Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (1): 178-192.doi: 10.19799/j.cnki.2095-4239.2023.0784
Previous Articles Next Articles
Yuhang LI( ), Zhuo HAN, Xufei AN, Danfeng ZHANG, Guorui ZHENG, Ming LIU(
), Zhuo HAN, Xufei AN, Danfeng ZHANG, Guorui ZHENG, Ming LIU( ), Yanbing HE(
), Yanbing HE( )
)
Received:2023-11-01
Revised:2023-11-06
Online:2024-01-05
Published:2024-01-22
Contact:
Ming LIU, Yanbing HE
E-mail:l-yh23@mails.tsinghua.edu.cn;liuming@sz.tsinghua.edu.cn;he.yanbing@sz.tsinghua.edu.cn
CLC Number:
Yuhang LI, Zhuo HAN, Xufei AN, Danfeng ZHANG, Guorui ZHENG, Ming LIU, Yanbing HE. Progress of ion transport in solid-state battery research based on solid state nuclear magnetic resonance[J]. Energy Storage Science and Technology, 2024, 13(1): 178-192.
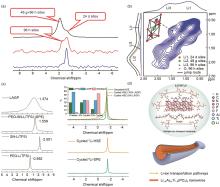
Fig. 2
(a) 6Li MAS NMR spectra for Li6-3y Al y La3Zr1.5W0.5O with single pulse (black line) and saturation recovery method (red and blue line)[20]; (b) 6Li-6Li 2D-EXSY of Li6-3y Al y La3Zr1.5W0.5O[20]; (c) The 6,7 Li NMR and isotope exchange result of PEO-LAGP[21]; (d)The pathway of Li-ion transport in the PVDF-LATP SSE[3]"
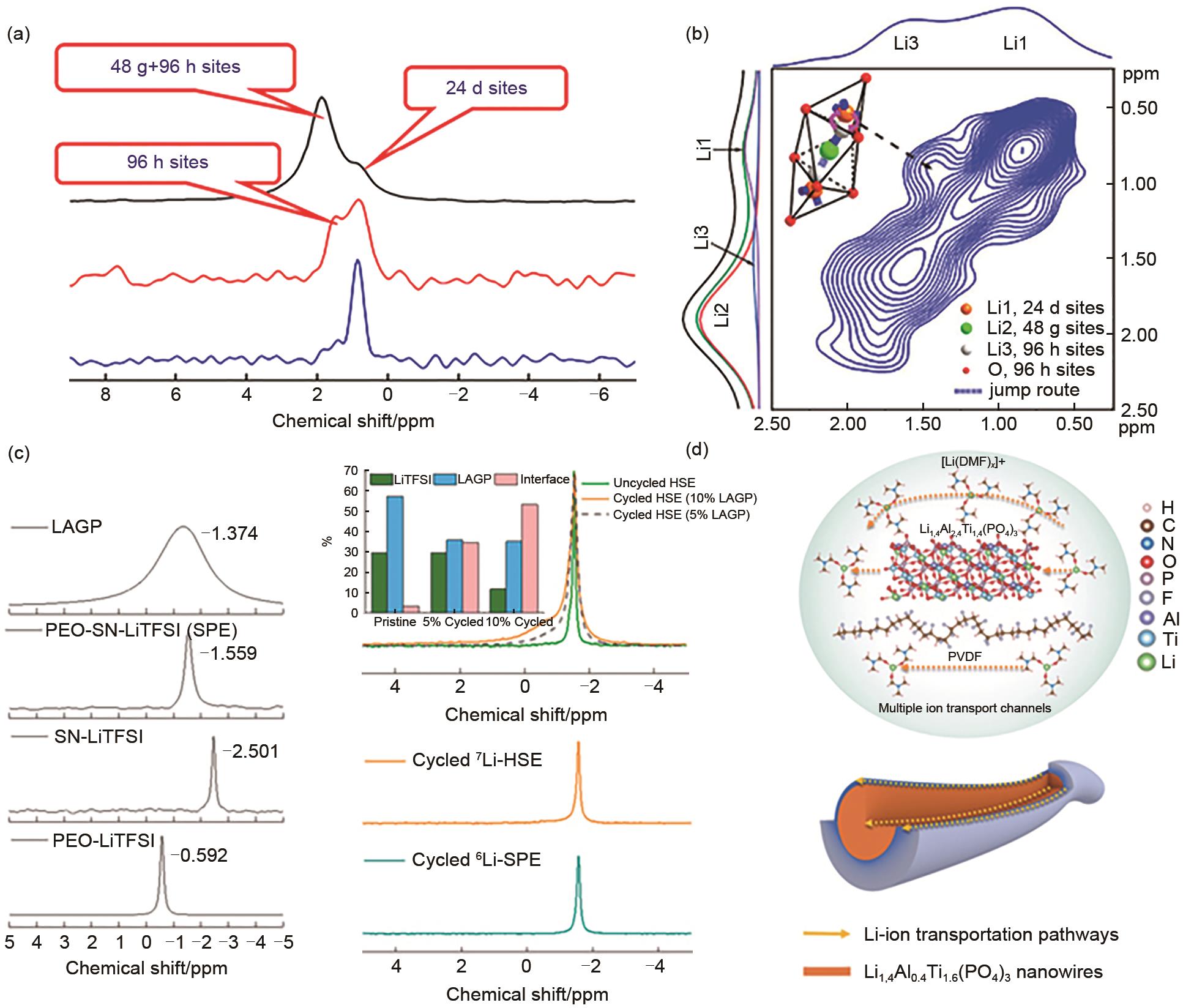
Fig. 5
Schematic of Li-ion transport mechanism in all-solid-state cathode. (a) Transport paths in the traditional cathode, and (b) composite cathode with La2Zr2O7 (LZONs), (c) Li-ion transport paths in traditional cathode within the cavities, and (d) PEO binder, (e) Lithium salt dissociation in PEO binder, (f) "Solid-polymer-solid" elastic Li-ion transport paths in composite cathode within cavities embedded with LZONs, and (g) PEO@LZONs composite binder, (h) Lithium salt dissociation in PEO@LZONs binder and adsorption for TFSI- anion, (i) Li-ion transport tunnel in LZONs, (j) 7Li NMR spectra of discharge LiFePO4(LFP), composite cathode (PLL-LFP) without PEO binder and spectral decomposition[46]"


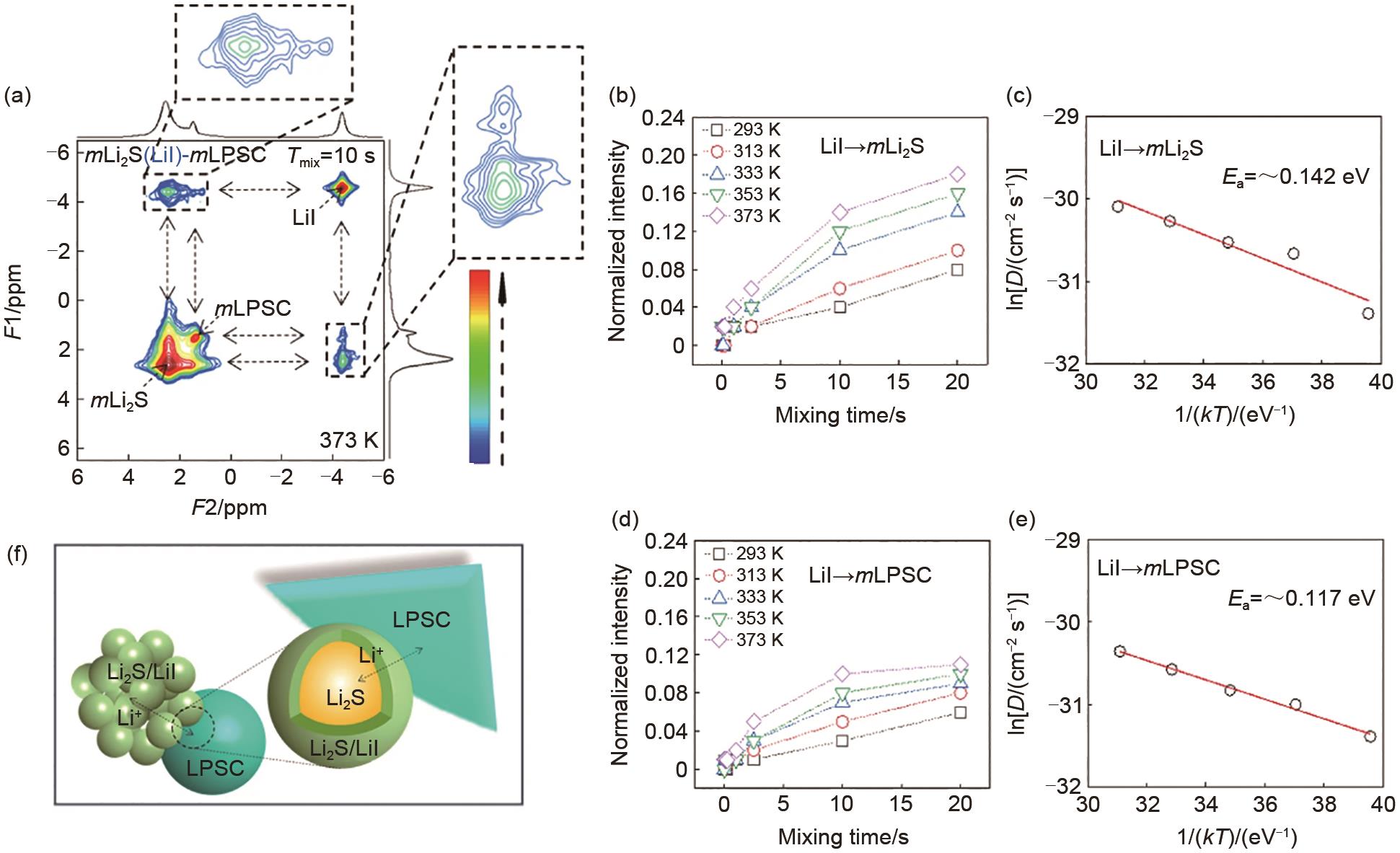
Fig. 7
(a) 2D 6Li-6Li exchange spectra of the mixture of LPSC and Li2S-LiI powders; (b), (c) Evolution of the cross-peak intensity as a function of Tmix obtained from the temperature-dependent 2D-EXSY measurements; (d), (e) Temperature dependence of the diffusion coefficient obtained from fitting the data in (b), (d); (f) Schematic of the proposed Li+ transport mechanism in the cathodic mixtures[62]"
| 1 | LI S, ZHANG S Q, SHEN L, et al. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries[J]. Advanced Science, 2020, 7(5): 1903088. |
| 2 | LEI D N, HE Y B, HUANG H J, et al. Cross-linked beta alumina nanowires with compact gel polymer electrolyte coating for ultra-stable sodium metal battery[J]. Nature Communications, 2019, 10: 4244. |
