Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (5): 1496-1515.doi: 10.19799/j.cnki.2095-4239.2023.0854
• Energy Storage Materials and Devices • Previous Articles Next Articles
Wanrui LI( ), Wenjun LI, Xiaoqing WANG, Shengli LU(
), Wenjun LI, Xiaoqing WANG, Shengli LU( ), Xilian XU(
), Xilian XU( )
)
Received:2023-11-28
Revised:2023-12-22
Online:2024-05-28
Published:2024-05-28
Contact:
Shengli LU, Xilian XU
E-mail:222203855035@zust.edu.cn;lushengli@zust.edu.cn;xuxilian@zust.edu.cn
CLC Number:
Wanrui LI, Wenjun LI, Xiaoqing WANG, Shengli LU, Xilian XU. Research progress of manganese/vanadium-based oxide heterostructure cathodes for zinc-ion batteries[J]. Energy Storage Science and Technology, 2024, 13(5): 1496-1515.
Table 1
Metal-doped manganese/vanadium-based oxide heterostructures and their zinc storage properties"
| Cathodes | Voltage/V | Capacity (mAh/g@A/g) | Cycling performance (cycles@A/g) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| Ag-V2O5 | 0.2~1.6 | 200@0.2, 96@1 | 80(700@3) | 3 mol/L Zn(CF3SO3)2 | [ |
| Ag-V2O5 | 0.2~1.6 | 426@0.1, 326.1@5 | 270(2000@5) | 2 mol/L Zn(CF3SO3)2 | [ |
| Al-VOH | 0.2~1.6 | 380@0.05, 245@4 | 236.5(3000@4) | 3 mol/L Zn(CF3SO3)2 | [ |
| Mg-MnO2 | 1.0~1.8 | 370@0.6, 172@6 | 95(10000@1.5) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
δ-Ca0.25V2O5·nH2O/ δ-Zn0.25V2O5·nH2O | 0.6~1.6 | 340@0.2C, 289@1C | 68(3000@80C) | 1 mol/L ZnSO4 | [ |
| FeVO | 0.2~1.6 | 214.4@0.5, 201.6@3 | 221.6(650@1) | 3 mol/L Zn(CF3SO3)2 | [ |
| Li3(V6O16) | 0.3~1.8 | 350@0.1, 189.8@1 | 189.8(1000@1) | 3 mol/L Zn(CF3SO3)2 | [ |
| Na6V10O28 | 0.2~1.9 | 279.5@0.1, 60@2 | 143(2000@2) | 3 mol/L Zn(CF3SO3)2 | [ |
| NH4V4O10 | 0.4~1.4 | 361.6@1, 252.8@10 | 255.5(1000@10) | 2 mol/L ZnSO4 | [ |
| δ-Ni0.25V2O5 | 0.3~1.7 | 402@0.2, 218.3@5 | 214(1200@5) | 3 mol/L ZnSO4 | [ |
