Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (5): 1348-1363.doi: 10.19799/j.cnki.2095-4239.2023.0257
• Special Issue on Key Materials and Recycling Technologies for Energy Storage Batteries • Previous Articles Next Articles
Shangzhuo LI( ), Yutong LONG, Zhaomeng LIU(
), Yutong LONG, Zhaomeng LIU( ), Xuanwen GAO, Wenbin LUO
), Xuanwen GAO, Wenbin LUO
Received:2023-04-21
Revised:2023-05-09
Online:2023-05-05
Published:2023-05-29
Contact:
Zhaomeng LIU
E-mail:2171602@stu.neu.edu.cn;liuzhaomeng@smm.neu.edu.cn
CLC Number:
Shangzhuo LI, Yutong LONG, Zhaomeng LIU, Xuanwen GAO, Wenbin LUO. Advances toward polyanionic cathode materials for potassium-ion batteries[J]. Energy Storage Science and Technology, 2023, 12(5): 1348-1363.

Fig. 2
(a) Schematic diagram of the synthesis mechanism of K3V2(PO4)3/C[36]; (b) N2 adsorption-desorption isotherm at K3V2(PO4)3/C (Illustration: Pore size distribution curve of K3V2(PO4)3/C)[36]; (c) Synthesis diagram of preparation process and mechanism of K3V2(PO4)3/C bundling nanowires[39]; (d) XRD diagram of K3V2(PO4)3/C bundled nanowires at 700—900℃[39]; (e) In-situ XRD plot and voltage overlay plot during 100 mAh/g charging and discharging[39]; (f) Long-life cycling performance of K3V2(PO4)3/C bundled nanowires at 1000 and 2000 mA/g[39]; (g) Rietveld refinement diagram of SXRD of K3V2(PO4)3 structure[43]; (h) The unit structure of K3V2(PO4)3 evolves with CV curve in the range of 1.0—4.0 V[43]"
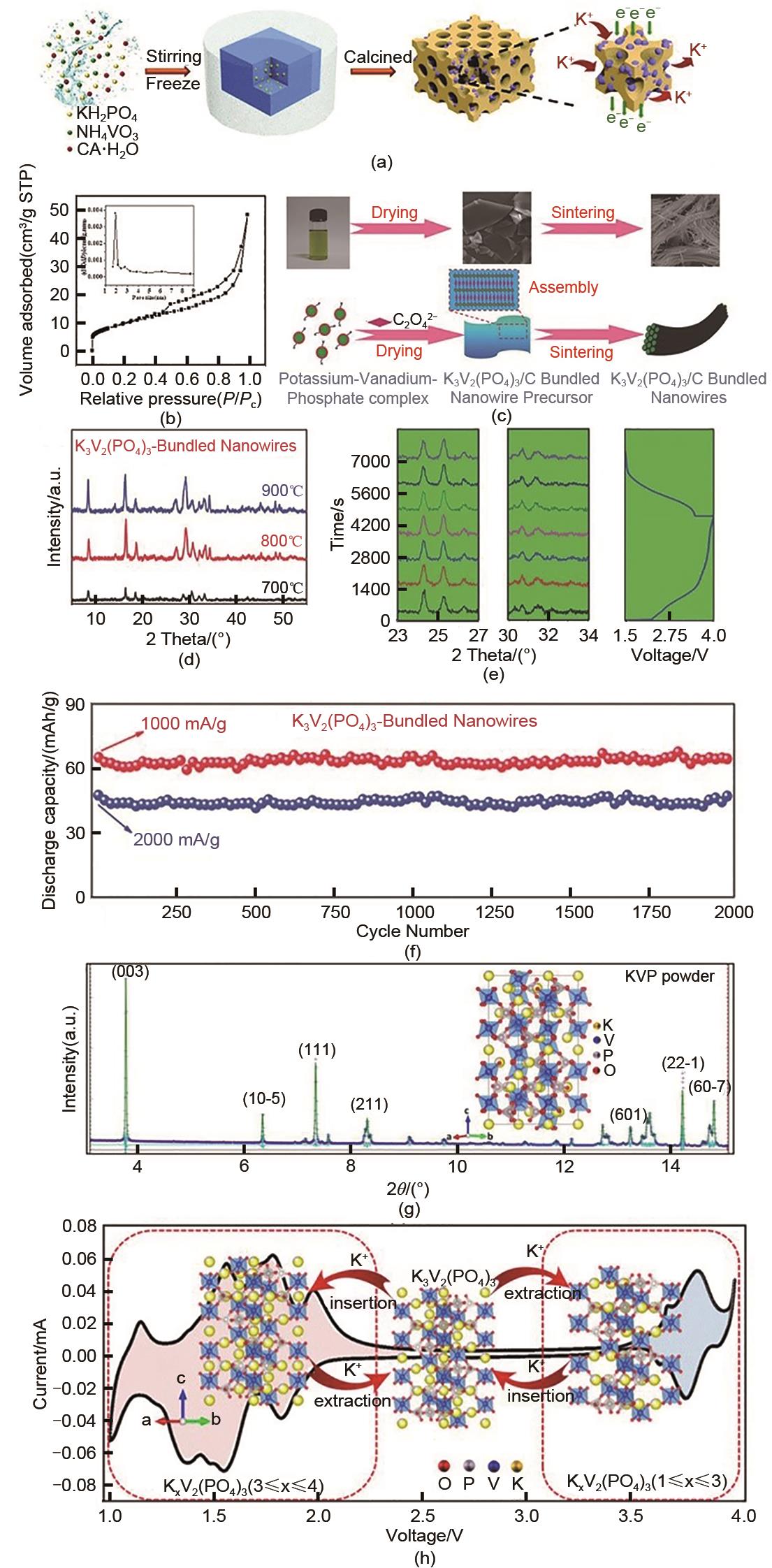
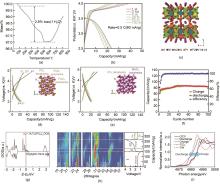
Fig. 3
(a) TGA curve for K3V3(PO4)4·H2O[44]; (b) Charge-discharge curve of K3V3(PO4)4·H2O from the 1st to the 100th cycle[44]; (c) Perspective views of K3V3(PO4)4·H2O potential K+ diffusion channels parallel to the (a) x-z basal plane [001][44]; (d) Schematic diagram of the charge-discharge curve and crystal structure of KFePO4 when the current density is 7.1 mA/g[48]; (e) Schematic diagram of the charge-discharge curve and crystal structure of KMnPO4 when the current density is 7.1 mA/g[48]; (f) N2 adsorption-desorption isotherms for KFePO4/C (Illustration: Pore size distribution curve of KFePO4/C)[49]; (g) DOS of K3Ti2(PO4)3[53]; (h) Structural evolution of KTP/C: a) Two-dimensional images of the manipulation of XRD data at the beginning of the first charge and discharge and the second discharge[53]; (i) XANES spectra of Ti K-edge measured at OCV, end of discharge (1 V) and charge (4 V) for KTP/C electrode and references (TiO2 and TiN)[53]"

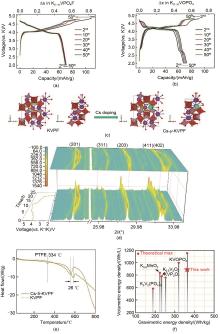
Fig. 5
(a) Charge/discharge profiles and cycle performance of KVPO4F[62]; (b) Charge/discharge profiles and cycle performance of KVOPO4[62]; (c) K-ion migration path 3 and K migration barrier in Cs-y-KVPF[63]; (d) Contour maps of in situ XRD patterns collected during the first charge/discharge of the Cs-5-KVPF electrode at a voltage ranging from 2.5 to 5.0 V[63]; (e) DSC curves of KVPF and Cs-5-KVPF electrodes inthe fully charged state[63]; (f) Comparison of the energy density of KVPF@C-PMS with other cathode materials[64]"

