Energy Storage Science and Technology ›› 2020, Vol. 9 ›› Issue (5): 1350-1369.doi: 10.19799/j.cnki.2095-4239.2020.0179
Previous Articles Next Articles
Hongming YI1,2( ), Zhiqiang LYU1,2, Huamin ZHANG1, Mingming SONG3, Qiong ZHENG1(
), Zhiqiang LYU1,2, Huamin ZHANG1, Mingming SONG3, Qiong ZHENG1( ), Xianfeng LI1(
), Xianfeng LI1( )
)
Received:2020-05-17
Revised:2020-05-28
Online:2020-09-05
Published:2020-09-08
Contact:
Qiong ZHENG,Xianfeng LI
E-mail:yihm@dicp.ac.cn;zhengqiong@dicp.ac.cn;lixianfeng@dicp.ac.cn
CLC Number:
Hongming YI, Zhiqiang LYU, Huamin ZHANG, Mingming SONG, Qiong ZHENG, Xianfeng LI. Recent progress and application challenges in V-based polyanionic compounds for cathodes of sodium-ion batteries[J]. Energy Storage Science and Technology, 2020, 9(5): 1350-1369.
Table 1
Main characteristic parameters and performance data of some typical V-based polyanionic compounds"
| 材料体系 | 材料名称 | 晶体结构类型 | 氧化还原电对 | 氧化还原电位/V | 理论 比容量/mA·h·g-1 | 理论能量密度 /W·h·kg-1 | 储钠机理 | 钠扩散系数 /cm·s-1 | 电化学性能 |
|---|---|---|---|---|---|---|---|---|---|
正磷 酸盐 | Na3V2(PO4)3[ | rhombohedral (NASICON, | V4+/V3+ | 3.4 | 117.6 | 400 | bi-phase reaction | 1.06×10-11 /CV | 107.1 mA·h/g at 0.1 C |
| Na3V2(PO4)3[ | rhombohedral (NASICON, | V4+/V3+ | 3.4 | 117.6 | 400 | bi-phase reaction | 9.6×10-11 /CV | 117 mA·h/g at 1 C 82 mA·h/g at 100 C | |
| NaVOPO4[ | monoclinic | V5+/V4+ | 3.6 | 145 | 522 | single-phase reaction | 1-3×10–11 /GITT | 101 mA·h/g at 5 C | |
| NaVOPO4[ | orthorhombic | V5+/V4+ | 3.3 | 145 | 479 | — | — | 115 mA·h/g at 0.1 C | |
| KVOPO4[ | orthorhombic | V5+/V3+ | 2.56 | 266.7 | 683 | 9.6 × 10-12 /CV | 235 mA·h/g at 0.05 C | ||
| NaVOPO4[ | tetragonal (layered structure) | — | — | — | — | — | — | — | |
| NaVOPO4[ | triclinic | V5+/V4+ | 3.5 | 145 | 508 | multi-phase reactions | 4.9 × 10-11 /EIS | 144 mA·h/g at 0.05 C 58 mA·h/g at 5 C | |
| VOPO4[ | tetragonal (layered structure) | V5+/V4+ | 3.4 | 165.5 | 563 | — | — | 150 mA·h/g at 0.05 C | |
| Na3V3(PO4)4[ | monoclinic (layered structure, C2/c) | V4+/V3+ | 3.9 | 44.5 | 174 | — | — | >40 mA·h/g at 0.6 C 36.9 mA·h/g at 3 C | |
| Na3V(PO4)2[ | monoclinic (layered structure, C2/c) | V4+/V3+ | 3.5 | 90 | 315 | — | — | >90 mA·h/g at 0.2 C 71 mA·h/g at 15 C | |
| Na4VO(PO4)2[ | orthorhombic (Pbca) | V5+/V4+ | 3.5 | 78 | 273 | single-phase reaction | — | 41.3 mA·h/g at 10 C | |
氟磷 酸盐 | NaVPO4F[ | tetragonal (NASICON, I4/mmm) | V4+/V3+ | 3.7 | 142.5 | 485 | — | — | 120.9 mA·h/g at 0.05 C 70.1 mA·h/g at 0.5 C |
| NaVPO4F[ | monoclinic (NASICON, C2/c) | V4+/V3+ | 3.4 | 142.5 | 484.5 | single-phase reaction | — | 135 mA·h/g at 0.2 C 112 mA·h/g at 30 C | |
| Na3V2(PO4)2F3[ | tetragonal (NASICON, P42/mnm) | V4+/V3+ | 3.9 | 128.3 | 500 | bi-phase reaction and single-phase reaction | 10-12 /EIS | 125 mA·h/g at 0.2 C 104 mA·h/g at 40 C 41 mA·h/g at 200 C | |