| Mg0.34V2O5·0.84H2O | 0.1~1.8 | 353@0.1, 264@1 | 84(2000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| Na0.33V2O5 | 0.2~1.6 | 367.1@0.1, 96.4@2 | 218.4(1000@1) | 3 mol/L Zn(CF3SO3)2 | [ |
| Zn0.3V2O5·1.5H2O | 0.3~1.6 | 426@0.2, 265.2@10 | 214(20000@10) | 3 mol/L Zn(CF3SO3)2 | [ |
| Cu0.06MnO2·1.7H2O | 0.8~1.9 | 493.3@0.1, 350@0.5 | 363.7(150@0.5) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| Bi0.09MnO2·1.5H2O | 0.8~1.9 | 175.5@0.1, 116.1@1 | 114.5(1100@1) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| Cu-Mn3O4 | 0.8~1.9 | 250@0.1, 45@1 | 110(900@0.6) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| KMgVOH | 0.2~1.5 | 423@0.1, 318@4 | 222(2000@4) | 3 mol/L Zn(CF3SO3)2 | [ |
| Al2.65V6O13·2.07H2O | 0.2~1.4 | 571.7@1, 205.7@5 | 183.5(2000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| AlMO | 0.8~1.8 | 311.2@0.1, 145.2@5 | 125.3(15000@4) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| Na6[V10O28]·nH2O | 0.2~1.5 | 228.5@0.1, 84.6@10 | 119.4(3000@10) | 2 mol/L Zn(CF3SO3)2 | [ |
| NaV8O20·xH2O | 0.4~1.4 | 417@0.1, 111@80 | 214(1600@10) | 3 mol/L Zn(CF3SO3)2 | [ |
| δ-K0.49V2O5 | 0.3~1.5 | 361@0.2, 150@5 | 154(2000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| Ag0.33V2O5 | 0.1~1.6 | 311@0.1, 107@5 | 144(500@2) | 3 mol/L Zn(CF3SO3)2 | [ |
| Cu0.95V2O5 | 0.2~1.6 | 405@0.1, 195@5 | 200(1000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| HNaV6O16·4H2O | 0.2~1.6 | 444@0.5, 328@5 | 307(1000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
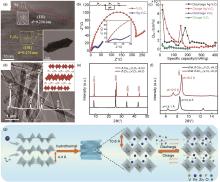
Fig. 3
(a)—(c) Structural and property characterization of Ag-V2O5: (a) TEM and HRTEM image, (b) EIS plots, (c) The calculated Zn2+ diffusion coefficients (DZn2+) vs. capacity plots[77]; (d)—(f) Structural characterization of CVO: (d) TEM images, EDS spectrum and Crystal structure, (e)—(f) XRD pattern[80]; (g) Schematic illustration of the one-pot preparation process and the crystal model of FeVO[81]"