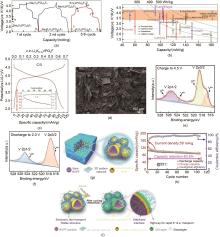
Fig. 6
(a) Voltage profiles of de-sodiation of Na3V2(PO4)2F3 and the following potassiation/de- potassiation[65]; (b) Compassion of the voltage and capacities of as-prepared K3V2(PO4)2F3 with different types of cathodes reported until now[65]; (c) A typical charge-discharge curve of Li x K0.15VPO4F at 0.2 C.(The inset demonstrates the capacity retention and Coulomb efficiency in the cycling ability test at 1 C rate)[67]; (d) SEM image of K1+δ VOPO4F[68];(e) Ex situ XPS of K1+δ VOPO4F charge to 4.5 V[68]; (f) Ex situ XPS of K1+δ VOPO4F discharge to 2.0 V[68]; (g) Schematic illustration for designing KVPF@3DC composite material[69]; (h) The cycling performance of KVPF@3DC at elevated temperature (55 ℃) with the current density of 50 mA/g[69]; (i) Schematic diagram of the interface change during repeated charge-discharge process at elevated temperature of KVPF@3DC[69]"


Fig. 7
(a) BVEL of o-KFeSO4F[71]; (b) BVEL of m-KFeSO4F[71]; (c) Normalized Fe K-edge XANES spectra of KFMg005SF,a charged KFMg005SF electrode, and a discharged KFMg005SF electrode[73]; (d) In-situ XRD patterns in the 2h range of 14.5°–17.5°, 27.2°–29.9°, and 31.0°–34.2° and the concerned charge/discharge profiles at 10 mA/g[74]; (e) GITT profiles and the relevant D(K+) values of KFSF@CNTs/DEG[74]; (f) Representative charge–discharge profiles of the full cell[74]"

Table 1
Electrochemical performance and spatial configuration of polyanionic cathode materials for potassium-ion batteries in recent years[46, 54, 62, 65, 71]"
| Materials | Structure | Theoretical capacity /(mAh/g) | Actual capacity /(mAh/g) | Average dischargepotential (vs. K+/K) /V |
|---|---|---|---|---|
| KVPO4F | KTiOPO4(KTP)-type orthorhombic (Pna21) | 131 | 90 | 4.13 |
| KVOPO4 | KTP-type orthorhombic (Pna21) | 133 | 80 | 4 |
| KVP2O7 | KAlP2O7-type monoclinic (P21/c) | 102 | 61 | 4.15 |
| K3V2(PO4)3 | Unknown | 106 | 54 | 3.5 |
| K3V2(PO4)2F3 | Orthorhombic (Cmcm) | 115 | 104 | 3.7 |
| Amorphous-FePO4 | — | 178 | 156 | 2.64 |
| Orthorhombic KFeSO4F | KTP-type orthorhombic (Pna21) | 128 | 100 | 3.6 |
| Monoclinic KFeSO4F | Layered monoclinic (C2/c) | 128 | 50 | 3.5(vs. Li) |
| 1 | ZHANG Q, WANG Z J, ZHANG S L, et al. Cathode materials for potassium-ion batteries: Current status and perspective[J]. Electrochemical Energy Reviews, 2018, 1(4): 625-658. |
| 2 | KOMABA S, HASEGAWA T, DAHBI M, et al. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors[J]. Electrochemistry Communications, 2015, 60: 172-175. |
| 3 | LIU Z, WANG J, JIA X, et al. Graphene armored with a crystal carbon shell for ultrahigh-performance potassium ion batteries and aluminum batteries[J]. ACS Nano, 2019, 13(9): 10631-10642. |
| 4 | LIU Z, WANG J, DING H, et al. Carbon nanoscrolls for aluminum battery[J]. ACS Nano, 2018, 12(8): 8456-8466. |
| 5 | JIAN Z, LUO W, JI X. Carbon electrodes for K-ion batteries[J]. J Am Chem Soc, 2015, 137(36): 11566-11569. |
