Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (7): 2119-2133.doi: 10.19799/j.cnki.2095-4239.2023.0212
Previous Articles Next Articles
Chong XU( ), Ning XU, Zhimin JIANG, Zhongkai LI, Yang HU, Hong YAN, Guoqiang MA(
), Ning XU, Zhimin JIANG, Zhongkai LI, Yang HU, Hong YAN, Guoqiang MA( )
)
Received:2023-04-10
Revised:2023-05-20
Online:2023-07-05
Published:2023-07-25
Contact:
Guoqiang MA
E-mail:xuchong01@sinochem.com;maguoqiang@sinochem.com
CLC Number:
Chong XU, Ning XU, Zhimin JIANG, Zhongkai LI, Yang HU, Hong YAN, Guoqiang MA. Mechanisms of gas evolution and suppressing strategies based on the electrolyte in lithium-ion batteries[J]. Energy Storage Science and Technology, 2023, 12(7): 2119-2133.

Fig. 1
(a), (b) Schematic for the diffusion of R-H+ from cathode to anode and the effect of temperature and voltage on the evolution of H2[25]; (c) Generation of H2 by a "double crossover-double catalysis" process during solvent decomposition[38] and (d) through chemical reaction between PVDF and Li dendrites on lithiated graphite anode[24]"


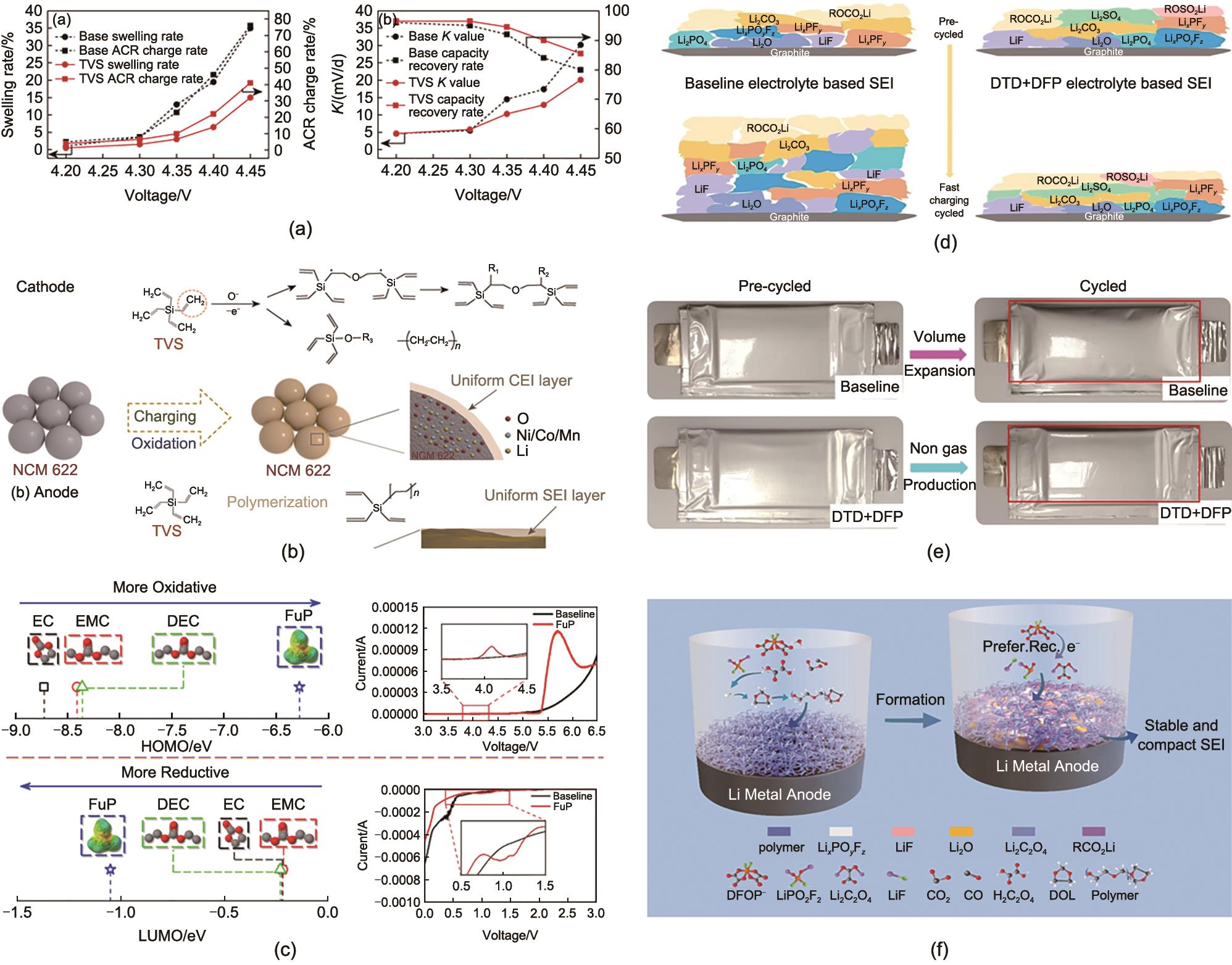
Fig. 10
(a) TVS reduces gas generation in lithium ion battery and; (b) The mechanism of TVS forming films on cathode and anode[76]; (c) Theoretical and practical decomposition potential of FuP[77]; (d) SEI component and (e) volume change of DTD+LiDFP based battery during cycling[78]; (f) Organic-inorganic hybrid SEI induced by a LiDFOP and DOL[79]"
| 1 | WOODY M, ARBABZADEH M, LEWIS G M, et al. Strategies to limit degradation and maximize Li-ion battery service lifetime—Critical review and guidance for stakeholders[J]. Journal of Energy Storage, 2020, 28: doi: 10. 1016/j. est. 2020. 101231. |
| 2 | FENG X N, REN D S, HE X M, et al. Mitigating thermal runaway of lithium-ion batteries[J]. Joule, 2020, 4(4): 743-770. |
| 3 | 陈晓霞, 刘凯, 王保国. 高安全性锂电池电解液研究与应用[J]. 储能科学与技术, 2020, 9(2): 583-592. |
| CHEN X X, LIU K, WANG B G. Research on high-safety electrolytes and their application in lithium-ion batteries[J]. Energy Storage Science and Technology, 2020, 9(2): 583-592. | |
| 4 | KONG L C, LI Y, FENG W. Strategies to solve lithium battery thermal runaway: From mechanism to modification[J]. Electrochemical Energy Reviews, 2021, 4(4): 633-679. |
| 5 | HU D Z, SU Y F, CHEN L, et al. The mechanism of side reaction induced capacity fading of Ni-rich cathode materials for lithium ion batteries[J]. Journal of Energy Chemistry, 2021, 58: 1-8. |
| 6 | BAO Y H, HONG G Q, CHEN Y, et al. Customized kirigami electrodes for flexible and deformable lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(1): 780-788. |
| 7 | KWADE A, HASELRIEDER W, LEITHOFF R, et al. Current status and challenges for automotive battery production technologies[J]. Nature Energy, 2018, 3(4): 290-300. |
