Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (8): 2504-2525.doi: 10.19799/j.cnki.2095-4239.2023.0237
• Energy Storage Materials and Devices • Previous Articles Next Articles
Zhihao LIU1( ), Tong DU2, Ruirui LI3, Tao DENG1,2,3(
), Tong DU2, Ruirui LI3, Tao DENG1,2,3( )
)
Received:2023-04-17
Revised:2023-04-21
Online:2023-08-05
Published:2023-08-23
Contact:
Tao DENG
E-mail:liuzhihao_1212@126.com;d82t722@cqjtu.edu.cn
CLC Number:
Zhihao LIU, Tong DU, Ruirui LI, Tao DENG. Developments of wide temperature range, high voltage and safe EC-free electrolytes[J]. Energy Storage Science and Technology, 2023, 12(8): 2504-2525.
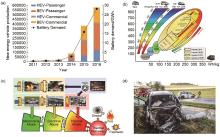
Fig. 4
Current status of electric vehicle development and thermal runaway accidents. (a) Demand for lithium-ion batteries for electric vehicles; (b) Roadmap for lithium-ion batteries for electric vehicles; (c) Lithium-ion battery accident[11]; (d) Schematic diagram of the fire and combustion of an electric vehicle in Germany"
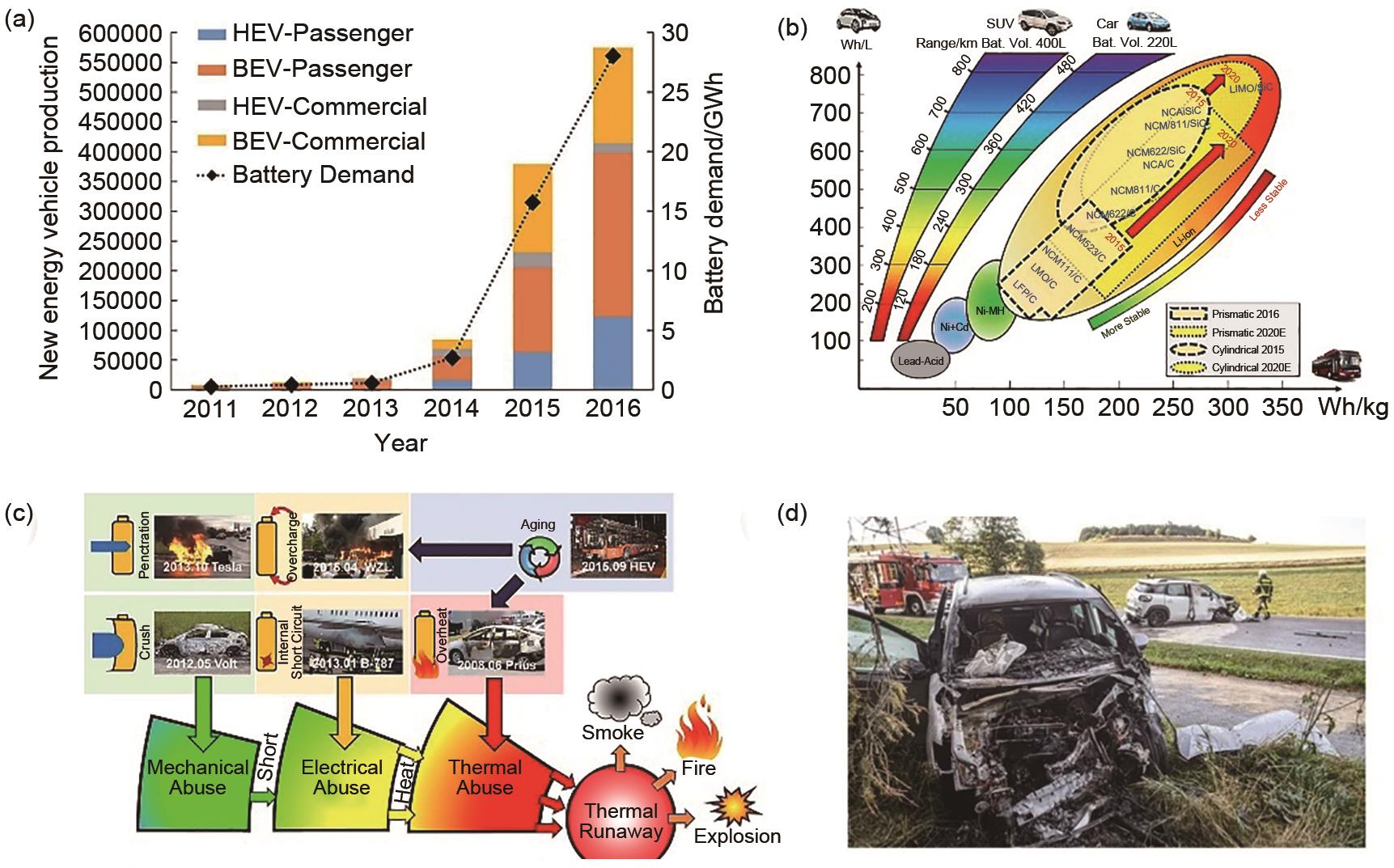
Fig. 5
Thermal runaway reaction mechanism and chronological analysis (a) Internal characteristics of different stages of battery thermal runaway[30]; (b) Schematic diagram of chemical crosstalk between positive and negative electrodes[35]; (c) Schematic of the causes of LIB fire accidents[10];(d) Thermal runaway mechanism graph of 38 Ah battery[46]"
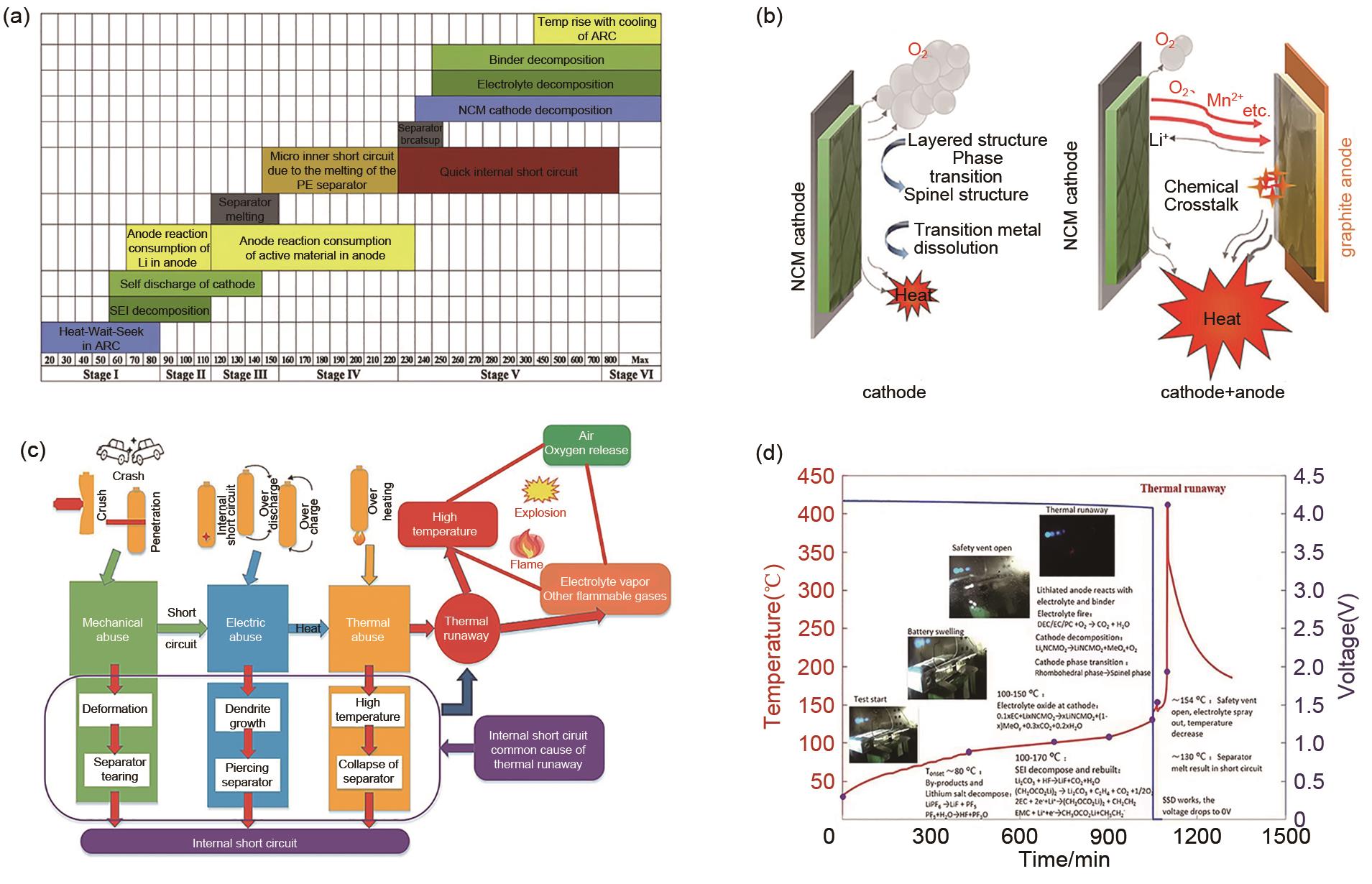

Fig. 6
EC-free based electrolyte lithium battery thermal safety characteristics test (a) Comparison of thermal runaway characteristics of conventional electrolytes and (b) perfluorinated electrolytes[28]; The electrical performance of SL-based batteries and conventional batteries at (c) 25 ℃ and (d) 60 ℃/70 ℃ [68]; (e) Characteristic stages of the heating test process for double-salt electrolyte batteries and (f) voltage and internal temperature changes[22]"
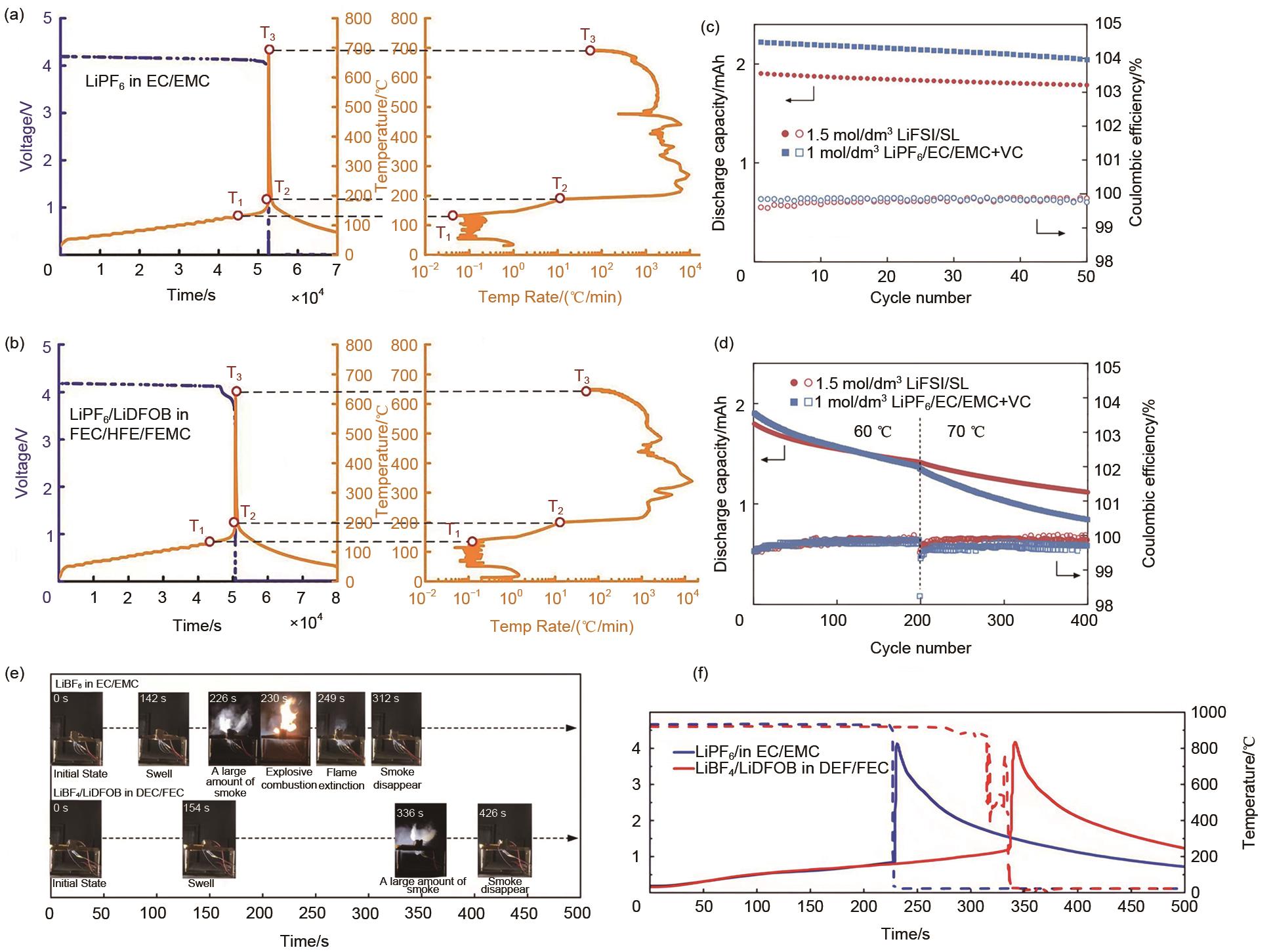
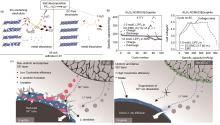
Fig. 8
High voltage Failure Mechanisms. (a) Schematic illustration of the proposed degradation pathways for Ni-rich cathodes with EC-containing and EC-free electrolytes; (b) Mechanism of rollover fading of EC-based electrolyte[73]; (c) Schematics showing the effect of the suppressed nickel ions dissolution on the morphology of the anode SEI layer[40]"
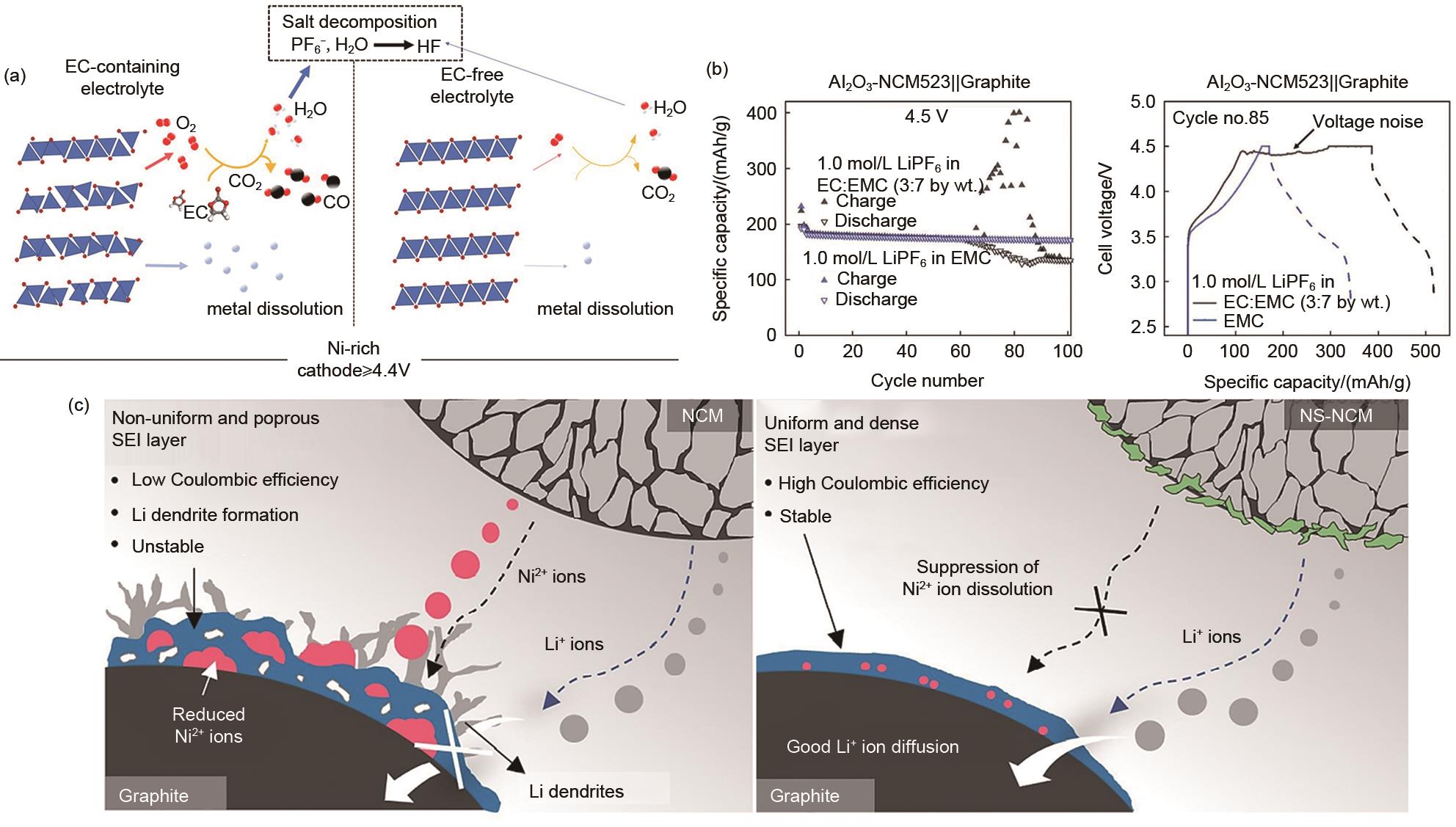
Table 1
Different EC-free electrolyte formulations and properties"
| 正极|负极 | 电解液 | 性能 | 机理 | 参考文献 |
|---|---|---|---|---|
| 石墨/金属锂 | 3.2 mol/dm3 LiTFSA/DMSO 1∶2 | 抑制了溶剂插层效应,放电容量达300 mAh/g | 调节溶剂化结构 | [ |
| NCM111|石墨 | 1 mol/L LiPF6 in MP∶VC (95∶5,质量比) | 提高离子电导率和低温性能。在-14 ℃下,以5 C的放电倍率循环20次,仍可保持约220 MWh的容量 | 低黏度、低熔点溶剂形成SEI膜 | [ |
| NCM442|石墨 | 1 mol/L LiPF6 in EMC:FEC (95∶5,质量比) | 在不同含量的添加剂中具有最佳的循环性能和最小的阻抗增长 | 找到能够有效钝化石墨负极的最佳添加剂的含量 | [ |
| NCM111|石墨 | 1 mol/L LiFSI in ADN:DMC 1∶1 | 在20 ℃时达到5.8 ms/cm的电导率 | 使用线性溶剂来提高离子传导性 | [ |
| NCM442|石墨 | 1 mol/L LiPF6 in EMC+2% SA | 改善60 ℃下的储存和循环性能,限制气体释放 | CEI膜的形成抑制了界面上的副反应 | [ |
| LiCoO2|石墨 | 1.5 mol/dm3 LiFSI in SL | 提高循环性能和高温稳定性 | LiFSI和SL的热稳定性优于传统电解液 | [ |
| NMC442|石墨 | 1 mol/L LiPF6 in DEC/FEC (1∶1,质量比) | 在500次循环后,与使用EC-DEC-FEC(45∶45∶10质量比)的电池相比,使用碳酸二乙酯(DEC)-FEC(1∶1质量比)的电池容量保持率增加了88% | 调节反应途径以减少不利于锂迁移的聚烯烃的生成 | [ |
| NCM532|石墨 | 1 mol/L LiPF6 in EMC∶FEC (95∶5,质量比) | 线性碳酸盐,如EMC和DMC比环状碳酸盐EC具有更高的氧化稳定性。 | 机理待解析 | [ |
| LiNi0.94Co0.06O2|石墨 | 1.0 mol/L LiFSI-0.5 mol/L LiPF6/EMC+3% VC | 抑制自产热,提高热稳定性 | 添加适当浓度的添加剂以提高导电性 | [ |
| LiCoO2|石墨 | 1.0 mol/L LiDFOB ADN/DMC (1∶1,质量比)+2% FEC | 降低界面阻抗,提高负极稳定性 | 形成富含LiF的高氧化稳定性的SEI膜 | [ |
| LCO|石墨 | LiFSI/MA/FE(1∶1.5∶2,摩尔比) | 在-50 ℃时可提供76%的室温容量 | 使用低熔点、低黏度的溶剂 | [ |
| NMA90|石墨 | 1.5 mol/L LiPF6 in EMC with FEC/TDI(20∶1,质量比) | 具有高离子传导性、低界面阻抗和良好钝化能力的CEI膜 | 溶剂与添加剂协同工作 | [ |
| NCM532|石墨 | 1.0 mol/L LiPF6 in EMC with 1% LiDFP | 电解液抑制过渡金属的溶解,改善高压电性能 | 降解的LiPF6有效地捕获了溶解的过渡金属,并抑制了有害串扰的影响 | [ |
| NCM811|石墨 | 0.6 mol/L LiBF4 and 0.6 mol/L LiDFOB in DEC/FEC, 2∶1, 体积比) | 将电池热失控(TR)的触发温度提高31.1 ℃,热失控最高温度降低76.1 ℃ | 清除了易燃的EC成分,抑制了电解液分解等副反应 | [ |
| NMC811|石墨 | 0.8 mol/L LiFSI-0.1 mol/L LiTFSI-0.6 mol/L LiPF6 in EMC | 在4.5 V的条件下,经过200次循环,保持大约82.1%的容量 | 形成稳定CEI膜,在高工作电位下有效稳定NMC811界面 | [ |
| NMC811|石墨 | 1.0 mol/L LiPF6 in PC/NMP/DMC (2∶1∶3,体积比) | 提高PC和石墨负极的兼容性 | 将NMP溶剂分子引入Li+的溶解层,从而降低溶剂化结构中PC的浓度 | [ |
| NMC811|石墨 | 1.0 mol/L LiPF6-0.2 mol/L LiDFOB in FEC/ EMC/TFA (1∶3∶1,体积比) | 在4.6 V电压下具有卓越的循环性(在200次循环中保持81.4%,0.5 C)和倍率性能(5 C时放电容量为154.5 mAh/g) | 不同溶剂和锂盐的协同分解 | [ |
| NMC811|石墨 | 1.0 mol/L LiPF6-0.02 mol/L LiDFOB in FEC/HFE/FEMC (2∶2∶6,体积比) | 与传统电解液相比,热失控的触发温度提高了12.5 ℃,热失控的最高温度降低了41.2 ℃ | 在正极表面形成了一个密集而均匀的含有F和B无机化合物的界面膜 | [ |