| 6 | PRAMUDITA J C, SEHRAWAT D, GOONETILLEKE D, et al. An initial review of the status of electrode materials for potassium‐ion batteries[J]. Advanced Energy Materials, 2017, 7(24): 1602911. |
| 7 | KIM H, SEO D H, KIM J C, et al. Investigation of potassium storage in layered P3-type K0.5MnO2 cathode[J]. Advanced Materials, 2017, 29(37): 1702480. |
| 8 | NAVEEN N, PARK W B, HAN S C, et al. Reversible K+-insertion/deinsertion and concomitant Na+-redistribution in P'3-Na0.52CrO2 for high-performance potassium-ion battery cathodes[J]. Chemistry of Materials, 2018, 30(6): 2049-2057. |
| 9 | ZHU Y H, YANG X, SUN T, et al. Recent progresses and prospects of cathode materials for non-aqueous potassium-ion batteries[J]. Electrochemical Energy Reviews, 2018, 1(4): 548-566. |
| 10 | WANG B Q, HAN Y, WANG X, et al. Prussian blue analogs for rechargeable batteries[J]. iScience, 2018, 3: 110-133. |
| 11 | ZHANG Z Y, LI M L, GAO Y, et al. Fast potassium storage in hierarchical Ca0.5Ti2(PO4)3@C microspheres enabling high-performance potassium-ion capacitors[J]. Advanced Functional Materials, 2018, 28(36): 1802684. |
| 12 | MASESE T, YOSHII K, KATO M, et al. A high voltage honeycomb layered cathode framework for rechargeable potassium-ion battery: P2-type K2/3Ni1/3Co1/3Te1/3O2[J]. Chemical Communications (Cambridge, England), 2019, 55(7): 985-988. |
| 13 | ZHANG H Y, XI K Y, JIANG K Z, et al. Enhanced K-ion kinetics in a layered cathode for potassium ion batteries[J]. Chemical Communications (Cambridge, England), 2019, 55(55): 7910-7913. |
| 14 | WU X Y, LEONARD D P, JI X L. Emerging non-aqueous potassium-ion batteries: Challenges and opportunities[J]. Chemistry of Materials, 2017, 29(12): 5031-5042. |
| 15 | 任重民, 王斌, 陈帅帅, 等. 层状正极材料力学劣化及改善措施[J]. 储能科学与技术, 2022, 11(3): 948-956 |
| REN Z M, WANG B, CHEN S S, et al. Mechanics-induced degradation on layer-structured cathodes and remedies to address it[J]. Energy Storage Science and Technology, 2022, 11(3): 948-956 | |
| 16 | XUE L G, LI Y T, LÜ X J, et al. Low-cost high-energy potassium cathode[J]. Journal of the American Chemical Society, 2017, 139(6): 2164-2167. |
| 17 | BIE X F, KUBOTA K, HOSAKA T, et al. A novel K-ion battery: Hexacyanoferrate(ii)/graphite cell[J]. Journal of Materials Chemistry A, 2017, 5(9): 4325-4330. |
| 18 | MANTHIRAM A, GOODENOUGH J B. Vanishing of superconductivity at a transition from itinerant-electron to small-polaron conduction in nominal Bi4– xPbx(Sr3Ca)Ca2– xYxCu4O16[J]. Applied Physics Letters, 1988, 53(26): 2695-2697. |
| 19 | NISHIMURA S I, NAKAMURA M, NATSUI R, et al. New lithium iron pyrophosphate as 3.5 V class cathode material for lithium ion battery[J]. Journal of the American Chemical Society, 2010, 132(39): 13596-13597. |
| 20 | RECHAM N, CHOTARD J N, DUPONT L, et al. A 3.6 V lithium-based fluorosulphate insertion positive electrode for lithium-ion batteries[J]. Nature Materials, 2010, 9(1): 68-74. |
| 21 | BARPANDA P, LANDER L, NISHIMURA S I, et al. Polyanionic insertion materials for sodium-ion batteries[J]. Advanced Energy Materials, 2018, 8(17): 1703055. |