| 3 | YANG K, CHEN L K, MA J B, et al. Stable interface chemistry and multiple ion transport of composite electrolyte contribute to ultra-long cycling solid-state LiNi0.8Co0.1Mn0.1O2/lithium metal batteries[J]. Angewandte Chemie International Edition, 2021, 60(46): 24668-24675. |
| 4 | KAMAYA N, HOMMA K, YAMAKAWA Y, et al. A lithium superionic conductor[J]. Nature Materials, 2011, 10(9): 682-686. |
| 5 | ZHU J P, XIANG Y X, ZHAO J, et al. Insights into the local structure, microstructure and ionic conductivity of silicon doped NASICON-type solid electrolyte Li1.3Al0.3Ti1.7P3O12[J]. Energy Storage Materials, 2022, 44: 190-196. |
| 6 | MI J S, CHEN L K, MA J B, et al. Defect strategy in solid-state lithium batteries[J]. Small Methods, 2023: 2301162. |
| 7 | LI C, SHEN M, HU B, JAP-CS. Solid-state NMR and EPR methods for metal ion battery research [J]. Acta Physico-Chimica Sini, 2020, 36(4):1902019. |
| 8 | ZHANG H, SHEN Y, YU Y, et al. Advances in the application of solid-state nuclear magnetic resonance for the study of ion diffusion mechanism in battery materials[J]. Energy Storage Science and Technology, 2020, 9: 78. |
| 9 | SHI Y, TANG M J A P C S. NMR/EPR Investigation of rechargeable batteries[J]. Acta Phys Chim Sin, 2019, 36: 1-9. |
| 10 | 钟贵明, 刘子庚, 王大为, 等. 锂/钠离子电池材料的固体核磁共振研究进展[J]. 电化学, 2016, 22(3): 231-243. |
| ZHONG G M, LIU Z G, WANG D W, et al. Recent progress in solid-state NMR study of electrode/electrolyte materials for lithium/sodium ion batteries[J]. Journal of Electrochemistry, 2016, 22(3): 231-243. | |
| 11 | GANAPATHY S, YU C A, VAN ECK E R H, et al. Peeking across grain boundaries in a solid-state ionic conductor[J]. ACS Energy Letters, 2019, 4(5): 1092-1097. |
| 12 | DAWSON J A, CANEPA P, CLARKE M J, et al. Toward understanding the different influences of grain boundaries on ion transport in sulfide and oxide solid electrolytes[J]. Chemistry of Materials, 2019, 31(14): 5296-5304. |
| 13 | MIARA L J, ONG S P, MO Y F, et al. Effect of Rb and Ta doping on the ionic conductivity and stability of the garnet Li7+2 x-Y(La3- xRbx)(Zr2- yTay)O12 (0≤x≤0.375, 0≤y≤1) superionic conductor-a first principles investigation[J]. ECS Meeting Abstracts, 2013, (8): 576. |
| 14 | MURUGAN R, THANGADURAI V, WEPPNER W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12[J]. Angewandte Chemie International Edition, 2007, 46(41): 7778-7781. |
| 15 | DAWSON J A, CANEPA P, FAMPRIKIS T, et al. Atomic-scale influence of grain boundaries on Li-ion conduction in solid electrolytes for all-solid-state batteries[J]. Journal of the American Chemical Society, 2018, 140(1): 362-368. |
