储能科学与技术 ›› 2024, Vol. 13 ›› Issue (7): 2206-2223.doi: 10.19799/j.cnki.2095-4239.2024.0376
收稿日期:2024-04-28
修回日期:2024-06-08
出版日期:2024-07-28
发布日期:2024-07-23
通讯作者:
余彦
E-mail:wlifeng@mail.ustc.edu.cn;yanyumse@ustc.edu.cn
作者简介:王立锋(1997—),男,博士研究生,研究方向为钠离子电池电解液的设计,E-mail:wlifeng@mail.ustc.edu.cn;
基金资助:
Lifeng WANG( ), Naiqing REN, Hai YANG, Yu YAO, Yan YU(
), Naiqing REN, Hai YANG, Yu YAO, Yan YU( )
)
Received:2024-04-28
Revised:2024-06-08
Online:2024-07-28
Published:2024-07-23
Contact:
Yan YU
E-mail:wlifeng@mail.ustc.edu.cn;yanyumse@ustc.edu.cn
摘要:
发展大规模储能技术是实现清洁能源的高效利用,进而实现国家碳中和目标的关键。相较于目前广泛应用的锂离子电池,钠离子电池(sodium ion batteries,SIBs)原材料资源丰度高且成本低,是非常有潜力的一种大规模储能技术。近年来,SIBs在室温下表现出优异的电化学性能,但其在低温下的应用面临着诸多挑战,这极大地限制了其在极端环境下的应用。缓慢的钠离子扩散速率和较差的电荷转移动力学是导致SIBs低温下性能差的主要原因,而这与控制体相和界面离子传输的电解液密切相关。本文首先从电解液角度简要阐述了SIBs低温性能衰退的原因;然后,从传统电解液优化和新型低温电解液两个方面综述了低温电解液的研究进展,系统地总结了低温SIBs电解液中有关碳酸酯类溶剂、醚类溶剂、添加剂和溶剂化结构的相关研究;最后,对低温电解液的发展前景予以展望。
中图分类号:
王立锋, 任乃青, 杨海, 姚雨, 余彦. 低温钠离子电池电解液研究进展[J]. 储能科学与技术, 2024, 13(7): 2206-2223.
Lifeng WANG, Naiqing REN, Hai YANG, Yu YAO, Yan YU. Advances in low-temperature electrolytes for sodium-ion batteries[J]. Energy Storage Science and Technology, 2024, 13(7): 2206-2223.
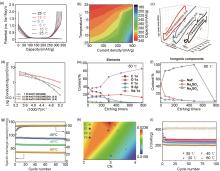
图5
(a) HCP在-25 ℃到25 ℃温度范围内的充放电曲线;(b) HCP电极在不同温度下的容量保持[52];(c) 不同电解液在0 ℃至-150 ℃之间的DSC曲线;(d) 不同电解液在20 ℃到-80 ℃温度范围内离子电导率随温度变化的曲线;(e) -80 ℃下形成的SEI中C 1s、O 1s、F 1s、S 2p和Na 1s元素的含量;(f) SEI中主要的无机成分NaF、Na2SO4 和Na2SO3 的含量;(g) -20 ℃、-40 ℃和-60 ℃、22 mA/g电流密度下电池的长循环性能[27];(h) 理论计算得到的三种电解液中Na+ —O键的BCP电子密度:(Ⅰ) N-THF中的O,(Ⅱ) THF中的O,(Ⅲ) N-mixTHF中的O,(Ⅳ) 2MeTHF中的O;(i) 电极在N-mixTHF在不同温度下的长循环性能(-20 ℃以上电流密度为100 mA/g,-40 ℃和-60 ℃下电流密度为50 mA/g)[54]"
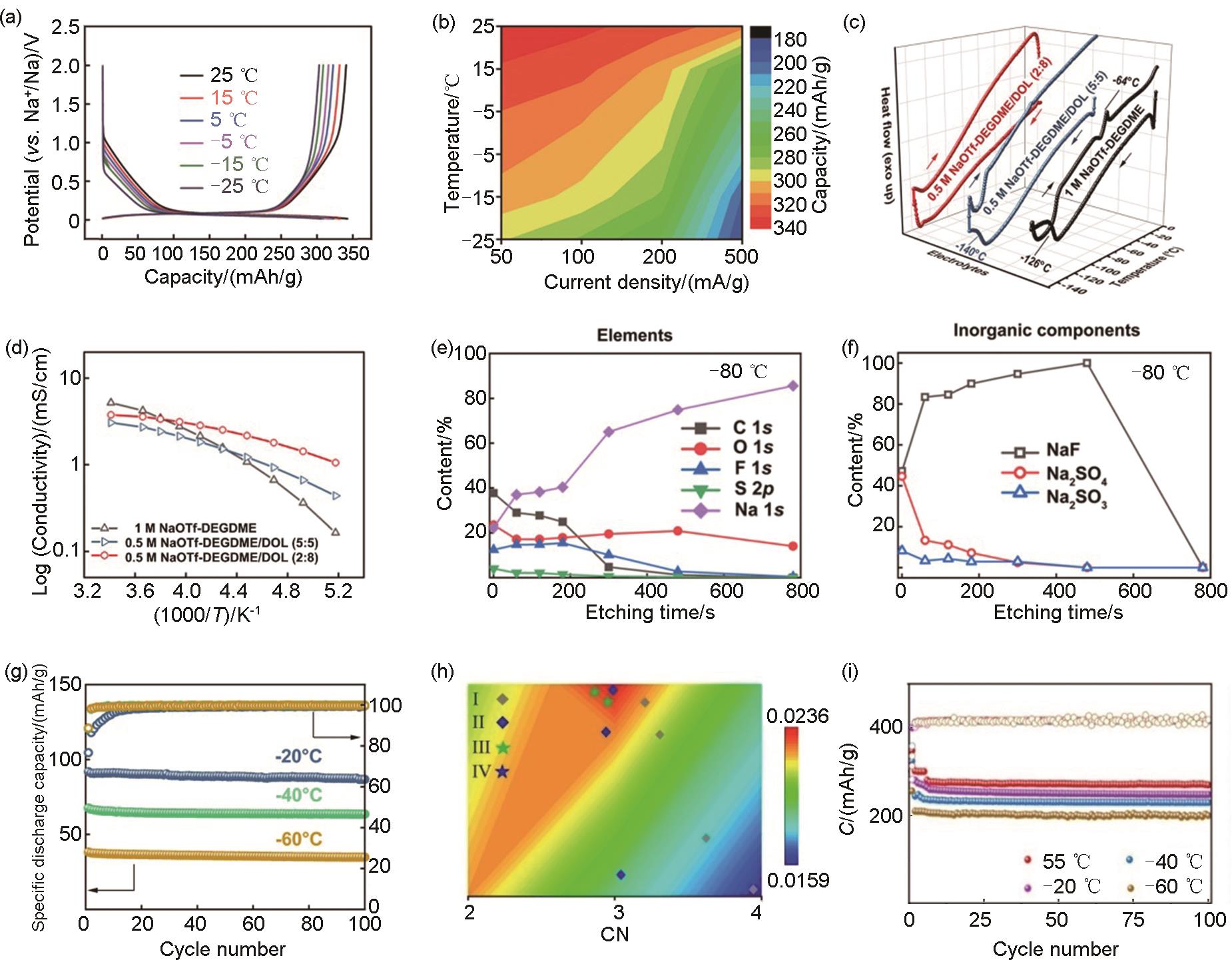
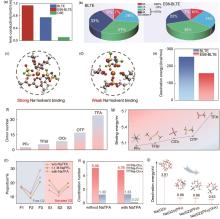
图6
(a) CSE、BLTE和ES6-BLTE电解液在-40 ℃下的离子电导率;(b) BLTE和ES6-BLTE的MD模拟得到的溶剂化结构中Na+ 周围FSI- 的数量(A代表与每个Na+ 配位的FSI-的数量,在SSPIs结构中,Na+ 仅在第一溶剂化鞘层中与溶剂配位;在CIPs结构中,Na+ 与一个FSI-配位;在AGGs结构中,Na+ 与多个FSI- 配位);从MD模拟结果中提取的 (c) BLTE和 (d) ES6-BLTE电解液最可能的溶剂化结构;(e)DFT计算得到的BLTE和ES6-BLTE电解液的去溶剂化能[61];(f) 常用阴离子的供体数;(g) Na+ 与阴离子的结合能;(h) 不同电解液中游离G2和溶剂化G2的比例;(i) 不含和含有NaTFA的电解液的MD模拟得到的配位数;(j) 四种代表性溶剂化结构的去溶剂化能[33]"
| 1 | MANTHIRAM A. A reflection on lithium-ion battery cathode chemistry[J]. Nature Communications, 2020, 11(1): 1550. DOI: 10.1038/s41467-020-15355-0. |
| 2 | CHEN S Q, SHEN L F, VAN AKEN P A, et al. Dual-functionalized double carbon shells coated silicon nanoparticles for high performance lithium-ion batteries[J]. Advanced Materials, 2017, 29(21): 1605650. DOI: 10.1002/adma.201605650. |
| 3 | ZHU C B, SONG K P, VAN AKEN P A, et al. Carbon-coated Na3V2(PO4)3 embedded in porous carbon matrix: An ultrafast Na-storage cathode with the potential of outperforming Li cathodes[J]. Nano Letters, 2014, 14(4): 2175-2180. DOI: 10.1021/nl500548a. |