| 22 | GOVER R K B, BRYAN A, BURNS P, et al. The electrochemical insertion properties of sodium vanadium fluorophosphate, Na3V2(PO4)2F3[J]. Solid State Ionics, 2006, 177(17/18): 1495-1500. |
| 23 | BIANCHINI M, BRISSET N, FAUTH F, et al. Na3V2(PO4)2F3 revisited: A high-resolution diffraction study[J]. Chemistry of Materials, 2014, 26(14): 4238-4247. |
| 24 | BIANCHINI M, FAUTH F, BRISSET N, et al. Comprehensive investigation of the Na3V2(PO4)2F3-NaV2(PO4)2F3 system by operando high resolution synchrotron X-ray diffraction[J]. Chemistry of Materials, 2015, 27(8): 3009-3020. |
| 25 | MU J J, LIU Z M, LAI Q S, et al. An industrial pathway to emerging presodiation strategies for increasing the reversible ions in sodium-ion batteries and capacitors[J]. Energy Materials, 2022, 2(6): 200043. |
| 26 | BARPANDA P, YE T, NISHIMURA S I, et al. Sodium iron pyrophosphate: A novel 3.0V iron-based cathode for sodium-ion batteries[J]. Electrochemistry Communications, 2012, 24: 116-119. |
| 27 | BARPANDA P, OYAMA G, NISHIMURA S I, et al. A 3.8-V earth-abundant sodium battery electrode[J]. Nature Communications, 2014, 5(1): 1-8. |
| 28 | CHEN M Z, CORTIE D, HU Z, et al. A novel graphene oxide wrapped Na2Fe2(SO4)3/C cathode composite for long life and high energy density sodium-ion batteries[J]. Advanced Energy Materials, 2018, 8(27): 1800944. |
| 29 | CHEN M Z, CHEN L N, HU Z, et al. Carbon-coated Na3.32Fe2.34(P2O7)2 cathode material for high-rate and long-life sodium-ion batteries[J]. Advanced Materials, 2017, 29(21): 1605535. |
| 30 | CHEN M Z, HUA W B, XIAO J, et al. NASICON-type air-stable and all-climate cathode for sodium-ion batteries with low cost and high-power density[J]. Nature Communications, 2019, 10: 1480. |
| 31 | YAN G C, MARIYAPPAN S, ROUSSE G, et al. Higher energy and safer sodium ion batteries via an electrochemically made disordered Na3V2(PO4)2F3 material[J]. Nature Communications, 2019, 10(1): 1-12. |
| 32 | KIM J, YOON G, KIM H, et al. Na3V(PO4)2: A new layered-type cathode material with high water stability and power capability for Na-ion batteries[J]. Chemistry of Materials, 2018, 30(11): 3683-3689. |
| 33 | HE G, KAN W H, MANTHIRAM A. Delithiation/lithiation behaviors of three polymorphs of LiVOPO4[J]. Chemical Communications, 2018, 54(94): 13224-13227. |
| 34 | TAO D, WANG S P, LIU Y C, et al. Lithium vanadium phosphate as cathode material for lithium ion batteries[J]. Ionics, 2015, 21(5): 1201-1239. |
| 35 | FANG Y J, ZHANG J X, XIAO L F, et al. Phosphate framework electrode materials for sodium ion batteries[J]. Advanced Science, 2017, 4(5): 1600392. |
| 36 | HAN J, LI G N, LIU F, et al. Investigation of K3V2(PO4)3/C nanocomposites as high-potential cathode materials for potassium-ion batteries[J]. Chemical Communications, 2017, 53(11): 1805-1808. |
| 37 | WU X L, JIANG L Y, CAO F F, et al. LiFePO4 nanoparticles embedded in a nanoporous carbon matrix: Superior cathode material for electrochemical energy-storage devices[J]. Advanced Materials, 2009, 21(25/26): 2710-2714. |