| 8 | ZHANG S, MA J, HU Z L, et al. Identifying and addressing critical challenges of high-voltage layered ternary oxide cathode materials[J]. Chemistry of Materials, 2019, 31(16): 6033-6065. |
| 9 | GELDASA F T, KEBEDE M A, SHURA M W, et al. Identifying surface degradation, mechanical failure, and thermal instability phenomena of high energy density Ni-rich NCM cathode materials for lithium-ion batteries: A review[J]. RSC Advances, 2022, 12(10): 5891-5909. |
| 10 | 梁浩斌, 杜建华, 郝鑫, 等. 锂电池膨胀形成机制研究现状[J]. 储能科学与技术, 2021, 10(2): 647-657. |
| LIANG H B, DU J H, HAO X, et al. A review of current research on the formation mechanism of lithium batteries[J]. Energy Storage Science and Technology, 2021, 10(2): 647-657. | |
| 11 | DING J F, XU R, YAN C, et al. A review on the failure and regulation of solid electrolyte interphase in lithium batteries[J]. Journal of Energy Chemistry, 2021, 59: 306-319. |
| 12 | CUI J, SHI C, ZHAO J B. Research progress on the effect of mechanical pressure on the performance of lithium batteries[J]. CIESC Journal, 2021, 72(7): 3511-3523. |
| 13 | LOULI A J, ELLIS L D, DAHN J R. Operando pressure measurements reveal solid electrolyte interphase growth to rank Li-ion cell performance[J]. Joule, 2019, 3(3): 745-761. |
| 14 | 张慧敏, 王京, 王一博, 等. 锂离子电池SEI多尺度建模研究展望[J]. 储能科学与技术, 2023, 12(2): 366-382. |
| ZHANG H M, WANG J, WANG Y B, et al. Multiscale modeling of the SEI of lithium-ion batteries[J]. Energy Storage Science and Technology, 2023, 12(2): 366-382. | |
| 15 | TAKENAKA N, BOUIBES A, YAMADA Y, et al. Frontiers in theoretical analysis of solid electrolyte interphase formation mechanism[J]. Advanced Materials, 2021, 33(37): 2100574. |
| 16 | HEISKANEN S K, KIM J, LUCHT B L.Generation and evolution of the solid electrolyte interphase of lithium-ion batteries[J]. Joule, 2019, 3(10): 2322-2333. |
| 17 | SHAN X Y, ZHONG Y, ZHANG L J, et al. A brief review on solid electrolyte interphase composition characterization technology for lithium metal batteries: Challenges and perspectives[J]. The Journal of Physical Chemistry C, 2021, 125(35): 19060-19080. |
| 18 | LIU X, REN D S, HSU H J, et al. Thermal runaway of lithium-ion batteries without internal short circuit[J]. Joule, 2018, 2(10): 2047-2064. |
| 19 | WANG Y, FENG X N, PENG Y, et al. Reductive gas manipulation at early self-heating stage enables controllable battery thermal failure[J]. Joule, 2022, 6(12): 2810-2820. |
| 20 | JONES P K, STIMMING U, LEE A A. Impedance-based forecasting of lithium-ion battery performance amid uneven usage[J]. Nature Communications, 2022, 13: 4806. |
| 21 | LI B, PAREKH M H, PALANISAMY M, et al. In situ thermal runaway detection in lithium-ion batteries with an integrated internal sensor[J]. ACS Applied Energy Materials, 2020, 3(8): 7997-8008. |