| Na3V2(PO4)2O2F[ | tetragonal (I4/mmm) | V5+/V4+ | 3.8 | 130 | 494 | two completely reversible bi-phasic reaction | — | 127.4 mA·h/g at 0.2 C 84.1 mA·h/g at 40 C | |
| Na3V2(PO4)2O1.6F1.4[ | tetragonal (P42/mnm) | V5+/V3.8+ | 3.8 | 155.6 | 592 | single-phase reaction | 2.4×10-12 /EIS | 134 mA·h/g at 0.1 C | |
焦磷 酸盐 | Na7V3(P2O7)4[ | monoclinic (C2/c) | V4+/V3+ | 4.13 | 79.6 | 329 | single-phase reaction | — | 67.2 mA·h/g at 8 C |
| Na2(VO)P2O7[ | tetragonal (P21/c) | V5+/V4+ | 3.8 | 93.4 | 355 | — | — | 80 mA·h/g at 0.05 C | |
| NaVP2O7[ | monoclinic | V4+/V3+ | 3.9 | 108.1 | 421 | — | — | 104 mA·h/g at 0.1 C | |
混合聚 阴离子型 | Na7V4(P2O7)4(PO4)[ | tetragonal (P | V4+/V3+ | 3.85 | 92.7 | 357 | bi-phasic reaction | — | 92 mA·h/g at 0.05 C 70.2 mA·h/g at 10 C |
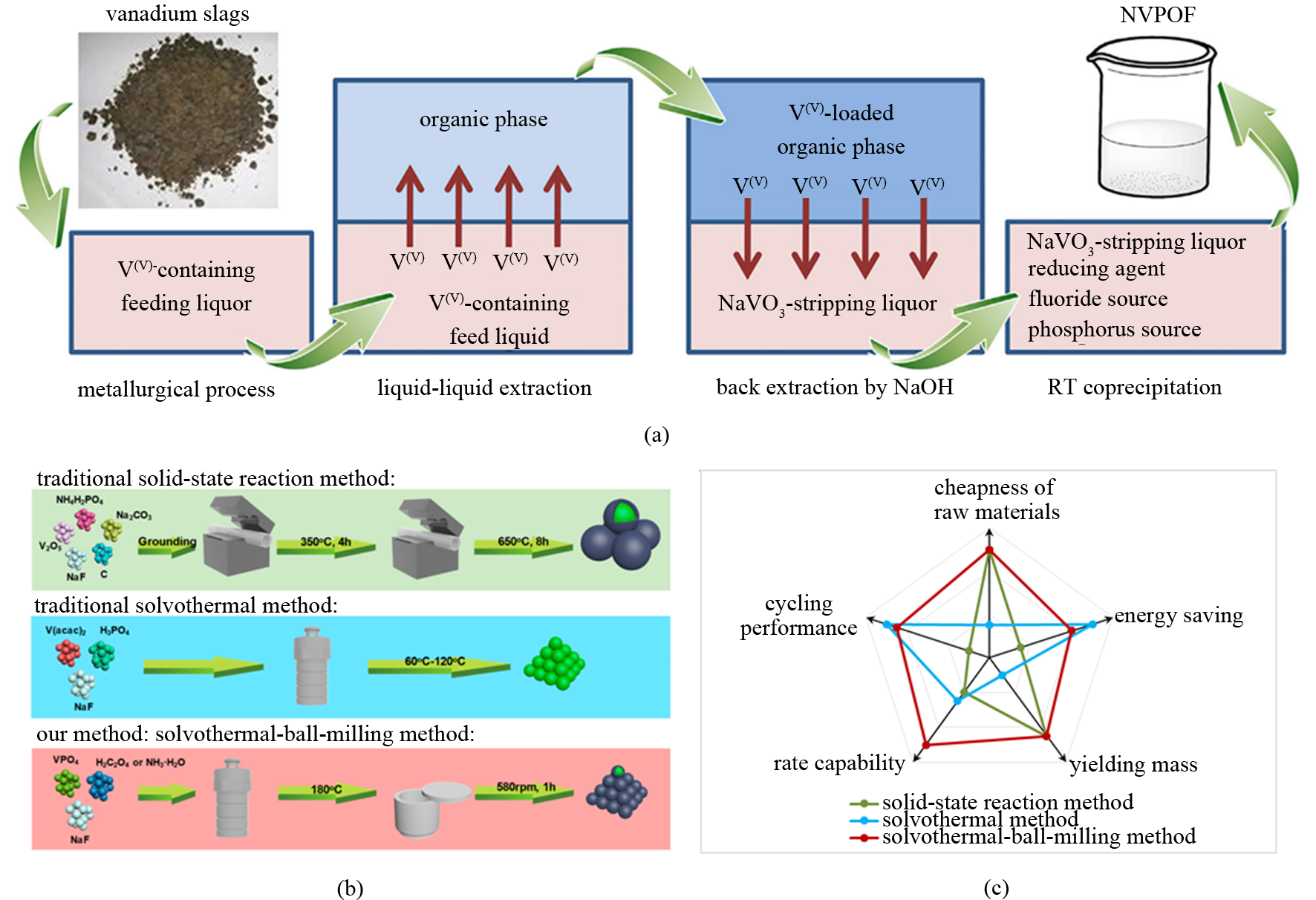
Fig.14
(a) schematic of integration of extraction-separation and materials-preparation; (b) schematic illustration of traditional solid-state method, solvothermal method, and solvothermal-ball-milling method; (c) parameter comparison of traditional solid-state method, solvothermal method, and solvothermal-ball-milling method"
| 1 | ROJO T, HU Y S, FORSYTH M, et al. Sodium-ion batteries[J]. Advanced Energy Materials, 2018, 8(17): doi:10.1002/adma.201800880. |
| 2 | YABUUCHI N, KUBOTA K, DAHBI M, et al. Research development on sodium-ion batteries[J]. Chemical Reviews, 2014, 114(23): 11636-11682. |
| 3 | PAN Huilin, HU Yongsheng, CHEN Liquan. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage[J]. Energy & Environmental Science, 2013, 6(8): 2338-2360. |
| 4 | HWANG J Y, MYUNG S T, SUN Y K. Sodium-ion batteries: Present and future[J]. Chemical Society Reviews, 2017, 46(12): 3529-3614. |
| 5 | CHE Haiying, CHEN Suli, XIE Yingying, et al. Electrolyte design strategies and research progress for room-temperature sodium-ion batteries[J]. Energy & Environmental Science, 2017, 10(5): 1075-1101. |
| 6 | XIANG Xingde, ZHANG Kai, CHEN Jun. Recent advances and prospects of cathode materials for sodium-ion batteries[J]. Advanced Materials, 2015, 27(36): 5343-5364. |
| 7 | 容晓晖, 陆雅翔, 戚兴国, 等. 钠离子电池: 从基础研究到工程化探索[J]. 储能科学与技术, 2020, 9(2): 515-522. |
| RONG Xiaohui, LU Yaxiang, QI Xingguo, et al. Na-ion batteries: From fundamental research to engineering exploration[J]. Energy Storage Science and Technology, 2020, 9(2): 515-522. | |
| 8 | BAURE A, SONG J, VAIL S, et al. The scale-up and commercialization of nonaqueous Na-ion battery technologies[J]. Advanced Energy Materials, 2018, 8(17): doi: 10.1002/aenm.201702869. |
| 9 | DAI Zhengfei, MANI U, TAN Huiteng, et al. Advanced cathode materials for sodium-ion batteries: What determines our choices?[J]. Small Methods, 2017, 1: doi: 10.1002/smtd.201700098. |