| 16 | VINOD CHANDRAN C, SYLKE P, ELENA W, et al. Solid-state NMR investigations on the structure and dynamics of the ionic conductor Li1+ xAlxTi2- x(PO4)3 (0.0≤x≤1.0)[J]. The Journal of Physical Chemistry C, 2016, 120(16): 8436-8442. |
| 17 | BIAO J, HAN B, CAO Y D, et al. Inhibiting formation and reduction of Li2CO3 to LiCx at grain boundaries in garnet electrolytes to prevent Li penetration[J]. Advanced Materials, 2023, 35(12): 2208951. |
| 18 | GEIGER C A, ALEKSEEV E, LAZIC B, et al. Crystal chemistry and stability of "Li7La3Zr2O12" garnet: A fast lithium-ion conductor[J]. Inorganic Chemistry, 2011, 50(3): 1089-1097. |
| 19 | WANG D W, ZHONG G M, PANG W K, et al. Toward understanding the lithium transport mechanism in garnet-type solid electrolytes: Li+ ion exchanges and their mobility at octahedral/tetrahedral sites[J]. Chemistry of Materials, 2015, 27(19): 6650-6659. |
| 20 | XIANG Y X, ZHENG G R, ZHONG G M, et al. Toward understanding of ion dynamics in highly conductive lithium ion conductors: Some perspectives by solid state NMR techniques[J]. Solid State Ionics, 2018, 318: 19-26. |
| 21 | LIU M, CHENG Z, GANAPATHY S, et al. Tandem interface and bulk Li-ion transport in a hybrid solid electrolyte with microsized active filler[J]. ACS Energy Letters, 2019, 4(9): 2336-2342. |
| 22 | ZHENG J, TANG M X, HU Y Y. Lithium ion pathway within Li7La3Zr2O12-polyethylene oxide composite electrolytes[J]. Angewandte Chemie International Edition, 2016, 55(40): 12538-12542. |
| 23 | ZHENG J, HU Y Y. New insights into the compositional dependence of Li-ion transport in polymer-ceramic composite electrolytes[J]. ACS Applied Materials & Interfaces, 2018, 10(4): 4113-4120. |
| 24 | PAN K C, ZHANG L, QIAN W W, et al. A flexible ceramic/polymer hybrid solid electrolyte for solid-state lithium metal batteries[J]. Advanced Materials, 2020, 32(17): 2000399. |
| 25 | ZHAO L, YU X N, JIAO J Y, et al. Building cross-phase ion transport channels between ceramic and polymer for highly conductive composite solid-state electrolyte[J]. Cell Reports Physical Science, 2023, 4(5): 101382. |
| 26 | MI J S, MA J B, CHEN L K, et al. Topology crafting of polyvinylidene difluoride electrolyte creates ultra-long cycling high-voltage lithium metal solid-state batteries[J]. Energy Storage Materials, 2022, 48: 375-383. |
| 27 | SIMON F J, HANAUER M, HENSS A, et al. Properties of the interphase formed between argyrodite-type Li6PS5Cl and polymer-based PEO10: LiTFSI[J]. ACS Applied Materials & Interfaces, 2019, 11(45): 42186-42196. |
| 28 | SIMON F J, HANAUER M, RICHTER F H, et al. Interphase formation of PEO20: LiTFSI-Li6PS5Cl composite electrolytes with lithium metal[J]. ACS Applied Materials & Interfaces, 2020, 12(10): 11713-11723. |