| 38 | SUN C W, RAJASEKHARA S, GOODENOUGH J B, et al. Monodisperse porous LiFePO4 microspheres for a high power Li-ion battery cathode[J]. Journal of the American Chemical Society, 2011, 133(7): 2132-2135. |
| 39 | WANG X P, NIU C J, MENG J S, et al. Novel K3V2(PO4)3/C bundled nanowires as superior sodium-ion battery electrode with ultrahigh cycling stability[J]. Advanced Energy Materials, 2015, 5(17): 1500716. |
| 40 | GOKULAKRISHNAN N, PANDURANGAN A, SINHA P K. Catalytic wet peroxide oxidation technique for the removal of decontaminating agents ethylenediaminetetraacetic acid and oxalic acid from aqueous solution using efficient Fenton type Fe-MCM-41 mesoporous materials[J]. Industrial & Engineering Chemistry Research, 2009, 48(3): 1556-1561. |
| 41 | NASRI S, MEGDICHE M, GARGOURI M. Electrical conduction and dielectric properties of a newly synthesized single phase: Ag0.4Na0.6FeP2O7[J]. Physica B: Condensed Matter, 2014, 451: 120-127. |
| 42 | LIU H, STROBRIDGE F C, BORKIEWICZ O, et al. Capturing metastable structures during high-rate cycling of LiFePO4 nanoparticle electrodes[J]. Science, 2014, 344(6191): 1480. |
| 43 | ZHANG L, ZHANG B W, WANG C R, et al. Constructing the best symmetric full K-ion battery with the NASICON-type K3V2(PO4)3[J]. Nano Energy, 2019, 60: 432-439. |
| 44 | JENKINS T, ALARCO J A, MACKINNON I D R. Synthesis and characterization of a novel hydrated layered vanadium(III) phosphate phase K3V3(PO4)4 ·H2O: A functional cathode material for potassium-ion batteries[J]. ACS Omega, 2021, 6(3): 1917-1929. |
| 45 | SHIRATSUCHI T, OKADA S, YAMAKI J, et al. FePO4 cathode properties for Li and Na secondary cells[J]. Journal of Power Sources, 2006, 159(1): 268-271. |
| 46 | MATHEW V, KIM S, KANG J, et al. Amorphous iron phosphate: Potential host for various charge carrier ions[J]. NPG Asia Materials, 2014, 6(10): e138. |
| 47 | LIM S Y, LEE J H, KIM S, et al. Lattice water for the enhanced performance of amorphous iron phosphate in sodium-ion batteries[J]. ACS Energy Letters, 2017, 2(5): 998-1004. |
| 48 | HOSAKA T, SHIMAMURA T, KUBOTA K, et al. Polyanionic compounds for potassium-ion batteries[J]. The Chemical Record, 2019, 19(4): 735-745. |
| 49 | SULTANA I, RAHMAN M M, MATETI S, et al. Approaching reactive KFePO4 phase for potassium storage by adopting an advanced design strategy[J]. Batteries & Supercaps, 2020, 3(5): 450-455. |
| 50 | YANG S B, CUI G L, PANG S P, et al. Fabrication of cobalt and cobalt oxide/graphene composites: Towards high-performance anode materials for lithium ion batteries[J]. ChemSusChem, 2010, 3(2): 236-239. |
| 51 | WANG D W, LI F, LIU M, et al. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage[J]. Angewandte Chemie International Edition, 2009, 48(9): 1525. |
| 52 | HAN J, NIU Y B, BAO S J, et al. Nanocubic KTi2(PO4)3 electrodes for potassium-ion batteries[J]. Chemical Communications, 2016, 52(78): 11661-11664. |
| 53 | VORONINA N, JO J H, KONAROV A, et al. KTi2(PO4)3 electrode with a long cycling stability for potassium-ion batteries[J]. Small, 2020, 16(20): 2001090. |