| 10 | GUO Jinzhi, WANG Pengfei, WU Xinglong, et al. High-energy/power and low-temperature cathode for sodium-ion batteries: In situ XRD study and superior full-cell performance[J]. Advanced Materials, 2017, 29(33): doi: 10.1002/adma.201701968. |
| 11 | JIAN Zelang, HAN Wenze, LU Xia, et al. Superior electrochemical performance and storage mechanism of Na3V2(PO4)3 cathode for room-temperature sodium-ion batteries[J]. Advanced Energy Materials, 2013, 3(2): 156-160. |
| 12 | ZHU Changbao, KOPOLD P, AKEN P A VAN, et al. High power-high energy sodium battery based on threefold interpenetrating network[J]. Advanced Materials, 2016, 28(12): 2409-2416. |
| 13 | CHEN Chihyao, MATSUMOTO K, NOHIRA T, et al. Improved electrochemical performance of NaVOPO4 positive electrodes at elevated temperature in an ionic liquid electrolyte[J]. Journal of the Electrochemical Society, 2015, 162(10): A2093-A2098. |
| 14 | NI Yang, HE Guang. Stable cycling of β-VOPO4/NaVOPO4 cathodes for sodium-ion batteries[J]. Electrochim Acta, 2018, 292: 47-54. |
| 15 | DING Jia, LIN Yuhchieh, LIU Jue, et al. KVOPO4: A new high capacity multielectron Na-ion battery cathode[J]. Advanced Energy Materials, 2018, 8(21): doi: 10.1002/aenm.201800221. |
| 16 | APARICION P A, DAWSON J A, ISLAM M S, et al. Computational study of NaVOPO4 polymorphs as cathode materials for Na-ion batteries: Diffusion, electronic properties, and cation-doping behavior[J]. The Journal of Physical Chemistry C, 2018, 122 (45): 25829-25836. |
| 17 | FANG Yongjin, LIU Qi, XIAO Lifen, et al. A fully sodiated NaVOPO4 with layered structure for high-voltage and long-lifespan sodium-ion batteries[J]. Chemistry, 2018, 4(5): 1167-1180. |
| 18 | HE Guang, KAN Wanghay, MANTHIRAM A. A 3.4 V layered VOPO4 cathode for Na-ion batteries[J]. Chemistry of Materials, 2016, 28(2): 682-688. |
| 19 | LIU Rui, LIU Haodong, SHENG Tian, et al. A novel 3.9 V layered Na3V3(PO4)4 cathode material for sodium ion batteries[J]. ACS Applied Energy Materials, 2018, 1(8): 3603-3606. |
| 20 | KIM Jongsoon, YOON Gabin, KIM Hyungsub, et al. Na3V(PO4)2: A new layered-type cathode material with high water stability and power capability for Na-ion batteries[J]. Chemistry of Materials, 2018, 30(11): 3683-3689. |
| 21 | KIM Jongsoon, KIM Hyungsub, Seongsu LEE. High power cathode material Na4VO(PO4)2 with open framework for Na ion batteries[J]. Chemistry of Materials, 2017, 29(8): 3363-3366. |
| 22 | RUAN Yanli, WANG Kun, SONG Shidong, et al. Graphene modified sodium vanadium fluorophosphate as a high voltage cathode material for sodium ion batteries[J]. Electrochimica Acta, 2015, 160: 330-336. |
| 23 | LING Moxiang, LI Fan, YI Hongming, et al. Superior Na-storage performance of molten-state-blending-synthesized monoclinic NaVPO4F nanoplates for Na-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(47): 24201-24209. |