| 29 | ZHENG J, WANG P B, LIU H Y, et al. Interface-enabled ion conduction in Li10GeP2S12-poly (ethylene oxide) hybrid electrolytes[J]. ACS Applied Energy Materials, 2019, 2(2): 1452-1459. |
| 30 | LIU M, ZHANG S N, VAN ECK E R V, et al. Improving Li-ion interfacial transport in hybrid solid electrolytes[J]. Nature Nanotechnology, 2022, 17: 959-967. |
| 31 | SHI P R, MA J B, LIU M, et al. A dielectric electrolyte composite with high lithium-ion conductivity for high-voltage solid-state lithium metal batteries[J]. Nature Nanotechnology, 2023, 18(6): 602-610. |
| 32 | BROGIOLI D, LANGER F, KUN R, et al. Space-charge effects at the Li7La3Zr2O12/poly (ethylene oxide) interface[J]. ACS Applied Materials & Interfaces, 2019, 11(12): 11999-12007. |
| 33 | CHEN W P, DUAN H, SHI J L, et al. Bridging interparticle Li+ conduction in a soft ceramic oxide electrolyte[J]. Journal of the American Chemical Society, 2021, 143(15): 5717-5726. |
| 34 | LI J, CAI Y J, CUI Y Y, et al. Fabrication of asymmetric bilayer solid-state electrolyte with boosted ion transport enabled by charge-rich space charge layer for -20~70 ℃ lithium metal battery[J]. Nano Energy, 2022, 95: 107027. |
| 35 | WANG C, LIU M, THIJS M, et al. High dielectric Barium titanate porous scaffold for efficient Li metal cycling in anode-free cells[J]. Nature Communications, 2021, 12: 6536. |
| 36 | JIANG B B, IOCOZZIA J, ZHAO L, et al. Barium titanate at the nanoscale: Controlled synthesis and dielectric and ferroelectric properties[J]. Chemical Society Reviews, 2019, 48(4): 1194-1228. |
| 37 | KALININ S V, JOHNSON C Y, BONNELL D A. Domain polarity and temperature induced potential inversion on the BaTiO3(100) surface[J]. Journal of Applied Physics, 2002, 91(6): 3816-3823. |
| 38 | TAKADA K, OHTA N, ZHANG L Q, et al. Interfacial phenomena in solid-state lithium battery with sulfide solid electrolyte[J]. Solid State Ionics, 2012, 225: 594-597. |
| 39 | WU B B, WANG S Y, et al. Interfacial behaviours between lithium ion conductors and electrode materials in various battery systems[J]. Journal of Materials Chemistry A, 2016, 4(40): 15266-15280. |
| 40 | XIA S H, ZHAO Y, YAN J H, et al. Dynamic regulation of lithium dendrite growth with electromechanical coupling effect of soft BaTiO3 ceramic nanofiber films[J]. ACS Nano, 2021, 15(2): 3161-3170. |
| 41 | YU C, GANAPATHY S, DE KLERK N J J, et al. Unravelling Li-ion transport from picoseconds to seconds: Bulk versus interfaces in an argyrodite Li6PS5Cl-Li2S all-solid-state Li-ion battery[J]. Journal of the American Chemical Society, 2016, 138(35): 11192-11201. |