| 54 | PARK W B, HAN S C, PARK C, et al. KVP2O7 as a robust high-energy cathode for potassium-ion batteries: Pinpointed by a full screening of the inorganic registry under specific search conditions[J]. Advanced Energy Materials, 2018, 8(13): 1703099. |
| 55 | HE X D, LIAO J Y, CHEN T, et al. Spray drying derived wrinkled pea-shaped carbon-matrixed KVP2O7 as a cathode material for potassium-ion batteries[J]. Journal of Alloys and Compounds, 2021, 884: 161126. |
| 56 | KEE Y, DIMOV N, STAIKOV A, et al. Insight into the limited electrochemical activity of NaVP2O7[J]. RSC Advances, 2015, 5(80): 64991-64996. |
| 57 | WERNERT R, NGUYEN L H B, PETIT E, et al. Controlling the cathodic potential of KVPO4F through oxygen substitution[J]. Chemistry of Materials, 2022, 34(10): 4523-4535. |
| 58 | HE X D, ZHANG L M, JIANG C H, et al. Elevating cyclability of an advanced KVPO4F cathode via multi-component coating strategy for high-performance potassium-ion batteries[J]. Chemical Engineering Journal, 2022, 433: 134634. |
| 59 | WERNERT R, IADECOLA A, STIEVANO L, et al. Origin of vanadium site sequential oxidation in KxVPO4F1– yOy[J]. Chemistry of Materials, 2023, 35(2): 617-627. |
| 60 | PARK H, KIM M, KANG S, et al. Understanding the effects of oxygen defects on the redox reaction pathways in LiVPO4F by combining ab initio calculations with experiments[J]. Journal of Materials Chemistry A, 2019, 7(21): 13060-13070. |
| 61 | LIAO M C, CAO Y J, LI Z Y, et al. VPO4F fluorophosphates polyanion cathodes for high-voltage proton storage[J]. Angewandte Chemie International Edition, 2022, 61(32): e202206635. |
| 62 | CHIHARA K, KATOGI A, KUBOTA K, et al. KVPO4F and KVOPO4 toward 4 volt-class potassium-ion batteries[J]. Chemical Communications, 2017, 53(37): 5208-5211. |
| 63 | ZHAO J, QIN Y Y, LI L, et al. Pillar strategy enhanced ion transport and structural stability toward ultra-stable KVPO4F cathode for practical potassium-ion batteries[J]. Science Bulletin, 2023, 68(6): 593-602. |
| 64 | XIE C, LIU X W, HAN J, et al. Pomegranate-like KVPO4F@C microspheres as high-volumetric-energy-density cathode for potassium-ion batteries[J]. Small, 2022, 18(51): 2204348. |
| 65 | LIN X Y, HUANG J Q, TAN H, et al. K3V2(PO4)2F3 as a robust cathode for potassium-ion batteries[J]. Energy Storage Materials, 2019, 16: 97-101. |
| 66 | MATTS I L, DACEK S, PIETRZAK T K, et al. Explaining performance-limiting mechanisms in fluorophosphate Na-ion battery cathodes through inactive transition-metal mixing and first-principles mobility calculations[J]. Chemistry of Materials, 2015, 27(17): 6008-6015. |
| 67 | FEDOTOV S S, KHASANOVA N R, SAMARIN A S, et al. AVPO4F (A=Li, K): A 4 V cathode material for high-power rechargeable batteries[J]. Chemistry of Materials, 2016, 28(2): 411-415. |
| 68 | HE H Y, CAO K, GUO S, et al. Flower-like K1+ δVOPO4F crystallite with a layered framework structure as a robust cathode for potassium-ion batteries[J]. Journal of Power Sources, 2023, 564: 232862. |
| 69 | LIU Z M, WANG J, LU B G. Plum pudding model inspired KVPO4F@3DC as high-voltage and hyperstable cathode for potassium ion batteries[J]. Science Bulletin, 2020, 65(15): 1242-1251. |