| 24 | SONG Weixin, JI Xiaobo, WU Zhengping, et al. Exploration of ion migration mechanism and diffusion capability for Na3V2(PO4)2F3 cathode utilized in rechargeable sodium-ion batteries[J]. Journal of Power Sources, 2014, 256: 258-263. |
| 25 | YI Hongming, LING Moxiang, XU Wenbin, et al. VSC-doping and VSU-doping of Na3V2-xTix(PO4)2F3 compounds for sodium ion battery cathodes: Analysis of electrochemical performance and kinetic properties[J]. Nano Energy, 2018, 47: 340-352. |
| 26 | XU Junling, CHEN Jizhang, TAO Li, et al. Investigation of Na3V2(PO4)2O2F as a sodium ion battery cathode material: Influences of morphology and voltage window[J]. Nano Energy, 2019, 60: 510-519. |
| 27 | PARK Y U, SEO D H, KWON D H, et al. A new high-energy cathode for a Na-ion battery with ultrahigh stability[J]. Journal of the American Chemical Society, 2013, 135(37): doi: 10.1021/ja406016j. |
| 28 | KIM J, PARK I, KIM H, et al. Tailoring a new 4 V-class cathode material for Na-ion batteries[J]. Advanced Energy Materials, 2016, 6(6): doi: 10.1002/aenm.201502147. |
| 29 | BARPANDA P, LIU G D, AVDEEV M, et al. t-Na2(VO)P2O7: A 3.8 V pyrophosphate insertion material for sodium-ion batteries[J]. Chemelectrochem, 2014, 1(9): 1488-1491. |
| 30 | DROZHZHIN O A, TERTOV I V, ALEKSEEVA A M. β-NaVP2O7 as a superior electrode material for Na-ion batteries[J]. Chemistry of Materials, 2019, 31(18): 7463-7469. |
| 31 | FANG Wenying, AN Zhongxun, XU Jiaqiang, et al. Superior performance of Na7V4(P2O7)4PO4 in sodium ion batteries[J]. RSC Advances, 2018, 8(38): 21224-21228. |
| 32 | CHEN Shuangqiang, WU Chao, SHEN Laifa, et al. Challenges and perspectives for NASICON-type electrode materials for advanced sodium-ion batteries[J]. Advanced Materials, 2017, 29: doi: 10.1002/adma.201700431. |
| 33 | CUSHING B L, GOODENOUGH J B. Li2NaV2(PO4)3: A 3.7 V lithium-insertion cathode with the rhombohedral NASICON structure[J]. Journal of Solid State Chemistry, 2001, 162(2): 176-181. |
| 34 | SONG Weixin, JI Xiaobo, WU Zhengping, et al. First exploration of Na-ion migration pathways in the NASICON structure Na3V2(PO4)3[J]. Journal of Materials Chem. A, 2014, 2(15): doi: 10.1039/C4TA00230J. |
| 35 | SONG Jie, XU Maowen, WANG Long, et al. Exploration of NaVOPO4 as a cathode for a Na-ion battery[J]. Chemical Communications, 2013, 49(46): 5280-5282. |
| 36 | LIU Zigeng, HU Yanyan, DUNSTAN M T, et al. Local structure and dynamics in the Na ion battery positive electrode material Na3V2(PO4)2F3[J]. Chemistry of Materials, 2014, 26(8): 2513-2521. |
| 37 | BIANCHINI M, BRISSET N, FAUTH F, et al. Na3V2(PO4)2F3 revisited: A high-resolution diffraction study[J]. Chemistry of Materials, 2014, 26(14): 4238-4247. |
| 38 | XIANG Xingde, ZHANG Kai, CHEN Jun. Advances and prospects of cathode materials for sodium-ion batteries[J]. Advanced Materials, 2015, 27(36): 5343-5364. |