| 4 | LI W H, ZENG L C, WU Y, et al. Nanostructured electrode materials for lithium-ion and sodium-ion batteries via electrospinning[J]. Science China Materials, 2016, 59(4): 287-321. DOI: 10.1007/s40843-016-5039-6. |
| 5 | DUNN B, KAMATH H, TARASCON J M. Electrical energy storage for the grid: A battery of choices[J]. Science, 2011, 334(6058): 928-935. DOI: 10.1126/science.1212741. |
| 6 | THACKERAY M M, WOLVERTON C, ISAACS E D. Electrical energy storage for transportation—Approaching the limits of, and going beyond, lithium-ion batteries[J]. Energy & Environmental Science, 2012, 5(7): 7854-7863. DOI: 10.1039/C2EE21892E. |
| 7 | MACKANIC D G, CHANG T H, HUANG Z J, et al. Stretchable electrochemical energy storage devices[J]. Chemical Society Reviews, 2020, 49(13): 4466-4495. DOI: 10.1039/d0cs00035c. |
| 8 | ZHU C B, MU X K, VAN AKEN P A, et al. Single-layered ultrasmall nanoplates of MoS2 embedded in carbon nanofibers with excellent electrochemical performance for lithium and sodium storage[J]. Angewandte Chemie International Edition, 2014, 53(8): 2152-2156. DOI: 10.1002/anie.201308354. |
| 9 | PALOMARES V, SERRAS P, VILLALUENGA I, et al. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems[J]. Energy & Environmental Science, 2012, 5(3): 5884-5901. DOI: 10.1039/C2EE02781J. |
| 10 | KIM H, KIM H, DING Z, et al. Recent progress in electrode materials for sodium-ion batteries[J]. Advanced Energy Materials, 2016, 6(19): 1600943. DOI: 10.1002/aenm.201600943. |
| 11 | HWANG J Y, MYUNG S T, SUN Y K. Sodium-ion batteries: Present and future[J]. Chemical Society Reviews, 2017, 46(12): 3529-3614. DOI: 10.1039/c6cs00776g. |
| 12 | WU C, JIANG Y, KOPOLD P, et al. Peapod-like carbon-encapsulated cobalt chalcogenide nanowires as cycle-stable and high-rate materials for sodium-ion anodes[J]. Advanced Materials, 2016, 28(33): 7276-7283. DOI: 10.1002/adma.201600964. |
| 13 | ARMAND M, TARASCON J M. Building better batteries[J]. Nature, 2008, 451(7179): 652-657. DOI: 10.1038/451652a. |
| 14 | ZHU G L, WEN K C, LV W Q, et al. Materials insights into low-temperature performances of lithium-ion batteries[J]. Journal of Power Sources, 2015, 300: 29-40. DOI: 10.1016/j.jpowsour.2015.09.056. |
| 15 | CHEN P, WU C Y, WANG Z Y, et al. Synergistically boosting sodium-storage performance of Na3V2(PO4)3 by regulating Na sites and constructing 3D interconnected carbon nanosheet frameworks[J]. ACS Applied Energy Materials, 2022, 5(2): 2542-2552. DOI: 10.1021/acsaem.1c04061. |
| 16 | ZHANG H W, SONG J J, LI J Y, et al. Interlayer-expanded MoS2 nanoflowers vertically aligned on MXene@Dual-phased TiO2 as high-performance anode for sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(14): 16300-16309. DOI: 10.1021/acsami.2c02080. |
| 17 | RUI X H, ZHANG X H, XU S T, et al. A low-temperature sodium-ion full battery: Superb kinetics and cycling stability[J]. Advanced Functional Materials, 2021, 31(11): 2009458. DOI: 10.1002/adfm.202009458. |