| 22 | LI W F, WANG H W, ZHANG Y J, et al. Flammability characteristics of the battery vent gas: A case of NCA and LFP lithium-ion batteries during external heating abuse[J]. Journal of Energy Storage, 2019, 24: 100775. |
| 23 | 石爽, 吕娜伟, 马敬轩, 等. 不同类型气体探测对磷酸铁锂电池储能舱过充安全预警有效性对比[J]. 储能科学与技术, 2022, 11(8): 2452-2462. |
| SHI S, LYU N W, MA J X, et al. Comparative study on the effectiveness of different types of gas detection on the overcharge safety early warning of a lithium iron phosphate battery energy storage compartment[J]. Energy Storage Science and Technology, 2022, 11(8): 2452-2462. | |
| 24 | JIN Y, ZHENG Z K, WEI D H, et al. Detection of micro-scale Li dendrite via H2 gas capture for early safety warning[J]. Joule, 2020, 4(8): 1714-1729. |
| 25 | METZGER M, STREHLE B, SOLCHENBACH S, et al. Origin of H2 evolution in LIBs: H2O reduction vs. electrolyte oxidation[J]. Journal of the Electrochemical Society, 2016, 163(5): A798-A809. |
| 26 | GALUSHKIN N Е, YAZVINSKAYA N N, GALUSHKIN D N. Mechanism of gases generation during lithium-ion batteries cycling[J]. Journal of the Electrochemical Society, 2019, 166(6): A897-A908. |
| 27 | WANDT J, FREIBERG Ats, OGRODNIK A, et al. Singlet oxygen evolution from layered transition metal oxide cathode materials and its implications for lithium-ion batteries[J]. Materials Today, 2018, 21(8): 825-833. |
| 28 | ZHANG S S. Understanding of performance degradation of LiNi0.80Co0.10Mn0.10O2 cathode material operating at high potentials[J]. Journal of Energy Chemistry, 2020, 41: 135-141. |
| 29 | ZHANG J X, YANG J W, YANG L M, et al. Exploring the redox decomposition of ethylene carbonate-propylene carbonate in Li-ion batteries[J]. Materials Advances, 2021, 2(5): 1747-1751. |
| 30 | TENG X, ZHAN C, BAI Y, et al. In situ analysis of gas generation in lithium-ion batteries with different carbonate-based electrolytes[J]. ACS Applied Materials & Interfaces, 2015, 7(41): 22751-22755. |
| 31 | HOBOLD G M, KHURRAM A, GALLANT B M. Operando gas monitoring of solid electrolyte interphase reactions on lithium[J]. Chemistry of Materials, 2020, 32(6): 2341-2352. |
| 32 | RINKEL B L D, VIVEK J P, GARCIA-ARAEZ N, et al. Two electrolyte decomposition pathways at nickel-rich cathode surfaces in lithium-ion batteries[J]. Energy & Environmental Science, 2022, 15(8): 3416-3438. |
| 33 | MAO C Y, RUTHER R E, GENG L X, et al. Evaluation of gas formation and consumption driven by crossover effect in high-voltage lithium-ion batteries with Ni-rich NMC cathodes[J]. ACS Applied Materials & Interfaces, 2019, 11(46): 43235-43243. |
| 34 | HATSUKADE T, SCHIELE A, HARTMANN P, et al. Origin of carbon dioxide evolved during cycling of nickel-rich layered NCM cathodes[J]. ACS Applied Materials & Interfaces, 2018, 10(45): 38892-38899. |