| 39 | SONG Weixin, CAO Xiaoyu, WU Zhengping, et al. Investigation of the sodium ion pathway and cathode behavior in Na3V2(PO4)2F3 combined via a first principles calculation[J]. Langmuir, 2014, 30(41): 12438-12446. |
| 40 | BIANCHINI M, FAUTH F, BRISSET N, et al. Comprehensive investigation of the Na3V2(PO4)2F3-Na3V2(PO4)2F3 system by operando high resolution synchrotron X-ray diffraction[J]. Chemistry of Materials, 2015, 27(8): 3009-3020. |
| 41 | ZHAO Jianqing, HE Jianping, DING Xiaochun, et al. A novel sol-gel synthesis route to NaVPO4F as cathode material for hybrid lithium ion batteries[J]. Journal of Power Sources, 2010, 195(19): 6854-6859. |
| 42 | PARK Y U, SEO D H, KIM H, et al. A family of high-performance cathode materials for Na-ion batteries, Na3(VO1-xPO4)2F1+2x (0≤x≤1) combined first-principles and experimental study[J]. Advanced Function Materials, 2014, 24: 4603-4614 |
| 43 | QI Yuruo, MU Linqin, ZHAO Junmei, et al. Superior Na-storage performance of low-temperature-synthesized Na3(VO1-xPO4)2F1+2x(0≤x≤1) nanoparticles for Na-ion batteries[J]. Angewandte Chemie International Edition, 2015, 54(34): 9911-9916. |
| 44 | WERNER M, OLGA V. Crystal structure of a new sodium vanadyl(Ⅳ) fluoride phosphate Na3V2O2F(PO4)2[J]. Solid State Sciences, 2002, 4(4): 495-501. |
| 45 | SAUVAGE F, QUAREZ E, TARASCON J M, et al. Crystal structure and electrochemical properties vs Na+ of the sodium fluorophosphate Na1.5VOPO4F0.5[J]. Solid State Sciences, 2006, 8(10): 1215-1221. |
| 46 | SERRAS P, PALOMARES V, GONI A, et al. High voltage cathode materials for Na-ion batteries of general formula Na3V2O2x(PO4)2F3-2x[J]. Journal of Materials Chemistry, 2012, 22(41): 22301-22308. |
| 47 | XU Maowen, WANG Long, ZHAO Xin, et al. Na3V2O2(PO4)2F/graphene sandwich structure for high-performance cathode of a sodium-ion battery[J]. Physical Chemistry Chemical Physics, 2013, 15(31): doi: 10.1039/C3CP52408f. |
| 48 | DENG Chao, ZHANG Sen. 1D nanostructured Na7V4(P2O7)4(PO4) as high-potential and superior-performance cathode material for sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2014, 6(12): doi: 10.1021/am501072j. |
| 49 | ZHANG Bao, CHEN Hezhang, TONG Hui, et al. Synthesis and electrochemical performance of Ni doped Na3V2(PO4)3/C cathode materials for sodium ion batteries[J]. Journal of Alloys and Compounds, 2017, 728: 976-983. |
| 50 | BIANCHINI M, XIAO Penghao, WANG Yan, et al. Additional sodium insertion into polyanionic cathodes for higher-energy Na-ion batteries[J]. Advanced Energy Materials, 2017, 7(18): doi: 10.1002/aenm.201700514. |
| 51 | LIM S J, HAN D W, NAM D H, et al. Structural enhancement of Na3V2(PO4)3/C composite cathode materials by pillar ion doping for high power and long cycle life sodium-ion batteries[J]. Journal of Materials Chemistry A, 2014, 2(46): 19623-19632. |