| NMC622|石墨 | 1.0 mol/L LiPF6 in PC/TFA (3∶7,体积比)+2% FEC | 与传统电解液相比,这种电解液的热释放量减少到745.2 J/g,显示出良好的热稳定性 | 用含氟的电解液去除自由基 | [ |
| LNMO|石墨 | 1.0 mol/L LiPF6 in FEC/F-EMC/ F-EPE(3∶5∶2体积比) | 在55 ℃下循环250次后,CE约为99.5%,容量保持率为50% | 通过加入含氟电解液抑制电解液高温条件下分解 | [ |
| NMC442|石墨 | 1.5 mol/L LiPF6 in EMC +2% VC+1% TTSPi | 有效地减缓气体的产生,减少容量衰减,降低阻抗,改善循环性能 | 寻找最佳的盐浓度 | [ |
| NMC811|石墨 | 1.0 mol/L LiFSI /FEC∶TEP∶BTFE(10∶20∶70,体积比) | 将热失控的触发温度提高47.3 ℃,将热失控的最高温度降低71.8 ℃ | 在电极和电解液界面之间形成无机 界面膜 | [ |
| NMC811|石墨 | 1.4 mol/L LiFSI in DMC/VC/TTE (2∶0.2∶3,摩尔比) | 在60 ℃的条件下,容量仍然可以保持50次循环,而且减缓界面阻抗和溶液阻抗的增加 | 在正负电极上形成一层薄而稳定的无机界面膜 | [ |
| NMC811|石墨 | 1 mol/L LiFSI in PC/FB (1∶5,摩尔比) | 抑制了由PC和Li+共嵌入引起的石墨剥落,并实现了宽温度工作范围(-90~90 ℃) | FB削弱了Li+和PC之间的相互作用,并在负极表面形成了一层SEI保护膜 | [ |
| NMC811|石墨 | 2 mol/L LiFSI:EMC:TTE (2∶3.3∶3.3,摩尔比) | 在-40 ℃时保持78%的室温容量 | 削弱了溶剂分子和Li+之间的离子 偶极作用 | [ |
| NMC811|石墨 | 1 mol/L LiPF6 in FEC/AN (7∶3,体积比) | 使得811|Gr体系电池可以在8 C条件下运行,容量保持率是使用EC基电解液的3倍 | 采用了一种溶剂辅助的跳跃机制来减少Li+的解溶障碍 | [ |
| Li2CoPO4F|石墨 | 5.4 mol/L LiBF4 in PC/FEC (1∶1,摩尔比) | 在4.8 V电压下循环600次后容量保持率约为70% | 利用高浓度的电解液调节溶剂结构 | [ |
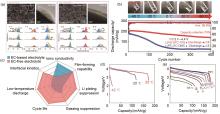
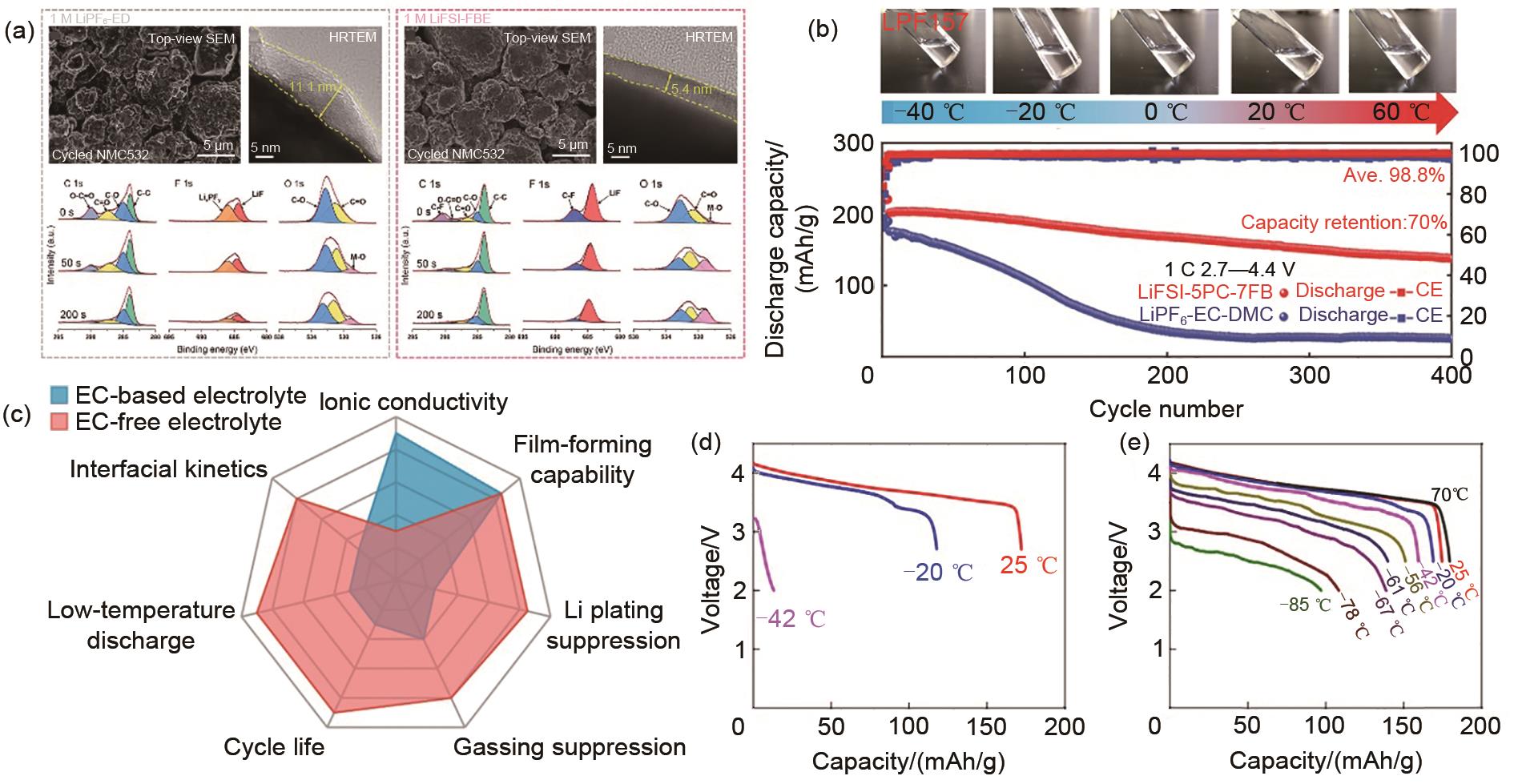
Fig. 12
Summary of low-temperature electrolyte performance (a) Comparison of the characterization of two electrolytes in the NMC532 system after 20 cycles[124]; (b) Low-temperature cycling electrical properties of PC-based electrolytes[113]; (c) Comparison network diagram of EC electrolyte and EC-free electrolyte performance[19]; (d) Discharge curves of conventional electrolytes and (e) D2-based electrolytes[93]"
| 1 | TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[M]//Materials for Sustainable Energy, Co-Published with Macmillan Publishers Ltd, UK, 2010: 171-179. |
| 2 | LU D B, LIN S X, HU S W, et al. Thermal behavior and failure mechanism of large format lithium-ion battery[J]. Journal of Solid State Electrochemistry, 2021, 25(1): 315-325. |
| 3 | CRABTREE G. Perspective: The energy-storage revolution[J]. Nature, 2015, 526(7575): doi: 10.1038/526S92a. |
| 4 | ZHOU K, XIE Q, LI B H, et al. An in-depth understanding of the effect of aluminum doping in high-nickel cathodes for lithium-ion batteries[J]. Energy Storage Materials, 2021, 34: 229-240. |
| 5 | LI W D, ERICKSON E M, MANTHIRAM A. High-nickel layered oxide cathodes for lithium-based automotive batteries[J]. Nature Energy, 2020, 5(1): 26-34. |
| 6 | FAN X L, WANG C S. High-voltage liquid electrolytes for Li batteries: Progress and perspectives[J]. Chemical Society Reviews, 2021, 50(18): 10486-10566. |
| 7 | 詹元杰, 武怿达, 马晓威, 等. 基于碳酸酯基电解液的4.5 V电池[J]. 储能科学与技术, 2020, 9(2): 319-330. |
| ZHAN Y J, WU Y D, MA X W, et al. 4.5 V Li-ion battery with a carbonate ester-based electrolyte[J]. Energy Storage Science and Technology, 2020, 9(2): 319-330. | |
| 8 | JIA H, XU W. Electrolytes for high-voltage lithium batteries[J]. Trends in Chemistry, 2022, 4(7): 627-642. |
| 9 | 陈晓霞, 刘凯, 王保国. 高安全性锂电池电解液研究与应用[J]. 储能科学与技术, 2020, 9(2): 583-592. |
| CHEN X X, LIU K, WANG B G. Research on high-safety electrolytes and their application in lithium-ion batteries[J]. Energy Storage Science and Technology, 2020, 9(2): 583-592. | |
| 10 | FENG X N, ZHENG S Q, REN D S, et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database[J]. Applied Energy, 2019, 246: 53-64. |
| 11 | FENG X N, OUYANG M G, LIU X, et al. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review[J]. Energy Storage Materials, 2018, 10: 246-267. |
| 12 | LIN W, ZHU M Y, FAN Y, et al. Low temperature lithium-ion batteries electrolytes: Rational design, advancements, and future perspectives[J]. Journal of Alloys and Compounds, 2022, 905: doi: 10.1016/j.jallcom.2022.164163. |
| 13 | FAN J A, TAN S. Studies on charging lithium-ion cells at low temperatures[J]. Journal of the Electrochemical Society, 2006, 153(6): doi: 10.1149/1.2190029. |
| 14 | GUPTA A, MANTHIRAM A. Designing advanced lithium-based batteries for low-temperature conditions[J]. Advanced Energy Materials, 2020, 10(38): doi: 10.1002/aenm.202001972. |
| 15 | ZHANG N, DENG T, ZHANG S Q, et al. Critical review on low-temperature Li-ion/metal batteries[J]. Advanced Materials, 2022, 34(15): doi: 10.1002/adma.202107899. |
| 16 | FONG R, VON SACKEN U, DAHN J R. Studies of lithium intercalation into carbons using nonaqueous electrochemical cells[J]. Journal of the Electrochemical Society, 1990, 137(7): 2009-2013. |
| 17 | PELED E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems-The solid electrolyte interphase model[J]. Journal of the Electrochemical Society, 1979, 126(12): 2047-2051. |