| 35 | RENFREW S E, MCCLOSKEY B D. Quantification of surface oxygen depletion and solid carbonate evolution on the first cycle of LiNi0.6Mn0.2Co0.2O2 electrodes[J]. ACS Applied Energy Materials, 2019, 2(5): 3762-3772. |
| 36 | ELLIS L D, ALLEN J P, THOMPSON L M, et al. Quantifying, understanding and evaluating the effects of gas consumption in lithium-ion cells[J]. Journal of the Electrochemical Society, 2017, 164(14): A3518-A3528. |
| 37 | 谢宏, 黄锴, 杜进桥, 等. 锂离子电池电解液痕量水污染的超声表象[J]. 储能科学与技术, 2022, 11(12): 4030-4037 |
| XIE H, HUANG K, DU J Q, et al. Studies on ultrasonic appearance of trace water contamination in lithium-ion battery electrolyte[J]. Energy Storage Science and Technology, 2022, 11(12): 4030-4037 | |
| 38 | WANG X Q, REN D S, LIANG H M, et al. Ni crossover catalysis: Truth of hydrogen evolution in Ni-rich cathode-based lithium-ion batteries[J]. Energy & Environmental Science, 2023, 16(3): 1200-1209. |
| 39 | ZHANG S S. Problems and their origins of Ni-rich layered oxide cathode materials[J]. Energy Storage Materials, 2020, 24: 247-254. |
| 40 | LYU Y C, WU X, WANG K, et al. An overview on the advances of LiCoO2 cathodes for lithium-ion batteries[J]. Advanced Energy Materials, 2021, 11(2): 2000982. |
| 41 | BOULINEAU A, SIMONIN L, COLIN J F, et al. First evidence of manganese-nickel segregation and densification upon cycling in Li-rich layered oxides for lithium batteries[J]. Nano Letters, 2013, 13(8): 3857-3863. |
| 42 | METZGER M, MARINO C, SICKLINGER J, et al. Anodic oxidation of conductive carbon and ethylene carbonate in high-voltage Li-ion batteries quantified by on-line electrochemical mass spectrometry[J]. Journal of the Electrochemical Society, 2015, 162(7): A1123-A1134. |
| 43 | JUNG R, METZGER M, MAGLIA F, et al. Chemical versus electrochemical electrolyte oxidation on NMC111, NMC622, NMC811, LNMO, and conductive carbon[J]. The Journal of Physical Chemistry Letters, 2017, 8(19): 4820-4825. |
| 44 | RENFREW S E, MCCLOSKEY B D. The role of electrolyte in the first-cycle transformations of LiNi0.6Mn0.2Co0.2O2[J]. Journal of the Electrochemical Society, 2019, 166(13): A2762-A2768. |
| 45 | RAMAKRISHNAN S, PARK B, WU J, et al. Extended interfacial stability through simple acid rinsing in a Li-rich oxide cathode material[J]. Journal of the American Chemical Society, 2020, 142(18): 8522-8531. |
| 46 | RENFREW S E, KAUFMAN L A, MCCLOSKEY B D. Altering surface contaminants and defects influences the first-cycle outgassing and irreversible transformations of LiNi0.6Mn0.2Co0.2O2[J]. ACS Applied Materials & Interfaces, 2019, 11(38): 34913-34921. |