| 52 | ZHENG Qiong, NI Xiao, LIN Le, et al. Towards enhanced sodium storage by investigation of the Li ion doping and rearrangement mechanism in Na3V2(PO4)3 for sodium ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(9): 4209-4218. |
| 53 | HU Pu, WANG Xiaofang, WANG Tianshi, et al. Boron substituted Na3V2(P1-xBxO4)3 cathode materials with enhanced performance for sodium-ion batteries[J]. Advanced Science, 2016, 3(12): doi: 10.1002/advs.201600112. |
| 54 | DUAN Wenchao, ZHU Zhiqiang, LI Hao, et al. Na3V2(PO4)3@C core-shell nanocomposites for rechargeable sodium-ion batteries[J]. Journal of Materials Chemistry A, 2014, 2(23): 8668-8675. |
| 55 | LIU Qiang, MENG Xing, WEI Zhixuan, et al. Core/double-shell structured Na3V2(PO4)2F3@C nanocomposite as the high power and long lifespancathode for sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2016, 8(46): 31709-31715. |
| 56 | RUI Xianhong, SUN Wenping, WU Chao, et al. An advanced sodium-ion battery composed of carbon coated Na3V2(PO4)3 in a porous graphene network[J]. Advanced Materials, 2015, 27(42): 6670-6675. |
| 57 | SHEN Wei, WANG Cong, XU Qunjie, et al. Nitrogen-doping-induced defects of a carbon coating layer facilitate Na-storage in electrode materials[J]. Advanced Energy Materials, 2015, 5(1): doi: 10.1002/aenm.201400982. |
| 58 | SHEN Wei, LI Hui, WANG Cong, et al. Improved electrochemical performance of the Na3V2(PO4)3 cathode by B-doping of the carbon coating layer for sodium-ion batteries[J]. Journal of Materials Chemistry A, 2015, 3(29): 15190-15201. |
| 59 | JIANG Yu, ZHOU Xuefeng, LI Dongjun, et al. Highly reversible Na storage in Na3V2(PO4)3 by optimizing nanostructure and rational surface engineering[J]. Advanced Energy Materials, 2018, 8(16): doi: 10.1002/aenm.201800068. |
| 60 | LIANG Xinghui, Xing OU, ZHENG Fenghua, et al. Surface modification of Na3V2(PO4)3 by nitrogen and sulfur dual-doped carbon layer with advanced sodium storage property[J]. ACS Applied Materials & Interfaces, 2017, 9(15): 13151-13162. |
| 61 | ZHANG Lulu, LIU Jing, WEI Cheng, et al. N/P-dual-doped carbon-coated Na3V2(PO4)2O2F microspheres as a high-performance cathode material for sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2020, 12: 3670-3680. |
| 62 | JIN Ting, LIU Yongchang, LI Yang, et al. Electrospun NaVPO4F/C nanofibers as self-standing cathode material for ultralong cycle life Na-ion batteries[J]. Advanced Energy Materials, 2017, 7(15): doi: 10.1002/aenm.201700087. |
| 63 | Zhiqiang LYU, LING Moxiang, YI Hongming, et al. Electrode design for high-performance sodium-ion batteries: Coupling nanorodsssembled Na3V2(PO4)3@C microspheres with a 3D conductive charge transport network[J]. ACS Applied Materials & Interfaces, 2020, 12(12): 13869-13877. |