| 42 | WANG C H, ADAIR K R, LIANG J W, et al. Solid-state plastic crystal electrolytes: Effective protection interlayers for sulfide-based all-solid-state lithium metal batteries[J]. Advanced Functional Materials, 2019, 29(26): 1900392. |
| 43 | SHI K, WAN Z P, YANG L, et al. In Situ construction of an ultra-stable conductive composite interface for high-voltage all-solid-state lithium metal batteries[J]. Angewandte Chemie International Edition, 2020, 132(29): 11882-11886. |
| 44 | HAO X G, ZHAO Q A, SU S M, et al. Constructing multifunctional interphase between Li1.4Al0.4Ti1.6(PO4)3 and Li metal by magnetron sputtering for highly stable solid-state lithium metal batteries[J]. Advanced Energy Materials, 2019, 9(34): 1901604. |
| 45 | WAN Z P, SHI K, HUANG Y F, et al. Three-dimensional alloy interface between Li6.4La3Zr1.4Ta0.6O12 and Li metal to achieve excellent cycling stability of all-solid-state battery[J]. Journal of Power Sources, 2021, 505: 230062. |
| 46 | MA J B, ZHONG G M, SHI P R, et al. Constructing a highly efficient "solid-polymer-solid" elastic ion transport network in cathodes activates the room temperature performance of all-solid-state lithium batteries[J]. Energy & Environmental Science, 2022, 15(4): 1503-1511. |
| 47 | WAN Z P, LEI D N, YANG W, et al. All-solid-state batteries: Low resistance-integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder[J]. Advanced Functional Materials, 2019, 29(1): 1805301. |
| 48 | LING H J, SHEN L, HUANG Y F, et al. Integrated structure of cathode and double-layer electrolyte for highly stable and dendrite-free all-solid-state Li-metal batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(51): 56995-57002. |
| 49 | XIAO G Y, XU H, BAI C, et al. Progress and perspectives of in situ polymerization method for lithium-based batteries[J]. Interdisciplinary Materials, 2023, 2(4): 609-634. |
| 50 | LIANG Z T, XIANG Y X, WANG K J, et al. Understanding the failure process of sulfide-based all-solid-state lithium batteries via operando nuclear magnetic resonance spectroscopy[J]. Nature Communications, 2023, 14: 259. |
| 51 | TAN D H S, WU E A, NGUYEN H, et al. Elucidating reversible electrochemical redox of Li6PS5Cl solid electrolyte[J]. ACS Energy Letters, 2019, 4(10): 2418-2427. |
| 52 | LEE Y G, FUJIKI S, JUNG C, et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes[J]. Nature Energy, 2020, 5(4): 299-308. |
| 53 | PAN H, ZHANG M H, CHENG Z, et al. Carbon-free and binder-free Li-Al alloy anode enabling an all-solid-state Li-S battery with high energy and stability[J]. Science Advances, 2022, 8(15): eabn4372. |