| 47 | RENFREW S E, MCCLOSKEY B D. Residual lithium carbonate predominantly accounts for first cycle CO2 and CO outgassing of Li-stoichiometric and Li-rich layered transition-metal oxides[J]. Journal of the American Chemical Society, 2017, 139(49): 17853-17860. |
| 48 | JUNG R, METZGER M, MAGLIA F, et al. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2 (NMC) cathode materials for Li-ion batteries[J]. Journal of the Electrochemical Society, 2017, 164(7): A1361-A1377. |
| 49 | WU Q S, MCDOWELL M T, QI Y. Effect of the electric double layer (EDL) in multicomponent electrolyte reduction and solid electrolyte interphase (SEI) formation in lithium batteries[J]. Journal of the American Chemical Society, 2023, 145(4): 2473-2484. |
| 50 | DAY R P, XIA J, PETIBON R, et al. Differential thermal analysis of Li-ion cells as an effective probe of liquid electrolyte evolution during aging[J]. Journal of the Electrochemical Society, 2015, 162(14): A2577-A2581. |
| 51 | YANG X W, WANG H W, LI M H, et al. Experimental study on thermal runaway behavior of lithium-ion battery and analysis of combustible limit of gas production[J]. Batteries, 2022, 8(11): 250. |
| 52 | WANG H B, XU H, ZHANG Z L, et al. Fire and explosion characteristics of vent gas from lithium-ion batteries after thermal runaway: A comparative study[J]. eTransportation, 2022, 13: 100190. |
| 53 | HAN J G, KIM K, LEE Y, et al. Scavenging materials: Scavenging materials to stabilize LiPF6-containing carbonate-based electrolytes for Li-ion batteries[J]. Advanced Materials, 2019, 31(20): 1804822. |
| 54 | SELF J, AIKEN C P, PETIBON R, et al. Survey of gas expansion in Li-ion NMC pouch cells[J]. Journal of the Electrochemical Society, 2015, 162(6): A796-A802. |
| 55 | WOTANGO A S, SU W N, LEGGESSE E G, et al. Improved interfacial properties of MCMB electrode by 1-(trimethylsilyl)imidazole as new electrolyte additive to suppress LiPF6 decomposition[J]. ACS Applied Materials & Interfaces, 2017, 9(3): 2410-2420. |
| 56 | KIM K, HWANG D, KIM S, et al. Cyclic aminosilane-based additive ensuring stable electrode-electrolyte interfaces in Li-ion batteries[J]. Advanced Energy Materials, 2020, 10(15): 2000012. |
| 57 | DENG B W, WANG H, GE W J, et al. Investigating the influence of high temperatures on the cycling stability of a LiNi0.6Co0.2Mn0.2O2 cathode using an innovative electrolyte additive[J]. Electrochimica Acta, 2017, 236: 61-71. |
| 58 | LIU G P, JIAO T P, CHENG Y, et al. Interfacial enhancement of silicon-based anode by a lactam-type electrolyte additive[J]. ACS Applied Energy Materials, 2021, 4(9): 10323-10332. |
| 59 | LIU G P, XU N B, ZOU Y, et al. Stabilizing Ni-rich LiNi0.83Co0.12Mn0.05O2 with cyclopentyl isocyanate as a novel electrolyte additive[J]. ACS Applied Materials & Interfaces, 2021, 13(10): 12069-12078. |