| 18 | WANG Y Y, HOU B H, GUO J Z, et al. An ultralong lifespan and low-temperature workable sodium-ion full battery for stationary energy storage[J]. Advanced Energy Materials, 2018, 8(18): 1703252. DOI: 10.1002/aenm.201703252. |
| 19 | XIA Y, QUE L F, YU F D, et al. Tailoring nitrogen terminals on MXene enables fast charging and stable cycling Na-ion batteries at low temperature[J]. Nano-Micro Letters, 2022, 14(1): 143. DOI: 10.1007/s40820-022-00885-7. |
| 20 | HWANG J Y, OH S M, MYUNG S T, et al. Radially aligned hierarchical columnar structure as a cathode material for high energy density sodium-ion batteries[J]. Nature Communications, 2015, 6: 6865. DOI: 10.1038/ncomms7865. |
| 21 | XIA X M, XU S T, TANG F, et al. A multifunctional interphase layer enabling superior sodium-metal batteries under ambient temperature and -40 ℃[J]. Advanced Materials, 2023, 35(11): 2209511. DOI: 10.1002/adma.202209511. |
| 22 | ZHENG J M, CHEN S R, ZHAO W G, et al. Extremely stable sodium metal batteries enabled by localized high-concentration electrolytes[J]. ACS Energy Letters, 2018, 3(2): 315-321. DOI: 10.1021/acsenergylett.7b01213. |
| 23 | ZHENG X Y, CAO Z, LUO W, et al. Solvation and interfacial engineering enable -40 ℃ operation of graphite/NCM batteries at energy density over 270 Wh·kg–1[J]. Advanced Materials, 2023, 35(10): 2210115. DOI: 10.1002/adma.202210115. |
| 24 | RUAN J F, LUO S N, WANG S F, et al. Enhancing the whole migration kinetics of Na+ in the anode side for advanced ultralow temperature sodium-ion hybrid capacitor[J]. Advanced Energy Materials, 2023, 13(34): 2301509. DOI: 10.1002/aenm.202301509. |
| 25 | SUN Y, LI J C, ZHOU H S, et al. Wide-temperature-range sodium-metal batteries: From fundamentals and obstacles to optimization[J]. Energy & Environmental Science, 2023, 16(11): 4759-4811. DOI: 10.1039/d3ee02082g. |
| 26 | LIANG H J, LIU H H, ZHAO X X, et al. Electrolyte chemistry toward ultrawide-temperature (-25 to 75 ℃) sodium-ion batteries achieved by phosphorus/silicon-synergistic interphase manipulation[J]. Journal of the American Chemical Society, 2024, 146(11): 7295-7304. DOI: 10.1021/jacs.3c11776. |
| 27 | WANG C L, THENUWARA A C, LUO J M, et al. Extending the low-temperature operation of sodium metal batteries combining linear and cyclic ether-based electrolyte solutions[J]. Nature Communications, 2022, 13(1): 4934. DOI: 10.1038/s41467-022-32606-4. |
| 28 | XU K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries[J]. Chemical Reviews, 2004, 104(10): 4303-4417. DOI: 10.1021/cr030203g. |
| 29 | AL-DAWSARI J N, BESSADOK-JEMAI A, WAZEER I, et al. Fitting of experimental viscosity to temperature data for deep eutectic solvents[J]. Journal of Molecular Liquids, 2020, 310: 113127. DOI: 10.1016/j.molliq.2020.113127. |