| 64 | YI Hongming, LI Dan, Zhiqiang LYU, et al. Constructing high-performance 3D porous self-standing electrodes with various morphologies and shapes by a flexible phase separation-derived method[J]. Journal of Materials Chemistry A, 2019, 7(39): 22550-22558. |
| 65 | ZHOU Weidong, XUE Leigang, Xujie LYU, et al. NaxMV(PO4)3 (M=Mn, Fe, Ni) structure and properties for sodium extraction[J]. Nano Letters, 2016, 16(12): 7836-7841. |
| 66 | DONG Jing, ZHANG Guoming, WANG Xiaoguang, et al. Cross-linked Na2VTi(PO4)3@C hierarchical nanofibers as high-performance bi-functional electrodes for symmetric aqueous rechargeable sodium batteries[J]. Journal of Materials Chemistry A, 2017, 5(35): 18725-18736. |
| 67 | KIM Y, HA K H, HO S M, et al. High-capacity anode materials for sodium-ion batteries[J]. Chemistry—A European Journal, 2014, 20: 11980-11992. |
| 68 | QI Yuruo, TONG Zizheng, ZHAO Junmei, et al. Scalable room-temperature synthesis of multi-shelled Na3(VOPO4)2F microsphere cathodes[J]. Joule, 2018, 2(11): 2348-2363. |
| 69 | YI Hongming, LIN Le, LING Moxiang, et al. Scalable and economic synthesis of high-performance Na3V2(PO4)2F3 by a solvothermal ball-milling method[J]. ACS Energy Letters, 2019, 4(7): 1565-1571. |
| 70 | DING C S, NOHIRA T, KURODA K, et al. NaFSA-C1C3pyrFSA ionic liquids for sodium secondary battery operating over a wide temperature range[J]. Journal of Power Sources, 2013, 238: 296-300. |
| 71 | KIM J, CHA H, LEE H, et al. Surface and interfacial chemistry in the nickel-rich cathode materials[J]. Batteries & Supercaps, 2020, 3(4): 309-322. |
| 72 | LI Jing, LI Hongyang, STONE W, et al. Development of electrolytes for single crystal NMC532/artificial graphite cells with long lifetime[J]. Journal of the Electrochemical Society, 2018, 165(3): A626-A635. |
| 73 | SCHIPPER F B, DIXIT H, ERICKSON M, et al. From surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium ion batteries[J]. Advanced Energy Materials, 2018, 8: doi: 10.1002/aenm.201701682. |
| 74 | LIU Wen, LI Xifei, XIONG Dongbin, et al. Significantly improving cycling performance of cathodes in lithium ion batteries: The effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2[J]. Nano Energy, 2018, 44: 111-120. |
| 75 | WU Feng, ZHU Na, BAI Ying, et al. Unveil the mechanism of solid electrolyte interphase on Na3V2(PO4)3 formed by a novel NaPF6/BMITFSI ionic liquid electrolyte[J]. Nano Energy, 2018, 51: 524-532. |
| [1] | Qiannan LIU, Weiping HU, Zhe HU. Research progress of phosphorus-based anode materials for sodium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(4): 1201-1210. |
| [2] | Chang SUN, Zerong DENG, Ningbo JIANG, Lulu ZHANG, Hui FANG, Xuelin YANG. Recent research progress of sodium vanadium fluorophosphate as cathode material for sodium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(4): 1184-1200. |
| [3] | Qiang CHEN, Min LI, Jingfa LI. Application of Prussian blue analogs and their derivatives in potassium ion batteries [J]. Energy Storage Science and Technology, 2021, 10(3): 1002-1015. |