| 70 | RECHAM N, ROUSSE G, SOUGRATI M T, et al. Preparation and characterization of a stable FeSO4F-based framework for alkali ion insertion electrodes[J]. Chemistry of Materials, 2012, 24(22): 4363-4370. |
| 71 | LANDER L, ROUSSE G, ABAKUMOV A M, et al. Structural, electrochemical and magnetic properties of a novel KFeSO4F polymorph[J]. Journal of Materials Chemistry A, 2015, 3(39): 19754-19764. |
| 72 | LING C, MIZUNO F. Mechanistic study of the electrochemical extraction of K+ from KFeSO4F[J]. Journal of Materials Chemistry A, 2013, 1(27): 8000-8006. |
| 73 | KUMAR P R, HOSAKA T, SHIMAMURA T, et al. Mg-doped KFeSO4F as a high-performance cathode material for potassium-ion batteries[J]. ACS Applied Energy Materials, 2022, 5(11): 13470-13479. |
| 74 | LIAO J Y, HU Q, DU Y C, et al. Robust carbon nanotube-interwoven KFeSO4F microspheres as reliable potassium cathodes[J]. Science Bulletin, 2022, 67(21): 2208-2215. |
| 75 | DONG J M, LIAO J Y, HE X D, et al. Graphene encircled KFeSO4F cathode composite for high energy density potassium-ion batteries[J]. Chemical Communications, 2020, 56(69): 10050-10053. |
| 76 | LIAO J Y, ZHANG X X, ZHANG Q H, et al. Synthesis of KVPO4F/carbon porous single crystalline nanoplates for high-rate potassium-ion batteries[J]. Nano Letters, 2022, 22(12): 4933-4940. |
| 77 | GU Z Y, GUO J Z, SUN Z H, et al. Air/water/temperature-stable cathode for all-climate sodium-ion batteries[J]. Cell Reports Physical Science, 2021, 2(12): 100665. |
| 78 | LIU Y, SUN C, NI Q, et al. Enhanced electrochemical performance of NASICON-type sodium ion cathode based on charge balance theory[J]. Energy Storage Materials, 2022, 53: 881-889. |
| 79 | 赵易飞, 杨振东, 李凤, 等. 氮掺杂碳包覆Na3V2(PO4)2F3钠离子电池正极材料的制备与性能[J]. 储能科学与技术, 2022, 11(6)1883-1891 |
| ZHAO Y F, YANG Z D, LI F, et al. Nitrogen-doped carbon-coated Na3V2(PO4)2F3 cathode materials for sodium-ion batteries: Preparation and electrochemical performance[J]. Energy Storage Science and Technology, 2022, 11(6)1883-1891 | |
| 80 | FEDOTOV S S, SAMARIN A S, NIKITINA V A, et al. Reversible facile Rb+ and K+ ions de/insertion in a KTiOPO4-type RbVPO4F cathode material[J]. Journal of Materials Chemistry A, 2018, 6(29): 14420-14430. |
| [1] | Chang LIU, Junjun YAO, Ying SUN, Daming FENG, Hongjie ZHENG, Tianyi MA. Coordinated preparation of pitch-based carbon microspheres for anode materials of high-rate potassium-ion batteries by emulsification/oxidation [J]. Energy Storage Science and Technology, 2023, 12(5): 1444-1452. |
| [2] | Jintao LI, Yue MU, Jing WANG, Jingyi QIU, Hai MING. Investigation of the structural evolution and interface behavior in cathode materials for Li-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(5): 1636-1654. |
| [3] | Yuwen ZHAO, Huan YANG, Junpeng GUO, Yi ZHANG, Qi SUN, Zhijia ZHANG. Application of magnetic metal elements in sodium ion batteries [J]. Energy Storage Science and Technology, 2023, 12(5): 1332-1347. |