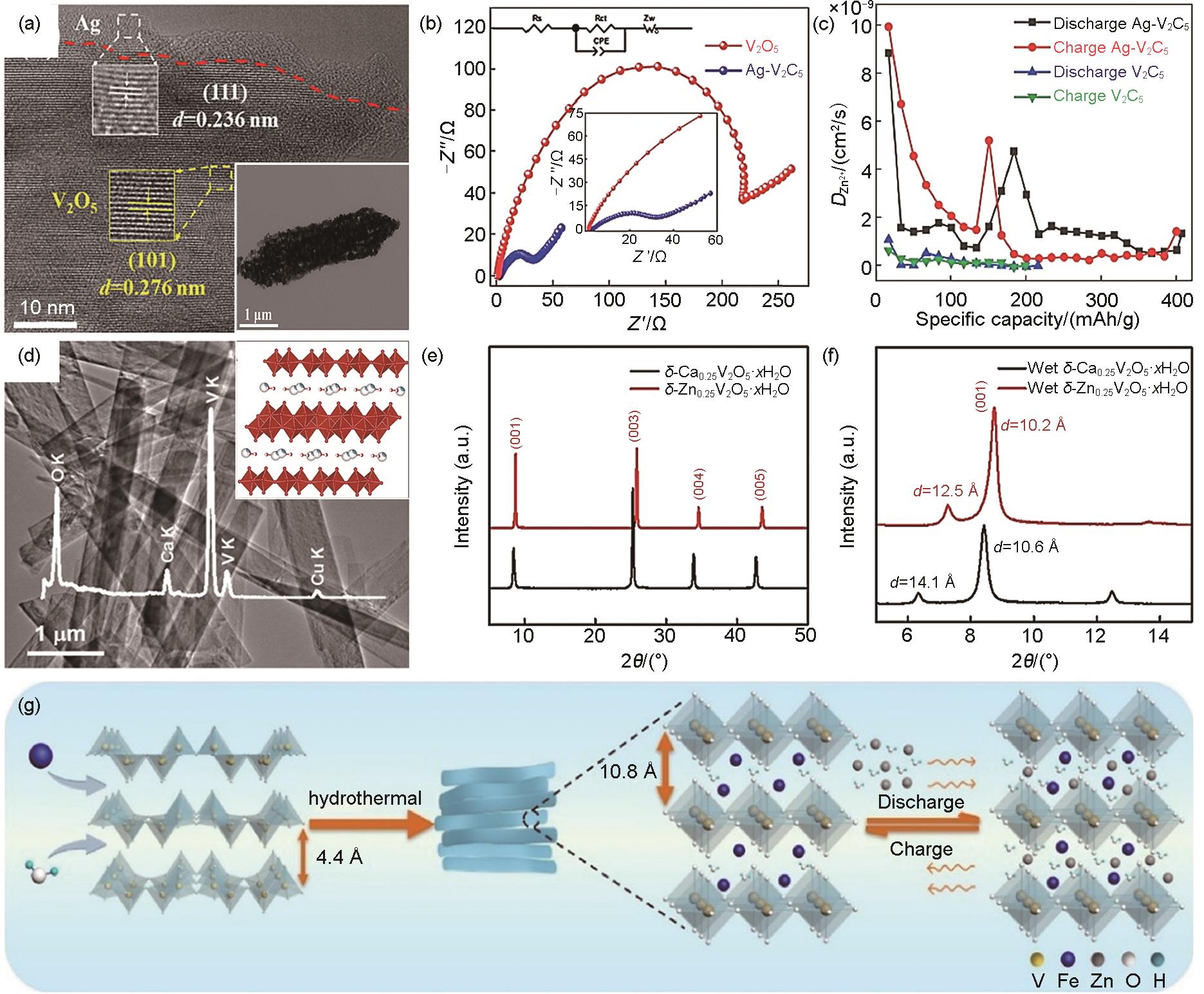
Fig. 4
(a)—(c) Structural characterization of VO2-rG: (a) SEM images, (b) HRTEM images, (c) Schematic diagram of the frame structure[98]; (d)—(g) Structural and property characterization of δ-MnO2-C NA: (d) Schematic illustration for the preparation process, (e) SEM images, (f) The contact angles on the surface of δ-MnO2-C NA electrodes, (g) Charge density differences of the δ-MnO2-C NA and δ-MnO2 with Zn2+ intercalated, Yellow and green colors represent depletion and accumulation of electrons, respectively[99]"
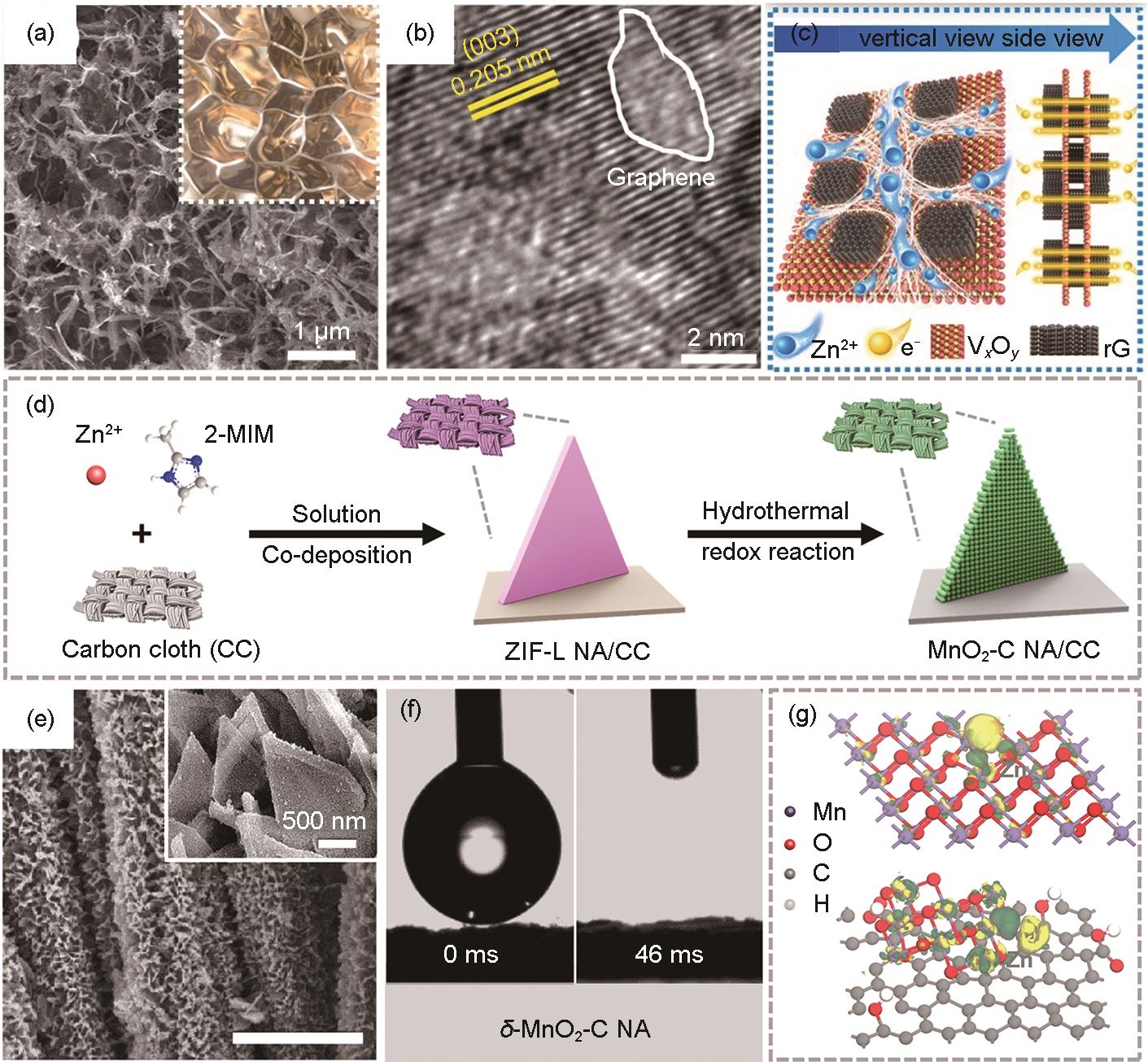
Table 2
The reported manganese/vanadium-based oxide/carbon materials heterostructure cathodes and their zinc storage properties"
| Cathodes | Voltage/V | Capacity (mAh/g@A/g) | Cycling performance (cycles@A/g) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| VOH-rG | 0.2~1.6 | 466@0.1, 190@20 | 267(5000@10) | 2 mol/L Zn(CF3SO3)2 | [ |
| δ-MnO2-C NA/CC | 0.8~1.8 | 346.7@0.5, 187.8@4 | 147(50000@4) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| 0.3~1.9 | 448@0.15, 170@10 | 176(1500@10) | 3 mol/L Zn(CF3SO3)2 | [ | |
| Cu0.26V2O5@C | 0.3~1.6 | 330@0.2, 163.8@2 | 175(500@2) | 3 mol/L Zn(CF3SO3)2 | [ |
| Ni0.006Ca0.0045VO2@C | 0.3~1.8 | 433.8@0.1, 158.8@5 | 74(4000@5) | 2 mol/L ZnSO4 | [ |
| VO2@C | 0.2~1.4 | 281@0.2, 202@5 | 195(1000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| Mn3O4@HCFs | 0.9~1.85 | 215.8@0.3, 115.7@2 | 225(1300@0.4) | 2 mol/L ZnSO4+0.15 mol/L MnSO4 | [ |
| MnO2@CNTs/CNHs | 1.0~1.9 | 343@0.3, 191.3@3 | 162.3(500@3) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| NiMn2O4@C | 1.0~1.85 | 139.7@0.05, 98.5@1.2 | 128.8(850@0.4) | 2 mol/L ZnSO4+0.15 mol/L MnSO4 | [ |