| 18 | PELED E, MENKIN S. Review—SEI: Past, present and future[J]. Journal of the Electrochemical Society, 2017, 164(7): doi: 10.1149/2.1441707jes. |
| 19 | YAO Y X, YAO N, ZHOU X R, et al. Ethylene-carbonate-free electrolytes for rechargeable Li-ion pouch cells at sub-freezing temperatures[J]. Advanced Materials, 2022, 34(45): doi: 10.1002/adma.202206448. |
| 20 | LI Q, LIU G, CHENG H R, et al. Low-temperature electrolyte design for lithium-ion batteries: Prospect and challenges[J]. Chemistry-A European Journal, 2021, 27(64): 15842-15865. |
| 21 | ZHAO Q, WU Y, YANG Z W, et al. A fluorinated electrolyte stabilizing high-voltage graphite/NCM811 batteries with an inorganic-rich electrode-electrolyte interface[J]. Chemical Engineering Journal, 2022, 440: doi: 10.1016/j.cej.2022.135939. |
| 22 | WU C J, WU Y, XU X D, et al. Synergistic dual-salt electrolyte for safe and high-voltage LiNi0.8Co0.1Mn0.1O2//graphite pouch cells[J]. ACS Applied Materials & Interfaces, 2022, 14(8): 10467-10477. |
| 23 | AURBACH D, EIN-ELI Y, MARKOVSKY B, et al. The study of electrolyte solutions based on ethylene and diethyl carbonates for rechargeable Li batteries: II. graphite electrodes[J]. Journal of the Electrochemical Society, 1995, 142(9): 2882-2890. |
| 24 | SMART M C, RATNAKUMAR B V, SURAMPUDI S. Use of organic esters as cosolvents in electrolytes for lithium-ion batteries with improved low temperature performance[J]. Journal of the Electrochemical Society, 2002, 149(4): doi: 10.1149/1.1453407. |
| 25 | SMART M C, RATNAKUMAR B V, SURAMPUDI S. Electrolytes for low-temperature lithium batteries based on ternary mixtures of aliphatic carbonates[J]. Journal of the Electrochemical Society, 1999, 146(2): 486-492. |
| 26 | YAMADA Y, USUI K, CHIANG C H, et al. General observation of lithium intercalation into graphite in ethylene-carbonate-free superconcentrated electrolytes[J]. ACS Applied Materials & Interfaces, 2014, 6(14): 10892-10899. |
| 27 | WU Y, REN D S, LIU X A, et al. High-voltage and high-safety practical lithium batteries with ethylene carbonate-free electrolyte[J]. Advanced Energy Materials, 2021, 11(47): doi: 10.1002/aenm. 202102299. |
| 28 | WU C J, WU Y, YANG X Y, et al. Thermal runaway suppression of high-energy lithium-ion batteries by designing the stable interphase[J]. Journal of the Electrochemical Society, 2021, 168(9): doi: 10.1149/1945-7111/ac285f. |
| 29 | LOGAN E R, TONITA E M, GERING K L, et al. A study of the transport properties of ethylene carbonate-free Li electrolytes[J]. Journal of the Electrochemical Society, 2018, 165(3): A705-A716. |
| 30 | REN D S, FENG X N, LIU L S, et al. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition[J]. Energy Storage Materials, 2021, 34: 563-573. |
| 31 | DENG K R, ZENG Q G, WANG D, et al. Nonflammable organic electrolytes for high-safety lithium-ion batteries[J]. Energy Storage Materials, 2020, 32: 425-447. |
| 32 | BALAKRISHNAN P G, RAMESH R, PREM KUMAR T. Safety mechanisms in lithium-ion batteries[J]. Journal of Power Sources, 2006, 155(2): 401-414. |
| 33 | HESS S, WOHLFAHRT-MEHRENS M, WACHTLER M. Flammability of Li-ion battery electrolytes: Flash point and self-extinguishing time measurements[J]. Journal of the Electrochemical Society, 2015, 162(2): A3084-A3097. |
| 34 | FENG X N, FANG M, HE X M, et al. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry[J]. Journal of Power Sources, 2014, 255: 294-301. |
| 35 | LIU X, REN D S, HSU H, et al. Thermal runaway of lithium-ion batteries without internal short circuit[J]. Joule, 2018, 2(10): 2047-2064. |
| 36 | SINHA N N, SMITH A J, BURNS J C, et al. The use of elevated temperature storage experiments to learn about parasitic reactions in wound LiCoO2/Graphite cells[J]. Journal of the Electrochemical Society, 2011, 158(11): A1194. |
| 37 | LI Y, LIU X, WANG L, et al. Thermal runaway mechanism of lithium-ion battery with LiNi0.8Mn0.1Co0.1O2 cathode materials[J]. Nano Energy, 2021, 85: doi: 10.1016/j.nanoen.2021.105878. |
| 38 | SHARIFI-ASL S, SOTO F A, NIE A M, et al. Facet-dependent thermal instability in LiCoO2[J]. Nano Letters, 2017, 17(4): 2165-2171. |
| 39 | WANG Y, FENG X N, PENG Y, et al. Reductive gas manipulation at early self-heating stage enables controllable battery thermal failure[J]. Joule, 2022, 6(12): 2810-2820. |
| 40 | KIM J, MA H, CHA H, et al. A highly stabilized nickel-rich cathode material by nanoscale epitaxy control for high-energy lithium-ion batteries[J]. Energy & Environmental Science, 2018, 11(6): 1449-1459. |
| 41 | OH P, OH S M, LI W D, et al. High-performance heterostructured cathodes for lithium-ion batteries with a Ni-rich layered oxide core and a Li-rich layered oxide shell[J]. Advanced Science, 2016, 3(11): doi: 10.1002/advs.201600184. |