| 54 | LIU S J, ZHOU L, HAN J A, et al. Super long-cycling all-solid-state battery with thin Li6PS5Cl-based electrolyte[J]. Advanced Energy Materials, 2022, 12(25): 2200660. |
| 55 | LI Y, ARNOLD W, HALACOGLU S, et al. Phase-transition interlayer enables high-performance solid-state sodium batteries with sulfide solid electrolyte[J]. Advanced Functional Materials, 2021, 31(28): 2101636. |
| 56 | XU R C, HAN F D, JI X, et al. Interface engineering of sulfide electrolytes for all-solid-state lithium batteries[J]. Nano Energy, 2018, 53: 958-966. |
| 57 | WANG C, SUN X L, YANG L, et al. In situ ion-conducting protective layer strategy to stable lithium metal anode for all-solid-state sulfide-based lithium metal batteries[J]. Advanced Materials Interfaces, 2021, 8(1): 2001698. |
| 58 | LUO S T, LIU X Y, ZHANG X A, et al. Nanostructure of the interphase layer between a single Li dendrite and sulfide electrolyte in all-solid-state Li batteries[J]. ACS Energy Letters, 2022, 7(9): 3064-3071. |
| 59 | YUAN Y, CHEN L K, LI Y H, et al. Functional LiTaO3 filler with tandem conductivity and ferroelectricity for PVDF-based composite solid-state electrolyte[J]. Energy Materials and Devices, 2023, 1(1): 9370004. |
| 60 | TAO J M, CHEN Y, BHARDWAJ A, et al. Combating Li metal deposits in all-solid-state battery via the piezoelectric and ferroelectric effects[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(41): e2211059119. |
| 61 | GU T, CHEN L K, HUANG Y F, et al. Engineering ferroelectric interlayer between Li1.3Al0.3Ti1.7(PO4)3 and lithium metal for stable solid-state batteries operating at room temperature[J]. Energy & Environmental Materials, 2023, 6: e12531. |
| 62 | LIU M, WANG C, ZHAO C L, et al. Quantification of the Li-ion diffusion over an interface coating in all-solid-state batteries via NMR measurements[J]. Nature Communications, 2021, 12: 5943. |
| 63 | BANERJEE A, TANG H M, WANG X F, et al. Revealing nanoscale solid-solid interfacial phenomena for long-life and high-energy all-solid-state batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(46): 43138-43145. |
| 64 | KOERVER R, AYGÜN I, LEICHTWEIß T, et al. Capacity fade in solid-state batteries: Interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and lithium thiophosphate solid electrolytes[J]. Chemistry of Materials, 2017, 29(13): 5574-5582. |
| 65 | KOERVER R, WALTHER F, AYGÜN I, et al. Redox-active cathode interphases in solid-state batteries[J]. Journal of Materials Chemistry A, 2017, 5(43): 22750-22760. |
| 66 | VARDAR G, BOWMAN W J, LU Q Y, et al. Structure, chemistry, and charge transfer resistance of the interface between Li7La3Zr2O12 electrolyte and LiCoO2 cathode[J]. Chemistry of Materials, 2018, 30(18): 6259-6276. |