| B-V2O3@C | 0.2~1.4 | 422@0.2, 349@2.5 | 84(2000@5) | 2 mol/L Zn(CF3SO3)2 | [ |
| V2O3@C | 0.3~1.5 | 350@0.1, 250@2 | 161(4000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| V2O3-C | 0.2~1.8 | 415@0.1, 349@1 | 260(100@0.1) | 3 mol/L Zn(CF3SO3)2 | [ |
| H-VO2@CC | 0.1~1.3 | 350@0.1, 192@4 | 150(4500@4) | 3 mol/L Zn(CF3SO3)2 | [ |
| V2O5@void@V2O5@CFs | 0.1~1.8 | 499@0.5, 387@16 | 455(100@4) | 3 mol/L Zn(CF3SO3)2 | [ |
| V2O5@CFC | 0.005~2.0 | 293@0.05, 91@4 | 154(1000@0.5) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| MnO@C | 0.3~1.8 | 456@0.05, 160@2 | 128(2000@2) | 2 mol/L ZnSO4+0.2 mol/L MnSO4 | [ |
| MnO@C | 0.8~1.8 | 210@0.1, 111@1 | 120.2(4500@1) | 3 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| V2O5@CNT | 0.2~1.7 | 312@1, 232@10 | 261(2000@1) | 1 mol/L ZnSO4 | [ |
| α-MnO2@CNT | 1.0~1.8 | 308.5@0.97C, 69.5@97.4C | 163(1000@32.5C) | 2 mol/L ZnSO4+0.2 mol/L MnSO4 | [ |
| Zn x MnO2@CNTs | 0.8~1.8 | 400@0.1, 148@3 | 300(100@1) | 3 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| CNTs@Mn3O4 | 0.8~1.8 | 310@0.1, 116@2 | 123(500@1) | 3 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| CuV2O6@RCNTs | 0.3~1.5 | 353@0.1, 121.5@5 | 174.7(1400@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| KV3O8·0.75H2O@SWCNT | 0.3~1.3 | 379@0.1, 206@5 | 200(10000@5) | 4 mol/L Zn(CF3SO3)2 | [ |
| MnO2@MNH-CNT | 1.0~1.8 | 236@0.4, 108@1.6 | 140(100@0.4) | 1 mol/L ZnSO4 | [ |
| V2O5@CNT | 0.2~1.6 | 375@0.5, 279@10 | 168.5(500@10) | 2 mol/L ZnSO4 | [ |
| ZnMn2O4@CNT | 0.4~1.8 | 220.3@0.1, 136.5@1 | 74.7(2000@3) | 1 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| α-MnO2@CNT HMs | 1.0~1.85 | 296@0.2, 80@3 | 82.9(10000@3) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| α-V2O5@CNT | 0.2~1.8 | 480@0.5, 353@15 | 158(1000@30) | 3 mol/L Zn(CF3SO3)2 | [ |