| 42 | TEUFL T, PRITZL D, KRIEG P, et al. Operating EC-based electrolytes with Li- and Mn-rich NCMs: The role of O2-release on the choice of the cyclic carbonate[J]. Journal of the Electrochemical Society, 2020, 167(11): doi: 10.1149/1945-7111/ab9e7f. |
| 43 | GALUSHKIN N Е, YAZVINSKAYA N N, GALUSHKIN D N. Mechanism of gases generation during lithium-ion batteries cycling[J]. Journal of the Electrochemical Society, 2019, 166(6): A897-A908. |
| 44 | BELHAROUAK I, KOENIG G M Jr, TAN T, et al. Performance degradation and gassing of Li4Ti5O12/LiMn2O4 lithium-ion cells[J]. Journal of the Electrochemical Society, 2012, 159(8): A1165-A1170. |
| 45 | WU Y, LIU X, WANG L, et al. Development of cathode-electrolyte-interphase for safer lithium batteries[J]. Energy Storage Materials, 2021, 37: 77-86. |
| 46 | XIA J, GLAZIER S L, PETIBON R, et al. Improving linear alkyl carbonate electrolytes with electrolyte additives[J]. Journal of the Electrochemical Society, 2017, 164(6): A1239-A1250. |
| 47 | XIA J, LIU Q Q, HEBERT A, et al. Succinic anhydride as an enabler in ethylene carbonate-free linear alkyl carbonate electrolytes for high voltage Li-ion cells[J]. Journal of the Electrochemical Society, 2017, 164(6): A1268-A1273. |
| 48 | JIANG F N, CHENG X B, YANG S J, et al. Thermoresponsive electrolytes for safe lithium-metal batteries[J]. Advanced Materials, 2023, 35(12): doi: 10.1002/adma.202209114. |
| 49 | ZHANG J N, WU H, DU X F, et al. Smart deep eutectic electrolyte enabling thermally induced shutdown toward high-safety lithium metal batteries[J]. Advanced Energy Materials, 2023, 13(3): doi: 10.1002/aenm.202202529. |
| 50 | EHTESHAMI N, IBING L, STOLZ L, et al. Ethylene carbonate-free electrolytes for Li-ion battery: Study of the solid electrolyte interphases formed on graphite anodes[J]. Journal of Power Sources, 2020, 451: doi: 10.1016/j.jpowsour.2020.227804. |
| 51 | KRUEGER S, KLOEPSCH R, LI J, et al. How do reactions at the anode/electrolyte interface determine the cathode performance in lithium-ion batteries?[J]. Journal of the Electrochemical Society, 2013, 160(4): A542-A548. |
| 52 | VETTER J, NOVÁK P, WAGNER M R, et al. Ageing mechanisms in lithium-ion batteries[J]. Journal of Power Sources, 2005, 147(1/2): 269-281. |
| 53 | ZHENG Y J, SHI Z H, REN D S, et al. In-depth investigation of the exothermic reactions between lithiated graphite and electrolyte in lithium-ion battery[J]. Journal of Energy Chemistry, 2022, 69: 593-600. |
| 54 | NAGASUBRAMANIAN G, FENTON K. Reducing Li-ion safety hazards through use of non-flammable solvents and recent work at Sandia National Laboratories[J]. Electrochimica Acta, 2013, 101: 3-10. |
| 55 | CHEN S Y, WANG Z X, ZHAO H L, et al. A novel flame retardant and film-forming electrolyte additive for lithium ion batteries[J]. Journal of Power Sources, 2009, 187(1): 229-232. |
| 56 | MCMILLAN R, SLEGR H, SHU Z X, et al. Fluoroethylene carbonate electrolyte and its use in lithium ion batteries with graphite anodes[J]. Journal of Power Sources, 1999, 81: 20-26. |
| 57 | CHAWLA N, BHARTI N, SINGH S. Recent advances in non-flammable electrolytes for safer lithium-ion batteries[J]. Batteries, 2019, 5(1): 19. |
| 58 | ZOU Y G, MA Z, LIU G, et al. Non-flammable electrolyte enables high-voltage and wide-temperature lithium-ion batteries with fast charging[J]. Angewandte Chemie, 2023, 135(8): doi: 10.1002/anie.202216189. |
| 59 | YAO K, ZHENG J P, LIANG R. Ethylene carbonate-free fluoroethylene car bonate-based electrolyte works better for freestanding Si-based composite paper anodes for Li-ion batteries[J]. Journal of Power Sources, 2018, 381: 164-170. |
| 60 | XU K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries[J]. Chemical Reviews, 2004, 104(10): 4303-4417. |
| 61 | XU K. Electrolytes and interphases in Li-ion batteries and beyond[J]. Chemical Reviews, 2014, 114(23): 11503-11618. |
| 62 | ZHANG S S. A review on electrolyte additives for lithium-ion batteries[J]. Journal of Power Sources, 2006, 162(2): 1379-1394. |
| 63 | CAMPION C L, LI W T, LUCHT B L. Thermal decomposition of LiPF6-based electrolytes for lithium-ion batteries[J]. Journal of the Electrochemical Society, 2005, 152(12): A2327. |
| 64 | YANG H, ZHUANG G V, ROSS P N. Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6[J]. Journal of Power Sources, 2006, 161(1): 573-579. |
| 65 | XIONG D J, HYNES T, DAHN J R. Dramatic effects of low salt concentrations on Li-ion cells containing EC-free electrolytes[J]. Journal of the Electrochemical Society, 2017, 164(9): A2089-A2100. |
