, Jianyao YAO, Zhaoyin WEN, Shufeng SONG, Ning HU. Solvothermal synthesis of three-dimensional petaloid garnet electrolyte and its application in solid polymer electrolytes [J]. Energy Storage Science and Technology, 2021, 10(3): 905-913. |
| [10] | SUN Huajun1,2, HONG Tingting1, LIU Xiaofang3, SUI Huiting2, LIU Pengdong1. Improvement of photovoltaic properties of bismuth ferrite film based solar cell using organic and inorganic interface layers [J]. Energy Storage Science and Technology, 2017, 6(6): 1340-. |
| [11] | ZHANG Qiang1,2, YAO Xiayin1,2, ZHANG Hongzhou3, ZHANG Lianqi3, XU Xiaoxiong1,2. Research progress on interfaces of all solid state lithium batteries [J]. Energy Storage Science and Technology, 2016, 5(5): 659-667. |
| [12] | XU Xiaoxiong, QIU Zhijun, GUAN Yibiao, HUANG Zhen, JIN Yi. All-solid-state lithium-ion batteries:State-of-the-art development and perspective [J]. Energy Storage Science and Technology, 2013, 2(4): 331-341. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