| [4] | Zhixiang CHENG, Wei CAO, Bo HU, Yunfang CHENG, Xin LI, Lihua JIANG, Kaiqiang JIN, Qingsong WANG. Thermal runaway and explosion propagation characteristics of large lithium iron phosphate battery for energy storage station [J]. Energy Storage Science and Technology, 2023, 12(3): 923-933. |
| [5] | Fangfang WANG, Xiangming FENG, Guangjin ZHAO, Dawei XIA, Yuxia HU, Weihua CHEN. Identification of retired power lithium-ion batteries of chemical systems by electrochemical impedance spectroscopy [J]. Energy Storage Science and Technology, 2023, 12(2): 609-614. |
| [6] | Kai ZHANG, Youlong XU. Research progress and development trend of sodium manganate cathode materials for sodium ion batteries [J]. Energy Storage Science and Technology, 2023, 12(1): 86-110. |
| [7] | Mengyang ZU, Meng ZHANG, Zikun LI, Ling HUANG. Cycle performance and degradation mechanism of Ni-Rich NCA, NCM, and NCMA [J]. Energy Storage Science and Technology, 2023, 12(1): 51-60. |
| [8] | Shaocong WANG, Wei LI, Ruiqin HUANG, Yifei GUO, Zheng LIU. Progress of the Jahn-Teller effect suppression method for manganese-based sodium-ion battery cathode materials [J]. Energy Storage Science and Technology, 2023, 12(1): 139-149. |
| [9] | Qunbin ZHANG, Tao DONG, Jingjing LI, Yanxia LIU, Haitao ZHANG. Research progress on the recovery and high-value utilization of spent electrolyte from lithium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(9): 2798-2810. |
| [10] | Laifeng SONG, Wenxin MEI, Zhuangzhuang JIA, Qingsong WANG. Analysis of thermal runaway characteristics of 280 Ah large LiFePO4 battery under adiabatic conditions [J]. Energy Storage Science and Technology, 2022, 11(8): 2411-2417. |
| [11] | Shuang SHI, Nawei LYU, Jingxuan MA, Kangyong YIN, Lei SUN, Ning ZHANG, Yang JIN. Comparative study on the effectiveness of different types of gas detection on the overcharge safety early warning of a lithium iron phosphate battery energy storage compartment [J]. Energy Storage Science and Technology, 2022, 11(8): 2452-2462. |
| [12] | Xingchu CAI, Yiming ZHU, Keshang JIANG, Xufeng XI, Yicao ZHANG, Weishi LIN. Application on perfluoro-2-methyl-3-pentanone in lithium battery premade energy storage cabin [J]. Energy Storage Science and Technology, 2022, 11(8): 2497-2504. |
| [13] | Xiaosa ZHANG, Hongyuan WANG, Zhenbiao LI, Zhimei XIA. New process of sulfated roasting-water leaching for treating electrode material of spent lithium iron phosphate batteries [J]. Energy Storage Science and Technology, 2022, 11(7): 2066-2074. |
| [14] | ZHOU Wei, FU Dongju, LIU Weifeng, CHEN Jianjun, HU Zhao, ZENG Xierong. Research progress on recycling technology of waste lithium iron phosphate power battery [J]. Energy Storage Science and Technology, 2022, 11(6): 1854-1864. |
| [15] | Lei LI, Zhao LI, Dan JI, Huichang NIU. Overcharge induced thermal runaway behaviors of pouch-type lithium-ion batteries with LFP and NCM cathodes: the differences and reasons [J]. Energy Storage Science and Technology, 2022, 11(5): 1419-1427. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