| 30 | ZHENG X Y, GU Z Y, FU J, et al. Knocking down the kinetic barriers towards fast-charging and low-temperature sodium metal batteries[J]. Energy & Environmental Science, 2021, 14(9): 4936-4947. DOI: 10.1039/D1EE01404H. |
| 31 | JIN Y, LE P M L, GAO P Y, et al. Low-solvation electrolytes for high-voltage sodium-ion batteries[J]. Nature Energy, 2022, 7: 718-725. DOI: 10.1038/s41560-022-01055-0. |
| 32 | WANG M, WANG Q C, DING X Y, et al. The prospect and challenges of sodium-ion batteries for low-temperature conditions[J]. Interdisciplinary Materials, 2022, 1(3): 373-395. DOI: 10.1002/idm2.12040. |
| 33 | ZHOU X Z, HUANG Y H, WEN B, et al. Regulation of anion-Na+ coordination chemistry in electrolyte solvates for low-temperature sodium-ion batteries[J]. Proceedings of the National Academy of Sciences of the United States of America, 2024, 121(5): e2316914121. DOI: 10.1073/pnas.2316914121. |
| 34 | GUO S H. A perspective on low-temperature electrolytes for sodium-ion batteries[J]. Energy Lab, 2023, 1: (3): 230003. DOI: 10.54227/elab.20230003. |
| 35 | HE M X, ZHU L J, YE G, et al. Tuning the electrolyte and interphasial chemistry for all-climate sodium-ion batteries[J]. Angewandte Chemie International Edition, 2024, 63(21): 2401051. DOI: 10.1002/anie.202401051. |
| 36 | WANG L F, REN N Q, YAO Y, et al. Designing solid electrolyte interfaces towards homogeneous Na deposition: Theoretical guidelines for electrolyte additives and superior high-rate cycling stability[J]. Angewandte Chemie International Edition, 2023, 62(6): e202214372. DOI: 10.1002/anie.202214372. |
| 37 | LI W H, HU S H, LUO X Y, et al. Confined amorphous red phosphorus in MOF-derived N-doped microporous carbon as a superior anode for sodium-ion battery[J]. Advanced Materials, 2017, 29(16): 1605820. DOI: 10.1002/adma.201605820. |
| 38 | DENG X H, ZHANG S, CHEN C, et al. Rational design of electrolytes operating at low temperatures: Does the co-solvent with a lower melting point correspond to better performance?[J]. Electrochimica Acta, 2022, 415: 140268. DOI: 10.1016/j.electacta.2022.140268. |
| 39 | ZHANG F, HE B J, XIN Y, et al. Emerging chemistry for wide-temperature sodium-ion batteries[J]. Chemical Reviews, 2024, 124(8): 4778-4821. DOI: 10.1021/acs.chemrev.3c00728. |
| 40 | GUO J Z, WANG P F, WU X L, et al. High-energy/power and low-temperature cathode for sodium-ion batteries: in situ XRD study and superior full-cell performance[J]. Advanced Materials, 2017, 29(33): 1701968. DOI: 10.1002/adma.201701968. |
| 41 | PONROUCH A, MARCHANTE E, COURTY M, et al. In search of an optimized electrolyte for Na-ion batteries[J]. Energy & Environmental Science, 2012, 5(9): 8572-8583. DOI: 10.1039/C2EE22258B. |
| 42 | LIU T F, WANG B, GU X X, et al. All-climate sodium ion batteries based on the NASICON electrode materials[J]. Nano Energy, 2016, 30: 756-761. DOI: 10.1016/j.nanoen.2016.09.024. |