| 67 | SCHWIETERT T K, ARSZELEWSKA V A, WANG C, et al. Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes[J]. Nature Materials, 2020, 19(4): 428-435. |
| 68 | CULVER S P, KOERVER R, ZEIER W, et al. On the functionality of coatings for cathode active materials in thiophosphate‐based all‐solid‐state batteries [J]. Advanced Energy Materials, 2019, 9(24): 1900626. |
| 69 | CHENG Z, LIU M, GANAPATHY S, et al. Revealing the impact of space-charge layers on the Li-ion transport in all-solid-state batteries[J]. Joule, 2020, 4(6): 1311-1323. |
| 70 | FAMPRIKIS T, CANEPA P, DAWSON J A, et al. Fundamentals of inorganic solid-state electrolytes for batteries[J]. Nature Materials, 2019, 18(12): 1278-1291. |
| 71 | ZOU Z Y, LI Y J, LU Z H, et al. Mobile ions in composite solids[J]. Chemical Reviews, 2020, 120(9): 4169-4221. |
| 72 | LIANG C C. Conduction characteristics of the lithium iodide-aluminum oxide solid electrolytes[J]. Journal of the Electrochemical Society, 1973, 120(10): 1289. |
| 73 | GUO X, MATEI I, JAMNIK J, et al. Defect chemical modeling of mesoscopic ion conduction in nanosized CaF2 / BaF2 multilayer heterostructures [J]. Physical Review B, 2007, 76(12): 125429. |
| 74 | BAIUTTI F, LOGVENOV G, GREGORI G, et al. High-temperature superconductivity in space-charge regions of lanthanum cuprate induced by two-dimensional doping[J]. Nature Communications, 2015, 6: 8586. |
| 75 | LU G Z, GENG F S, GU S Y, et al. Distinguishing the effects of the space-charge layer and interfacial side reactions on Li10GeP2S12-based all-solid-state batteries with stoichiometric-controlled LiCoO2[J]. ACS Applied Materials & Interfaces, 2022, 14(22): 25556-25565. |
| [1] | Yonghao HUANG, Guojing ZANG, Weiya ZHU, Youhao LIAO, Weishan LI. Enhancing interfacial stability between lithium-containing ceramic separator and 4.35 V LiNi0.8Co0.1Mn0.1O2 cathode through LiF additives [J]. Energy Storage Science and Technology, 2023, 12(8): 2361-2369. |
| [2] | Zhengguang ZHAO, Zhenying CHEN, Guangqun ZHAI, Xi ZHANG, Xiaodong ZHUANG. Preparation of Sc/O-doped sulfide electrolyte for all-solid-state batteries [J]. Energy Storage Science and Technology, 2023, 12(8): 2412-2423. |
| [3] | Zenghui HAO, Xunliang LIU, Yuan MENG, Nan MENG, Zhi WEN. Effect of electrode interface microstructure on the performance of solid-state lithium-ion battery [J]. Energy Storage Science and Technology, 2023, 12(7): 2095-2104. |
| [4] | Jiayi ZHANG, Suting WENG, Zhaoxiang WANG, Xuefeng WANG. Solid electrolyte interphase (SEI) on graphite anode correlated with thermal runaway of lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(7): 2105-2118. |