| 60 | SONG Y M, KIM C K, KIM K E, et al. Exploiting chemically and electrochemically reactive phosphite derivatives for high-voltage spinel LiNi0.5Mn1 5O4 cathodes[J]. Journal of Power Sources, 2016, 302: 22-30. |
| 61 | YIM T, WOO S G, LIM S H, et al. 5 V-class high-voltage batteries with over-lithiated oxide and a multi-functional additive[J]. Journal of Materials Chemistry A, 2015, 3(11): 6157-6167. |
| 62 | ZHENG J M, XIAO J, GU M, et al. Interface modifications by anion receptors for high energy lithium ion batteries[J]. Journal of Power Sources, 2014, 250: 313-318. |
| 63 | WU Y, REN D S, LIU X, et al. High-voltage and high-safety practical lithium batteries with ethylene carbonate-free electrolyte[J]. Advanced Energy Materials, 2021, 11(47): 2102299. |
| 64 | KANG G H, ZHONG G, MA J B, et al. Weakly solvated EC-free linear alkyl carbonate electrolytes for Ni-rich cathode in rechargeable lithium battery[J]. iScience, 2022, 25(12): 105710. |
| 65 | WANG Y K, LI Z M, HOU Y P, et al. Emerging electrolytes with fluorinated solvents for rechargeable lithium-based batteries[J]. Chemical Society Reviews, 2023, 52(8): 2713-2763. |
| 66 | 封迈, 陈楠, 陈人杰. 锂离子电池低温电解液的研究进展[J]. 储能科学与技术, 2023, 12(3): 792-807. |
| FENG M, CHEN N, CHEN R J. Research progress of low-temperature electrolyte for lithium-ion battery[J]. Energy Storage Science and Technology, 2023, 12(3): 792-807. | |
| 67 | LI Q, LIU X S, HAN X, et al. Identification of the solid electrolyte interface on the Si/C composite anode with FEC as the additive[J]. ACS Applied Materials & Interfaces, 2019, 11(15): 14066-14075. |
| 68 | SANG P F, CHEN Q L, WANG D Y, et al. Organosulfur materials for rechargeable batteries: Structure, mechanism, and application[J]. Chemical Reviews, 2023, 123(4): 1262-1326. |
| 69 | 毛舒岚, 武倩, 王卓雅, 等. 三元NCM锂离子电池高电压电解质的研究进展[J]. 储能科学与技术, 2020, 9(2): 538-550. |
| MAO S L, WU Q, WANG Z Y, et al. Research progress on high-voltage electrolytes for ternary NCM lithium-ion batteries[J]. Energy Storage Science and Technology, 2020, 9(2): 538-550. | |
| 70 | LIU H D, NAYLOR A J, MENON A S, et al. Understanding the roles of tris(trimethylsilyl) phosphite (TMSPi) in LiNi0.8Mn0.1Co0.1O2 (NMC811)/silicon-graphite (Si-gr) lithium-ion batteries[J]. Advanced Materials Interfaces, 2020, 7(15): 2000277. |
| 71 | HAN S Y, LIU Y, ZHANG H, et al. Succinonitrile as a high-voltage additive in the electrolyte of LiNi0.5Co0.2Mn0.3O2/graphite full batteries[J]. Surface and Interface Analysis, 2020, 52(6): 364-373. |
| 72 | WANG A P, WANG L, LIANG H M, et al. Lithium difluorophosphate as a widely applicable additive to boost lithium-ion batteries: A perspective[J]. Advanced Functional Materials, 2023, 33(8): 2211958. |