| 43 | LI Y Q, YANG Y, LU Y X, et al. Ultralow-concentration electrolyte for Na-ion batteries[J]. ACS Energy Letters, 2020, 5(4): 1156-1158. DOI: 10.1021/acsenergylett.0c00337. |
| 44 | DENG L, GOH K, YU F D, et al. Self-optimizing weak solvation effects achieving faster low-temperature charge transfer kinetics for high-voltage Na3V2(PO4)2F3 cathode[J]. Energy Storage Materials, 2022, 44: 82-92. DOI: 10.1016/j.ensm.2021.10.012. |
| 45 | PONROUCH A, PALACÍN M R. On the high and low temperature performances of Na-ion battery materials: Hard carbon as a case study[J]. Electrochemistry Communications, 2015, 54: 51-54. DOI: 10.1016/j.elecom.2015.03.002. |
| 46 | XU Y N, WEI Q L, XU C, et al. Layer-by-layer Na3V2(PO4)3 embedded in reduced graphene oxide as superior rate and ultralong-life sodium-ion battery cathode[J]. Advanced Energy Materials, 2016, 6(14): 1600389. DOI: 10.1002/aenm.201600389. |
| 47 | MA X H, WEI Y Y, WU Y D, et al. High crystalline Na2Ni[Fe(CN)6]particles for a high-stability and low-temperature sodium-ion batteries cathode[J]. Electrochimica Acta, 2019, 297: 392-397. DOI: 10.1016/j.electacta.2018.11.063. |
| 48 | CUI G J, WANG H, YU F P, et al. Scalable synthesis of Na3V2(PO4)3/C with high safety and ultrahigh-rate performance for sodium-ion batteries[J]. Chinese Journal of Chemical Engineering, 2022, 46: 280-286. DOI: 10.1016/j.cjche.2021.06.008. |
| 49 | YOO D J, YANG S, KIM K J, et al. Fluorinated aromatic diluent for high-performance lithium metal batteries[J]. Angewandte Chemie International Edition, 2020, 59(35): 14869-14876. DOI: 10.1002/anie.202003663. |
| 50 | WANG H P, ZHU C L, LIU J D, et al. Formation of NaF-rich solid electrolyte interphase on Na anode through additive-induced anion-enriched structure of Na+ solvation[J]. Angewandte Chemie International Edition, 2022, 61(38): e202208506. DOI: 10.1002/anie.202208506. |
| 51 | LIU X Y, ZHENG X Y, QIN X, et al. Temperature-responsive solid-electrolyte-interphase enabling stable sodium metal batteries in a wide temperature range[J]. Nano Energy, 2022, 103: 107746. DOI: 10.1016/j.nanoen.2022.107746. |
| 52 | HOU B H, WANG Y Y, NING Q L, et al. Self-supporting, flexible, additive-free, and scalable hard carbon paper self-interwoven by 1D microbelts: Superb room/low-temperature sodium storage and working mechanism[J]. Advanced Materials, 2019, 31(40): e1903125. DOI: 10.1002/adma.201903125. |
| 53 | ZHOU J, WANG Y Y, WANG J W, et al. Low-temperature and high-rate sodium metal batteries enabled by electrolyte chemistry[J]. Energy Storage Materials, 2022, 50: 47-54. DOI: 10.1016/j.ensm.2022.05.005. |
| 54 | FANG H Y, HUANG Y H, HU W, et al. Regulating ion-dipole interactions in weakly solvating electrolyte towards ultra-low temperature sodium-ion batteries[J]. Angewandte Chemie International Edition, 2024, 63(15): 2400539. DOI: 10.1002/anie.202400539. |