| 66 | AN K, TRAN Y H T, KWAK S, et al. Design of fire-resistant liquid electrolyte formulation for safe and long-cycled lithium-ion batteries[J]. Advanced Functional Materials, 2021, 31(48): doi: 10.1002/adfm.202106102. |
| 67 | REN X D, GAO P Y, ZOU L F, et al. Role of inner solvation sheath within salt-solvent complexes in tailoring electrode/electrolyte interphases for lithium metal batteries[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(46): 28603-28613. |
| 68 | HIRATA K, MORITA Y, KAWASE T, et al. Electrochemical performance of an ethylene carbonate-free electrolyte based on lithium bis (fluorosulfonyl) imide and sulfolane[J]. Journal of Power Sources, 2018, 395: 163-170. |
| 69 | SUN X G, ANGELL C A. New sulfone electrolytes for rechargeable lithium batteries. part I. oligoether-containing sulfones[J]. Electrochemistry Communications, 2005, 7(3): 261-266. |
| 70 | 李放放, 陈仕谋. 高压锂离子电池电解液添加剂研究进展[J]. 储能科学与技术, 2016, 5(4): 436-442. |
| LI F F, CHEN S M. Research progress on electrolyte additives for high voltage lithium-ion batteries[J]. Energy Storage Science and Technology, 2016, 5(4): 436-442. | |
| 71 | YANG L, RAVDEL B, LUCHT B L. Electrolyte reactions with the surface of high voltage LiNi0.5Mn1.5O4 cathodes for lithium-ion batteries[J]. Electrochemical and Solid-State Letters, 2010, 13(8): A95. |
| 72 | GUO K L, QI S H, WANG H P, et al. High-voltage electrolyte chemistry for lithium batteries[J]. Small Science, 2022, 2(5): doi: 10.1002/smsc.202100107. |
| 73 | LASZCZYNSKI N, SOLCHENBACH S, GASTEIGER H A, et al. Understanding electrolyte decomposition of graphite/NCM811 cells at elevated operating voltage[J]. Journal of the Electrochemical Society, 2019, 166(10): A1853-A1859. |
| 74 | ZHANG X H, ZOU L F, XU Y B, et al. Electrolytes: Advanced electrolytes for fast-charging high-voltage lithium-ion batteries in wide-temperature range (adv. energy mater. 22/2020)[J]. Advanced Energy Materials, 2020, 10(22): doi: 10.1002/aenm. 202070098. |
| 75 | KUNDURACI M, AMATUCCI G G. Synthesis and characterization of nanostructured 4.7 V LixMn1.5Ni0.5O4 spinels for high-power lithium-ion batteries[J]. Journal of the Electrochemical Society, 2006, 153(7): A1345. |
| 76 | CHOI N S, HAN J G, HA S Y, et al. Recent advances in the electrolytes for interfacial stability of high-voltage cathodes in lithium-ion batteries[J]. RSC Advances, 2015, 5(4): 2732-2748. |
| 77 | LI W D, DOLOCAN A, OH P, et al. Dynamic behaviour of interphases and its implication on high-energy-density cathode materials in lithium-ion batteries[J]. Nature Communications, 2017, 8(1): 1-10. |
| 78 | PIECZONKA N P W, LIU Z Y, LU P, et al. Understanding transition-metal dissolution behavior in LiNi0.5Mn1.5O4 high-voltage spinel for lithium ion batteries[J]. The Journal of Physical Chemistry C, 2013, 117(31): 15947-15957. |
| 79 | EDSTRÖM K, GUSTAFSSON T, THOMAS J O. The cathode-electrolyte interface in the Li-ion battery[J]. Electrochimica Acta, 2004, 50(2/3): 397-403. |
| 80 | MARKEVICH E, SHARABI R, GOTTLIEB H, et al. Reasons for capacity fading of LiCoPO4 cathodes in LiPF6 containing electrolyte solutions[J]. Electrochemistry Communications, 2012, 15(1): 22-25. |
| 81 | SLOOP S E, KERR J B, KINOSHITA K. The role of Li-ion battery electrolyte reactivity in performance decline and self-discharge[J]. Journal of Power Sources, 2003, 119: 330-337. |
| 82 | MANTHIRAM A. A reflection on lithium-ion battery cathode chemistry[J]. Nature Communications, 2020, 11: 1550. |
| 83 | XIA L, TANG B C, YAO L B, et al. Oxidation decomposition mechanism of fluoroethylene carbonate-based electrolytes for high-voltage lithium ion batteries: A DFT calculation and experimental study[J]. ChemistrySelect, 2017, 2(24): 7353-7361. |
| 84 | LI W D, DOLOCAN A, LI J Y, et al. Ethylene carbonate-free electrolytes for high-nickel layered oxide cathodes in lithium-ion batteries[J]. Advanced Energy Materials, 2019, 9(29): doi: 10.1002/aenm.201901152. |
| 85 | ZHANG T, PAILLARD E. Recent advances toward high voltage, EC-free electrolytes for graphite-based Li-ion battery[J]. Frontiers of Chemical Science and Engineering, 2018, 12(3): 577-591. |
| 86 | 贠娇娇, 刘红梅, 郑会元, 等. 无机添加剂用于锂离子电池电解液的研究进展[J]. 储能科学与技术, 2014, 3(6): 584-589. |
| YUN J J, LIU H M, ZHENG H Y, et al. Research progress on inorganic additives for lithium-ion battery electrolytes[J]. Energy Storage Science and Technology, 2014, 3(6): 584-589. | |
| 87 | XIA J, PETIBON R, XIONG D J, et al. Enabling linear alkyl carbonate electrolytes for high voltage Li-ion cells[J]. Journal of Power Sources, 2016, 328: 124-135. |