| [5] | Qixin GAO, Jingteng ZHAO, Guoxing LI. Research progress on fast-charging lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(7): 2166-2184. |
| [6] | Shenran ZHANG, Lihuan XU, Chang SU. Influence of different carbon contents on the electrochemical performance of SiO/C anode [J]. Energy Storage Science and Technology, 2023, 12(6): 1784-1793. |
| [7] | Wenchao SHI, Yu LIU, Bomian ZHANG, Qi LI, Chunhua HAN, Liqiang MAI. Research progress and prospect on electrolyte additives for stabilizing the zinc anode interface in aqueous batteries [J]. Energy Storage Science and Technology, 2023, 12(5): 1589-1603. |
| [8] | Yongshi YU, Xianming XIA, Hongyang HUANG, Yu YAO, Xianhong RUI, Guobin ZHONG, Wei SU, Yan YU. Research progress on sodium metal anode modified by artificial interface layer [J]. Energy Storage Science and Technology, 2023, 12(5): 1380-1391. |
| [9] | Wen ZHANG, Shuang LI, Cheng CHEN, Qiang SHEN. Effect of in situ solidification on the performance of silicon oxide anode [J]. Energy Storage Science and Technology, 2023, 12(4): 1045-1050. |
| [10] | Huimin ZHANG, Jing WANG, Yibo WANG, Jiaxin ZHENG, Jingyi QIU, Gaoping CAO, Hao ZHANG. Multiscale modeling of the SEI of lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(2): 366-382. |
| [11] | Xunchang JIANG, Minhui LIAO, Yang ZHOU, Daxiang YANG, Qiang WANG. Design and performance of nanofiber membrane-based elastic solid electrolyte [J]. Energy Storage Science and Technology, 2023, 12(11): 3307-3317. |
| [12] | Yansen ZHENG, Yongyin WANG, Jiuqing GUI, Zhuohao XIE, Yue XU, Qiaoying CAO, Yuehua XU, Yingliang LIU, Yeru LIANG. Preparation and performances of gelatin/polyethylene oxide composite electrolyte for high-voltage solid-state lithium batteries [J]. Energy Storage Science and Technology, 2023, 12(10): 3064-3074. |
| [13] | Pengbo ZHAI, Dongmei CHANG, Zhijie BI, Ning ZHAO, Xiangxin GUO. Research progress on key interfacial issues in lithium lanthanum zirconium oxide-based solid-state [J]. Energy Storage Science and Technology, 2022, 11(9): 2847-2865. |
| [14] | Jinghua WU, Jing YANG, Gaozhan LIU, Zhiyan WANG, Zhihua ZHANG, Hailong YU, Xiayin YAO, Xuejie HUANG. Review and prospective of solid-state lithium batteries in the past decade (2011—2021) [J]. Energy Storage Science and Technology, 2022, 11(9): 2713-2745. |
| [15] | Chaochao WEI, Chuang YU, Zhongkai WU, Linfeng PENG, Shijie CHENG, Jia XIE. Research progress of Li3PS4 solid electrolyte [J]. Energy Storage Science and Technology, 2022, 11(5): 1368-1382. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