| 55 | CHEN K A, SHEN X H, LUO L B, et al. Correlating the solvating power of solvents with the strength of ion-dipole interaction in electrolytes of lithium-ion batteries[J]. Angewandte Chemie, 2023, 135(47): e202312373. DOI: 10.1002/ange.202312373. |
| 56 | WANG X, YIN X P, FENG X C, et al. Rational design of Na0.67Ni0.2Co0.2Mn0.6O2 microsphere cathode material for stable and low temperature sodium ion storage[J]. Chemical Engineering Journal, 2022, 428: 130990. DOI: 10.1016/j.cej.2021.130990. |
| 57 | SHI Q H, QI R J, FENG X C, et al. Niobium-doped layered cathode material for high-power and low-temperature sodium-ion batteries[J]. Nature Communications, 2022, 13(1): 3205. DOI: 10.1038/s41467-022-30942-z. |
| 58 | ZHENG Y Q, SUN M Y, YU F D, et al. Utilizing weakly-solvated diglyme-based electrolyte to achieve a 10, 000-cycles durable Na3V2(PO4)2F3 cathode endured at -20 ℃[J]. Nano Energy, 2022, 102: 107693. DOI: 10.1016/j.nanoen.2022.107693. |
| 59 | ZHENG K X, XU S T, YAO Y, et al. Multi-component surface engineering of Na3V2(PO4)2O2F for low-temperature (-40 ℃) sodium-ion batteries[J]. Chemical Communications, 2022, 58(74): 10349-10352. DOI: 10.1039/d2cc03281c. |
| 60 | HUBBLE D, BROWN D E, ZHAO Y Z, et al. Liquid electrolyte development for low-temperature lithium-ion batteries[J]. Energy & Environmental Science, 2022, 15(2): 550-578. DOI: 10.1039/D1EE01789F. |
| 61 | ZHONG S E, YU Y S, YANG Y, et al. Molecular engineering on solvation structure of carbonate electrolyte toward durable sodium metal battery at -40 ℃[J]. Angewandte Chemie International Edition, 2023, 62(18): 2301169. DOI: 10.1002/anie.202301169. |
| 62 | SEOK J, HYUN J H, JIN A H, et al. Visualization of sodium metal anodes via Operando X-ray and optical microscopy: Controlling the morphological evolution of sodium metal plating[J]. ACS Applied Materials & Interfaces, 2022, 14(8): 10438-10446. DOI: 10.1021/acsami.1c24673. |
| 63 | CHENG H R, SUN Q J, LI L L, et al. Emerging era of electrolyte solvation structure and interfacial model in batteries[J]. ACS Energy Letters, 2022, 7(1): 490-513. DOI: 10.1021/acsenergylett.1c02425. |
| 64 | HAO H C, HUTTER T, BOYCE B L, et al. Review of multifunctional separators: Stabilizing the cathode and the anode for alkali (Li, Na, and K) metal-sulfur and selenium batteries[J]. Chemical Reviews, 2022, 122(9): 8053-8125. DOI: 10.1021/acs.chemrev.1c00838. |
| 65 | TAO L, RUSSELL J A, XIA D W, et al. Reversible switch in charge storage enabled by selective ion transport in solid electrolyte interphase[J]. Journal of the American Chemical Society, 2023, 145(30): 16538-16547. DOI: 10.1021/jacs.3c03429. |
| 66 | BOLLI C, GUÉGUEN A, MENDEZ M A, et al. Operando monitoring of F– formation in lithium ion batteries[J]. Chemistry of Materials, 2019, 31(4): 1258-1267. DOI: 10.1021/acs.chemmater.8b03810. |