| 88 | EHTESHAMI N, PAILLARD E. Ethylene carbonate-free, adiponitrile-based electrolytes compatible with graphite anodes[J]. ECS Transactions, 2017, 77(1): 11-20. |
| 89 | DOSE W M, LI W Q, TEMPRANO I, et al. Onset potential for electrolyte oxidation and Ni-rich cathode degradation in lithium-ion batteries[J]. ACS Energy Letters, 2022, 7(10): 3524-3530. |
| 90 | MA L, GLAZIER S L, PETIBON R, et al. A guide to ethylene carbonate-free electrolyte making for Li-ion cells[J]. Journal of the Electrochemical Society, 2016, 164(1): A5008-A5018. |
| 91 | LEE Y, LEE T K, KIM S, et al. Fluorine-incorporated interface enhances cycling stability of lithium metal batteries with Ni-rich NCM cathodes[J]. Nano Energy, 2020, 67: doi: 10.1016/j.nanoen.2019.104309. |
| 92 | ZENG G F, AN Y L, XIONG S L, et al. Nonflammable fluorinated carbonate electrolyte with high salt-to-solvent ratios enables stable silicon-based anode for next-generation lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(26): 23229-23235. |
| 93 | FAN X L, JI X, CHEN L, et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents[J]. Nature Energy, 2019, 4(10): 882-890. |
| 94 | CHOUDHURY S. Lithium fluoride additives for stable cycling of lithium batteries at high current densities[M]// Rational design of nanostructured polymer electrolytes and solid-liquid interphases for lithium batteries. Cham: Springer, 2019: 81-94. |
| 95 | JIA H P, ZOU L F, GAO P Y, et al. High-performance silicon anodes enabled by nonflammable localized high-concentration electrolytes[J]. Advanced Energy Materials, 2019, 9(31): doi: 10.1002/aenm.201900784. |
| 96 | CHEN S R, ZHENG J M, MEI D H, et al. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes[J]. Advanced Materials, 2018, 30(21): doi: 10.1002/adma.201706102. |
| 97 | SU C C, HE M N, REDFERN P C, et al. Oxidatively stable fluorinated sulfone electrolytes for high voltage high energy lithium-ion batteries[J]. Energy & Environmental Science, 2017, 10(4): 900-904. |
| 98 | CAO X A, JIA H, XU W, et al. Review—Localized high-concentration electrolytes for lithium batteries[J]. Journal of the Electrochemical Society, 2021, 168(1): doi: 10.1149/1945-7111/abd60e. |
| 99 | JIA H, XU Y B, BURTON S D, et al. Enabling ether-based electrolytes for long cycle life of lithium-ion batteries at high charge voltage[J]. ACS Applied Materials & Interfaces, 2020, 12(49): 54893-54903. |
| 100 | WU Z C, LI R H, ZHANG S Q, et al. Deciphering and modulating energetics of solvation structure enables aggressive high-voltage chemistry of Li metal batteries[J]. Chem, 2023, 9(3): 650-664. |
| 101 | WU Y, FENG X N, YANG M, et al. Thermal runaway of nonflammable localized high-concentration electrolytes for practical LiNi0.8 Mn0.1 Co0.1 O2 |Graphite-SiO pouch cells[J]. Advanced Science, 2022, 9(32): doi: 10.1002/advs.202204059. |
| 102 | KO S, YAMADA Y, YAMADA A. A 4.8 V reversible Li2CoPO4F/graphite battery enabled by concentrated electrolytes and optimized cell design[J]. Batteries & Supercaps, 2020, 3(9): 910-916. |
| 103 | HAN J G, JEONG M Y, KIM K, et al. An electrolyte additive capable of scavenging HF and PF5 enables fast charging of lithium-ion batteries in LiPF6-based electrolytes[J]. Journal of Power Sources, 2020, 446: doi: 10.1016/j.jpowsour.2019.227366. |
| 104 | LU D, LEI X C, WENG S T, et al. A self-purifying electrolyte enables high energy Li ion batteries[J]. Energy & Environmental Science, 2022, 15(8): 3331-3342. |
| 105 | KLEIN S, VAN WICKEREN S, RÖSER S, et al. Understanding the outstanding high-voltage performance of NCM523||Graphite lithium ion cells after elimination of ethylene carbonate solvent from conventional electrolyte[J]. Advanced Energy Materials, 2021, 11(14): doi: 10.1002/aenm.202003738. |
| 106 | KLEIN S, HARTE P, VAN WICKEREN S, et al. Re-evaluating common electrolyte additives for high-voltage lithium ion batteries[J]. Cell Reports Physical Science, 2021, 2(8): doi: 10.1016/j.xcrp.2021.100521. |
| 107 | KLEIN S, HARTE P, HENSCHEL J, et al. Graphite lithium-ion cells: On the beneficial impact of Li2CO3 as electrolyte additive in NCM523∥graphite lithium ion cells under high-voltage conditions (adv. energy mater. 10/2021)[J]. Advanced Energy Materials, 2021, 11(10): doi: 10.1002/aenm.202170039. |
| 108 | HAN J G, LEE J B, CHA A M, et al. Unsymmetrical fluorinated malonatoborate as an amphoteric additive for high-energy-density lithium-ion batteries[J]. Energy & Environmental Science, 2018, 11(6): 1552-1562. |
| 109 | VIDAL C, GROSS O, GU R, et al. xEV Li-ion battery low-temperature effects-Review[J]. IEEE Transactions on Vehicular Technology, 2019, 68(5): 4560-4572. |