| [4] | Yongli HENG, Zhenyi GU, Jinzhi GUO, Xinglong WU. Na3V2(PO4)3@C cathode material for aqueous zinc-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(3): 938-944. |
| [5] | Peng ZHANG, Xingqiang LAI, Junrong SHEN, Donghai ZHANG, Yongheng YAN, Rui ZHANG, Jun SHENG, Kangwei DAI. Research and industrialization progress of solid-state lithium battery [J]. Energy Storage Science and Technology, 2021, 10(3): 896-904. |
| [6] | Min'an YANG, Ning CHEN, Bo WANG, Qian ZHANG, Jingpei CHEN, Hailei ZHAO, Fushen LI. Gene law about cycle stability of cathode material for lithium-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(2): 462-469. |
| [7] | Zuhao ZHANG, Xiaokai DING, Dong LUO, Jiaxiang CUI, Huixian XIE, Chenyu LIU, Zhan LIN. Challenges and solutions of lithium-rich manganese-based layered oxide cathode materials [J]. Energy Storage Science and Technology, 2021, 10(2): 408-424. |
| [8] | Yue MU, Yun DU, Hai MING, Songtong ZHANG, Jingyi QIU. Methods of investigating structural evolution and interface behavior in cathode materials for Li-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(1): 7-26. |
| [9] | Wei ZHENG, Qiong LIU, Zhouguang LU. Modulating anionic redox reaction in layered transition metal oxides for sodium-ion batteries [J]. Energy Storage Science and Technology, 2020, 9(5): 1416-1427. |
| [10] | Yongsheng GAO, Guanghai CHEN, Xinran WANG, Ying BAI, Chuan WU. Safety of electrolytes for sodium-ion batteries: Strategies and progress [J]. Energy Storage Science and Technology, 2020, 9(5): 1309-1317. |
| [11] | Xingguo QI, Weigang WANG, Yongsheng HU, Qiang ZHANG. Surface modification research of layered oxide materials for sodium-ion batteries [J]. Energy Storage Science and Technology, 2020, 9(5): 1396-1401. |
| [12] | Xiaohui ZHU, Yuhang ZHUANG, Yang ZHAO, Mingzhu NI, Jing XU, Hui XIA. Development of layered cathode materials for sodium-ion batteries [J]. Energy Storage Science and Technology, 2020, 9(5): 1340-1349. |
| [13] | Quan ZHOU, Xingguo QI, Yaxiang LU, Xiaohui RONG, Fei TANG, Weihe KONG, Kun TANG, Liquan CHEN, Yongsheng HU. The necessity of establishing Na-ion battery standards [J]. Energy Storage Science and Technology, 2020, 9(5): 1225-1233. |
| [14] | Yun LU, Jianing LIANG, Yong ZHU, Zhengrong LI, Yezhou HU, Ke CHEN, Deli WANG. Recent progress in organics derived cathode materials for lithium sulfur batteries [J]. Energy Storage Science and Technology, 2020, 9(5): 1454-1466. |
| [15] | FAN Ersha, LI Li, LIN Jiao, ZHANG Xiaodong, CHEN Renjie, WU Feng. Low-temperature molten-salt-assisted recycling of spent LiNi1/3Co1/3Mn1/3O2 cathode materials [J]. Energy Storage Science and Technology, 2020, 9(2): 361-367. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