| 73 | KANG S, PARK K, PARK S-H, et al. Unraveling the role of LiFSI electrolyte in the superior performance of graphite anodes for Li-ion batteries[J]. Electrochimica Acta, 2018, 259: 949-954. |
| 74 | 詹元杰, 武怿达, 马晓威, 等. 基于碳酸酯基电解液的4.5 V电池[J]. 储能科学与技术, 2020, 9(2): 319-330. |
| ZHAN Y J, WU Y D, MA X W, et al. 4.5 V Li-ion battery with a carbonate ester-based electrolyte[J]. Energy Storage Science and Technology, 2020, 9(2): 319-330. | |
| 75 | WANG P, CUI X L, ZHAO D N, et al. Effects of soluble products decomposed from chelato-borate additives on formation of solid electrolyte interface layers[J]. Journal of Power Sources, 2022, 535: doi: 10. 1016/j. jpowsour. 2022. 231451. |
| 76 | 江依义, 沈旻, 宋半夏, 等. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| JIANG Y Y, SHEN M, SONG B X, et al. Effect of dual-functional electrolyte additive on high temperature and high voltage performance of Li-ion battery[J]. Journal of Inorganic Materials, 2022, 37(7): 710-716. | |
| 77 | ZHANG Z H, HU J G, HU Y, et al. Tri(2-furyl)phosphine-induced robust interphases for durable nickel-rich lithium-ion batteries[J]. Applied Surface Science, 2023, 624: 157027. |
| 78 | HU Y, ZHANG Z H, WANG H M. Fast-charging electrolyte: A multiple additives strategy with 1,3,2-dioxathiolane 2,2-dioxide and lithium difluorophosphate for commercial graphite/LiFePO4 pouch battery[J]. ChemistrySelect, 2022, 7(19): e202200740. |
| 79 | GUO L Y, HUANG F F, CAI M Z, et al. Organic-inorganic hybrid SEI induced by a new lithium salt for high-performance metallic lithium anodes[J]. ACS Applied Materials & Interfaces, 2021, 13(28): 32886-32893. |
| [1] | Zenghui HAO, Xunliang LIU, Yuan MENG, Nan MENG, Zhi WEN. Effect of electrode interface microstructure on the performance of solid-state lithium-ion battery [J]. Energy Storage Science and Technology, 2023, 12(7): 2095-2104. |
| [2] | Zhiwei CHEN, Weige ZHANG, Junwei ZHANG, Yanru ZHANG. Comprehensive health assessment and screening method of power battery pack based on visual characteristics of charge curves [J]. Energy Storage Science and Technology, 2023, 12(7): 2211-2219. |
| [3] | Jin LI, Qingsong WANG, Depeng KONG, Xiaodong WANG, Zhenhua YU, Yanfei LE, Xinyan HUANG, Zhenkai HU, Houfu WU, Huabin FANG, Caowei, Shaoyu ZHANG, Ping ZHUO, Ye CHEN, Ziting LI, Wenxin MEI, Yue ZHANG, Lixiang ZHAO, Liang TANG, Zonghou HUANG, Chi CHEN, Yanhu LIU, Yuxi CHU, Xiaoyuan XU, Jin ZHANG, Yikai LI, Rong FENG, Biao YANG, Bo HU, Xiaoying YANG. Research progress on the safety assessment of lithium-ion battery energy storage [J]. Energy Storage Science and Technology, 2023, 12(7): 2282-2301. |
| [4] | Jiayi ZHANG, Suting WENG, Zhaoxiang WANG, Xuefeng WANG. Solid electrolyte interphase (SEI) on graphite anode correlated with thermal runaway of lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(7): 2105-2118. |