| 110 | BI K, ZHAO S X, HUANG C, et al. Improving low-temperature performance of spinel LiNi0.5Mn1.5O4 electrode and LiNi0.5Mn1.5O4/Li4Ti5O12 full-cell by coating solid-state electrolyte Li-Al-Ti-P-O[J]. Journal of Power Sources, 2018, 389: 240-248. |
| 111 | RUI X H, JIN Y, FENG X Y, et al. A comparative study on the low-temperature performance of LiFePO4/C and Li3V2(PO4)3/C cathodes for lithium-ion batteries[J]. Journal of Power Sources, 2011, 196(4): 2109-2114. |
| 112 | WANG K J. Study on low temperature performance of Li ion battery[J]. OALib, 2017, 4(11): 1-12. |
| 113 | SMART M C, RATNAKUMAR B V, CHIN K B, et al. Lithium-ion electrolytes containing ester cosolvents for improved low temperature performance[J]. Journal of the Electrochemical Society, 2010, 157(12): A1361. |
| 114 | SHI P, FANG S H, LUO D, et al. A safe electrolyte based on propylene carbonate and non-flammable hydrofluoroether for high-performance lithium ion batteries[J]. Journal of the Electrochemical Society, 2017, 164(9): A1991-A1999. |
| 115 | ZHANG S S, XU K, ALLEN J L, et al. Effect of propylene carbonate on the low temperature performance of Li-ion cells[J]. Journal of Power Sources, 2002, 110(1): 216-221. |
| 116 | HU Z L, WANG C, WANG C, et al. Uncovering the critical impact of the solid electrolyte interphase structure on the interfacial stability[J]. InfoMat, 2022, 4(3): doi: 10.1002/inf2.12249. |
| 117 | QIN M S, LIU M C, ZENG Z Q, et al. Rejuvenating propylene carbonate-based electrolyte through nonsolvating interactions for wide-temperature Li-ions batteries[J]. Advanced Energy Materials, 2022, 12(48): doi: 10.1002/aenm.202201801. |
| 118 | ZHANG Z X, YAO T F, WANG E K, et al. Unlocking the low-temperature potential of propylene carbonate to -30 ℃ via N-methylpyrrolidone[J]. ACS Applied Materials & Interfaces, 2022, 14(40): 45484-45493. |
| 119 | LIU X W, SHEN X H, LI H, et al. Ethylene carbonate-free propylene carbonate-based electrolytes with excellent electrochemical compatibility for Li-ion batteries through engineering electrolyte solvation structure[J]. Advanced Energy Materials, 2021, 11(19): doi: 10.1002/aenm.202003905. |
| 120 | NAN B, CHEN L, RODRIGO N D, et al. Enhancing Li+ transport in NMC811||Graphite lithium-ion batteries at low temperatures by using low-polarity-solvent electrolytes[J]. Angewandte Chemie, 2022, 61(35): doi: 10.1002/anie. 202205967. |
| 121 | XU J, WANG X, YUAN N Y, et al. Graphite-based lithium ion battery with ultrafast charging and discharging and excellent low temperature performance[J]. Journal of Power Sources, 2019, 430: 74-79. |
| 122 | HUANG X T, LI R H, SUN C C, et al. Solvent-assisted hopping mechanism enables ultrafast charging of lithium-ion batteries[J]. ACS Energy Letters, 2022, 7(11): 3947-3957. |
| 123 | GHAUR A, PESCHEL C, DIENWIEBEL I, et al. Effective SEI formation via phosphazene-based electrolyte additives for stabilizing silicon-based lithium-ion batteries [J]. Advanced Energy Materials, 2023, 13(26): doi: 10.1002/aenm.202203503. |
| 124 | CAO Z, ZHENG X Y, ZHOU M, et al. Electrolyte solvation engineering toward high-rate and low-temperature silicon-based batteries[J]. ACS Energy Letters, 2022, 7(10): 3581-3592. |
| 125 | HUBBLE D, BROWN D E, ZHAO Y Z, et al. Liquid electrolyte development for low-temperature lithium-ion batteries[J]. Energy & Environmental Science, 2022, 15(2): 550-578. |
| 126 | FAN X L, CHEN L, BORODIN O, et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries[J]. Nature Nanotechnology, 2018, 13(8): 715-722. |
| 127 | HAREGEWOIN A M, WOTANGO A S, HWANG B J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives[J]. Energy & Environmental Science, 2016, 9(6): 1955-1988. |
| 128 | THENUWARA A C, SHETTY P P, KONDEKAR N, et al. Efficient low-temperature cycling of lithium metal anodes by tailoring the solid-electrolyte interphase[J]. ACS Energy Letters, 2020, 5(7): 2411-2420. |
| 129 | WOTANGO A S, SU W N, HAREGEWOIN A M, et al. Designed synergetic effect of electrolyte additives to improve interfacial chemistry of MCMB electrode in propylene carbonate-based electrolyte for enhanced low and room temperature performance[J]. ACS Applied Materials & Interfaces, 2018, 10(30): 25252-25262. |
| 130 | XIONG D J, BAUER M, ELLIS L D, et al. Some physical properties of ethylene carbonate-free electrolytes[J]. Journal of the Electrochemical Society, 2018, 165(2): A126-A131. |
| 131 | PETIBON R, HARLOW J, LE D B, et al. The use of ethyl acetate and methyl propanoate in combination with vinylene carbonate as ethylene carbonate-free solvent blends for electrolytes in Li-ion batteries[J]. Electrochimica Acta, 2015, 154: 227-234. |