| 67 | SONG X N, MENG T, DENG Y M, et al. The effects of the functional electrolyte additive on the cathode material Na0.76Ni0.3Fe0.4Mn0.3O2 for sodium-ion batteries[J]. Electrochimica Acta, 2018, 281: 370-377. DOI: 10.1016/j.electacta.2018.05.185. |
| 68 | WANG Q D, ZHAO C L, YAO Z P, et al. Entropy-driven liquid electrolytes for lithium batteries[J]. Advanced Materials, 2023, 35(17): e2210677. DOI: 10.1002/adma.202210677. |
| 69 | ZHANG W, XIA H R, ZHU Z Q, et al. Decimal solvent-based high-entropy electrolyte enabling the extended survival temperature of lithium-ion batteries to -130 ℃[J]. CCS Chemistry, 2021, 3(4): 1245-1255. DOI: 10.31635/ccschem.020.202000341. |
| 70 | TIAN Z N, ZOU Y G, LIU G, et al. Electrolyte solvation structure design for sodium ion batteries[J]. Advanced Science, 2022, 9(22): e2201207. DOI: 10.1002/advs.202201207. |
| 71 | YANG C Y, XIA J L, CUI C Y, et al. All-temperature zinc batteries with high-entropy aqueous electrolyte[J]. Nature Sustainability, 2023, 6: 325-335. DOI: 10.1038/s41893-022-01028-x. |
| 72 | YANG C, LIU X W, LIN Y, et al. Entropy-driven solvation toward low-temperature sodium-ion batteries with temperature-adaptive feature[J]. Advanced Materials, 2023, 35(28): 2301817. DOI: 10.1002/adma.202301817. |
| 73 | XIAO A W, LEE H J, CAPONE I, et al. Understanding the conversion mechanism and performance of monodisperse FeF2 nanocrystal cathodes[J]. Nature Materials, 2020, 19: 644-654. DOI: 10.1038/s41563-020-0621-z. |
| 74 | SUN H, ZHU G Z, ZHU Y M, et al. High-safety and high-energy-density lithium metal batteries in a novel ionic-liquid electrolyte[J]. Advanced Materials, 2020, 32(26): e2001741. DOI: 10.1002/adma.202001741. |
| 75 | KRUK D, JANCELEWICZ M, KLIMASZYK A, et al. Internal dynamics of ionic liquids over a broad temperature range—The role of the cation structure[J]. Materials, 2021, 15(1): 216. DOI: 10.3390/ma15010216. |
| 76 | SERRA MORENO J, MARESCA G, PANERO S, et al. Sodium-conducting ionic liquid-based electrolytes[J]. Electrochemistry Communications, 2014, 43: 1-4. DOI: 10.1016/j.elecom.2014.02.010. |
| 77 | LI P Y, HU N Q, WANG J Y, et al. Recent progress and perspective: Na ion batteries used at low temperatures[J]. Nanomaterials, 2022, 12(19): 3529. DOI: 10.3390/nano12193529. |
| 78 | DING C S, NOHIRA T, HAGIWARA R, et al. Electrochemical performance of hard carbon negative electrodes for ionic liquid-based sodium ion batteries over a wide temperature range[J]. Electrochimica Acta, 2015, 176: 344-349. DOI: 10.1016/j.electacta.2015.07.024. |
| 79 | BELLUSCI M, SIMONETTI E, DE FRANCESCO M, et al. Ionic liquid electrolytes for safer and more reliable sodium battery systems[J]. Applied Sciences, 2020, 10(18): 6323. DOI: 10.3390/app10186323. |