| [5] | Guiping ZHANG, Xiaoyan YAN, Bing WANG, Peixin YAO, Changjie HU, Yizhe LIU, Shuli LI, Jianjun XUE. Long life lithium iron phosphate battery and its materials and process [J]. Energy Storage Science and Technology, 2023, 12(7): 2134-2140. |
| [6] | Qixin GAO, Jingteng ZHAO, Guoxing LI. Research progress on fast-charging lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(7): 2166-2184. |
| [7] | Ronghan QIAO, Jing ZHU, Xiaoyu SHEN, Guanjun CEN, Junfeng HAO, Hongxiang JI, Mengyu TIAN, Zhou JIN, Yuanjie ZHAN, Yida WU, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Xuejie HUANG. Reviews of selected 100 recent papers for lithium batteries (Apr. 1, 2023 to May 31, 2023) [J]. Energy Storage Science and Technology, 2023, 12(7): 2333-2348. |
| [8] | Lingfeng HUANG, Dongmei HAN, Sheng HUANG, Shuanjin WANG, Min XIAO, Yuezhong MENG. Research progress of polymer electrolytes containing organoboron for lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(6): 1815-1830. |
| [9] | Birong TAN, Jianhua DU, Xianghu YE, Xin CAO, Chang QU. Overview of SOC estimation methods for lithium-ion batteries based on model [J]. Energy Storage Science and Technology, 2023, 12(6): 1995-2010. |
| [10] | Ya CHEN, Liyun FAN, Jingxue LI, Meisi LI, Chao XU, Yuanqi GU. Research on heat dissipation of lithium-ion batteries with secondary flow serpentine channel [J]. Energy Storage Science and Technology, 2023, 12(6): 1880-1889. |
| [11] | Xijiang SHEN, Qiangling DUAN, Peng QIN, Qingsong WANG, Jinhua SUN. Experimental study on thermal runaway mitigation and heat transfer characteristics of ternary lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(6): 1862-1871. |
| [12] | Lei LEI, Peng GAO, Nana FENG, Kunpeng CAI, Hai ZHANG, Yang ZHANG. The influences of multifactors in the synthesis progress on the characteristics of lithium lanthanum zirconate solid electrolytes [J]. Energy Storage Science and Technology, 2023, 12(5): 1625-1635. |
| [13] | Yongli YI, Ran YU, Wu LI, Yi JIN, Zheren DAI. Preparation of Mo, Al-doped Li7La3Zr2O12-based composite solid electrolyte and performance of all-solid-state batterys [J]. Energy Storage Science and Technology, 2023, 12(5): 1490-1499. |
| [14] | Shedong LI, Yingying SONG, Yuhua BIAN, Zhaomeng LIU, Xuanwen GAO, Wenbin LUO. Status and challenges in the development of room-temperature sodium-sulfur batteries [J]. Energy Storage Science and Technology, 2023, 12(5): 1315-1331. |
| [15] | Xuanchen WANG, Da WANG, Zhaomeng LIU, Xuanwen GAO, Wenbin LUO. Research progress and prospect of potassium ion battery electrolyte [J]. Energy Storage Science and Technology, 2023, 12(5): 1409-1426. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