| 132 | GU Y X, FANG S H, YANG L, et al. A non-flammable electrolyte for long-life lithium ion batteries operating over a wide-temperature range[J]. Journal of Materials Chemistry A, 2021, 9(27): 15363-15372. |
| 133 | LAZAR M L, LUCHT B L. Carbonate free electrolyte for lithium ion batteries containing γ-butyrolactone and methyl butyrate[J]. Journal of the Electrochemical Society, 2015, 162(6): A928-A934. |
| 134 | PHAM H Q, LEE H Y, HWANG E H, et al. Non-flammable organic liquid electrolyte for high-safety and high-energy density Li-ion batteries[J]. Journal of Power Sources, 2018, 404: 13-19. |
| [1] | Huan LIU, Na PENG, Qingwen GAO, Wenpeng LI, Zhirong YANG, Jingtao WANG. Crown ether-doped polymer solid electrolyte for high-performance all-solid-state lithium batteries [J]. Energy Storage Science and Technology, 2023, 12(8): 2401-2411. |
| [2] | Minghu WU, Chengpeng YUE, Fan ZHANG, Junxiao LI, Wei HUANG, Sheng HU, Jing TANG. Combined GRU-MLR method for predicting the remaining useful life of lithium batteries via multiscale decomposition [J]. Energy Storage Science and Technology, 2023, 12(7): 2220-2228. |
| [3] | Jin LI, Qingsong WANG, Depeng KONG, Xiaodong WANG, Zhenhua YU, Yanfei LE, Xinyan HUANG, Zhenkai HU, Houfu WU, Huabin FANG, Caowei, Shaoyu ZHANG, Ping ZHUO, Ye CHEN, Ziting LI, Wenxin MEI, Yue ZHANG, Lixiang ZHAO, Liang TANG, Zonghou HUANG, Chi CHEN, Yanhu LIU, Yuxi CHU, Xiaoyuan XU, Jin ZHANG, Yikai LI, Rong FENG, Biao YANG, Bo HU, Xiaoying YANG. Research progress on the safety assessment of lithium-ion battery energy storage [J]. Energy Storage Science and Technology, 2023, 12(7): 2282-2301. |
| [4] | Yuling LIU, Jinhao MENG, Qiao PENG, Tianqi LIU, Yang WANG, Yongxiang CAI. NSGA-II genetic algorithm-based optimization of the lithium battery equalization index [J]. Energy Storage Science and Technology, 2023, 12(6): 1946-1956. |
| [5] | Hai WANG, Yuhua BIAN, Jiadong WANG, Zhaoyang LIU, Jie ZHANG, Jian YAO, Xuanwen GAO, Zhaomeng LIU, Wenbin LUO. Retired lithium battery recycling and battery-grade lithium carbonate preparation [J]. Energy Storage Science and Technology, 2023, 12(5): 1453-1460. |
| [6] | Jialiang LIU, Cuijing GUO, Huanling WANG. Safety detection and verification of energy storage in lithium-ion battery based on fire fault tree model [J]. Energy Storage Science and Technology, 2023, 12(5): 1695-1704. |
| [7] | Chuan HU, Zhiwei HU, Zhendong LI, Shuai LI, Hao WANG, Liping WANG. Tailoring LiPF6-base electrolyte solvation structure toward a stable Lithium-rich manganese-based cathode interface [J]. Energy Storage Science and Technology, 2023, 12(5): 1604-1615. |
| [8] | Liyu ZHAO, Huanwu SUN, Shichuang LIU, Zhiyuan YAN. Energy consumption comparison and optimization of auxiliary power-battery heating system of heavy truck [J]. Energy Storage Science and Technology, 2023, 12(4): 1139-1147. |
| [9] | Kuijie LI, Ping LOU, Minyuan GUAN, Jinlong MO, Weixin ZHANG, Yuancheng CAO, Shijie CHENG. A review of multi-dimensional signal evolution and coupling mechanism of lithium-ion battery thermal runaway [J]. Energy Storage Science and Technology, 2023, 12(3): 899-912. |
| [10] | Mai FENG, Nan CHEN, Renjie CHEN. Research progress of low-temperature electrolyte for lithium-ion battery [J]. Energy Storage Science and Technology, 2023, 12(3): 792-807. |
| [11] | Xinhao ZHAO, Liang XU. Improved firefly optimization algorithm to optimize back propagation neural network for state of health estimation of power lithium ion batteries [J]. Energy Storage Science and Technology, 2023, 12(3): 934-940. |
| [12] | Wei LIU, Zhenming LI, Mingyang LIU, Cenyu YANG, Chao MEI, Ying LI. Review of high-temperature phase change heat storage material preparation and applications [J]. Energy Storage Science and Technology, 2023, 12(2): 398-430. |
| [13] | Yuhao ZHOU, Luoyun XÜ, Zhongping ZHANG, Lingchong LIU, Bin NAN, Haiqi ZHAO. Construction and simulation analysis of thermoelectric coupling model of lithium battery based on digital twin [J]. Energy Storage Science and Technology, 2023, 12(2): 536-543. |
| [14] | Lulu LI, Zhengshun TAO, Tinglong PAN, Weilin YANG, Guanyang HU. Research on fractional modeling and SOC estimation strategy for lithium batteries [J]. Energy Storage Science and Technology, 2023, 12(2): 544-551. |
| [15] | Qiantong LIU, Yuanxiu XING. Remaining life prediction of lithium-ion battery based on VMD-PSO-GRU model [J]. Energy Storage Science and Technology, 2023, 12(1): 236-246. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