| 80 | DING C S, NOHIRA T, FUKUNAGA A, et al. Charge-discharge performance of an ionic liquid-based sodium secondary battery in a wide temperature range[J]. Electrochemistry, 2015, 83(2): 91-94. DOI: 10.5796/electrochemistry.83.91. |
| 81 | MATSUMOTO K, CHEN C Y, KIKO T, et al. Intermediate-temperature operation of sodium secondary batteries with high rate capability and cyclability using ionic liquid electrolyte[J]. ECS Transactions, 2016, 75(15): 139-145. DOI: 10.1149/07515.0139ecst. |
| 82 | GAO Y, YAN Z F, GRAY J L, et al. Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions[J]. Nature Materials, 2019, 18(4): 384-389. DOI: 10.1038/s41563-019-0305-8. |
| 83 | YANG Y X, ZHONG Y R, SHI Q W, et al. Electrocatalysis in lithium sulfur batteries under lean electrolyte conditions[J]. Angewandte Chemie International Edition, 2018, 57(47): 15549-15552. DOI: 10.1002/anie.201808311. |
| 84 | HU X F, MATIOS E, ZHANG Y W, et al. Deeply cycled sodium metal anodes at low temperature and in lean electrolyte conditions[J]. Angewandte Chemie International Edition, 2021, 60(11): 5978-5983. DOI: 10.1002/anie.202014241. |
| 85 | LIU Y, LU S W, WANG Z C, et al. Weakly polar ether-aided ionic liquid electrolyte enables high-performance sodium metal batteries over wide temperature range[J]. Advanced Functional Materials, 2024: 2312295. DOI: 10.1002/adfm.202312295. |
| [1] | 李昌豪, 汪书苹, 杨献坤, 曾子琪, 周昕玥, 谢佳. 低温型锂离子电池中的非水电解质研究进展[J]. 储能科学与技术, 2024, 13(7): 2286-2299. |
| [2] | 谭仕荣, 尹文骥, 曾翠鸿, 黎小琼, 訚硕, 纪方力, 胡思江, 王红强, 李庆余. 高温淬火对钠离子电池锰基层状正极材料结构和性能的影响[J]. 储能科学与技术, 2024, 13(7): 2399-2406. |
| [3] | 姜森, 陈龙, 孙创超, 王金泽, 李如宏, 范修林. 低温锂电池电解液的发展及展望[J]. 储能科学与技术, 2024, 13(7): 2270-2285. |
| [4] | 陆洋, 闫帅帅, 马骁, 刘誌, 章伟立, 刘凯. 低温锂电池电解液的研究与应用[J]. 储能科学与技术, 2024, 13(7): 2224-2242. |
| [5] | 廖世接, 魏颖, 黄云辉, 胡仁宗, 许恒辉. 间二氟苯稀释剂稳定电极界面助力低温锂金属电池[J]. 储能科学与技术, 2024, 13(7): 2124-2130. |
| [6] | 李想, 刘德重, 袁开, 陈大鹏. 用于低温锂金属电池的固态电解质技术研究进展[J]. 储能科学与技术, 2024, 13(7): 2327-2347. |
| [7] | 王美龙, 薛煜瑞, 胡文茜, 杜可遇, 孙瑞涛, 张彬, 尤雅. 低温磷酸铁锂电池用全醚高熵电解液的设计研究[J]. 储能科学与技术, 2024, 13(7): 2131-2140. |
| [8] | 王浩天, 王永刚, 董晓丽. 基于有机电极材料的低温电池研究进展[J]. 储能科学与技术, 2024, 13(7): 2259-2269. |
| [9] | 黄嘉琦, 熊杰明, 谭恩忠, 孙心语, 程李巍, 王华. 重新审视低温钠金属半电池[J]. 储能科学与技术, 2024, 13(7): 2151-2160. |
| [10] | 徐雄文, 莫英, 周望, 姚环东, 洪娟, 雷化, 涂健, 刘继磊. 硬碳动力学特性对钠离子电池低温性能的影响及机制[J]. 储能科学与技术, 2024, 13(7): 2141-2150. |
| [11] | 马国政, 陈金伟, 熊兴宇, 杨振忠, 周钢, 胡仁宗. SnSb-Li4Ti5O12 复合负极材料低温高倍率储锂特性研究[J]. 储能科学与技术, 2024, 13(7): 2107-2115. |
| [12] | 王文涛, 魏一凡, 黄鲲, 吕国伟, 张思瑶, 唐昕雅, 陈泽彦, 林清源, 母志鹏, 王昆桦, 才华, 陈军. 低温锂离子电池测试标准及研究进展[J]. 储能科学与技术, 2024, 13(7): 2300-2307. |
| [13] | 林炜琦, 卢巧瑜, 陈宇鸿, 邱麟媛, 季钰榕, 管联玉, 丁翔. 低温钠离子电池正极材料研究进展[J]. 储能科学与技术, 2024, 13(7): 2348-2360. |
| [14] | 李征, 杨振忠, 王琼, 胡仁宗. 基于专利情报分析的锂离子电池用低温电解液的发展现状和研究进展[J]. 储能科学与技术, 2024, 13(7): 2317-2326. |
| [15] | 程广玉, 刘新伟, 刘硕, 顾海涛, 王可. 调控电解液溶剂组分实现LCO/C低温18650电池循环寿命显著提升[J]. 储能科学与技术, 2024, 13(7): 2171-2180. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