| S-ZnMn2O4/CNTs | 0.8~1.8 | 175.1@0.5, 128.4@1 | 91.7(800@1.5) | 2 mol/L ZnSO4+0.2 mol/L MnSO4 | [ |
| RGO@VO2 | 0.3~1.3 | 276@0.1, 120@35 | 240(1000@4) | 3 mol/L Zn(CF3SO3)2 | [ |
| CuV2O6@RGO | 0.3~1.6 | 427@0.1, 241@5 | 285(200@2) | 3 mol/L Zn(CF3SO3)2 | [ |
| MnO2@G | 1.0~1.9 | 317@0.1, 112@7.5 | 186(2000@2) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| β-MnO2@GO | 1.05~1.8 | 312.4@0.25C, 94.9@10C | 129.6(2000@4C) | 3 mol/L ZnSO4+0.2 mol/L MnSO4 | [ |
| γ-MnO2@G | 0.8~1.8 | 301@0.5, 95.8@10 | 64(300@1) | 2 mol/L ZnSO4+0.4 mol/L MnSO4 | [ |
| MnO2@rGO | 1.0~1.9 | 332.2@0.3, 172.3@6 | 165.4(500@6) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| Na/MnO2@GCF | 1.0~1.8 | 381.8@0.1, 94.8@3 | 188(1000@1) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| P-MnO2-x @VMG | 1.0~1.8 | 302.8@0.5, 150.1@10 | 185.8(1000@2) | 2 mol/L ZnSO4+0.2 mol/L MnSO4 | [ |
| ZnMn2O4@NG | 0.8~1.8 | 221@0.1, 110@1 | 74(2500@1) | 1 mol/L ZnSO4+0.05 mol/L MnSO4 | [ |
| rGO@HM-ZnMn2O4 | 0.8~1.8 | 188@0.3, 59@2 | 72.7(650@1) | 1 mol/L ZnSO4+0.05 mol/L MnSO4 | [ |
| δ-Na x V2O5·nH2O@G | 0.2~1.6 | 433.5@0.1, 244.1@5 | 215(1000@2) | 3 mol/L Zn(CF3SO3)2 | [ |
| H11Al2V6O23.2@G | 0.4~1.4 | 305.4@1, 180.6@10 | 131.7(400@2) | 2 mol/L ZnSO4 | [ |
| Na1.1V3O7.9@rGO | 0.4~1.4 | 220@0.3, 72@2 | 84.8(500@1) | 1 mol/L Zn(CF3SO3)2 | [ |
| Od-VO2@rG | 0.2~1.4 | 376@0.1, 116@20 | 186(5000@10) | 2 mol/L Zn(CF3SO3)2 | [ |
| Ov-PVO@G | 0.2~1.6 | 583.1@0.2, 372.3@20 | 448.3(3000@10) | 3 mol/L Zn(CF3SO3)2 | [ |
| V2O5@EGO | 0.2~1.6 | 462@0.2, 334@5 | 187(3000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| V2O5/VG/CC | 0.2~1.6 | 370@0.2, 216@50 | 278(5000@2) | 3 mol/L ZnSO4 | [ |
| Na5V12O32@G | 0.2~1.6 | 220@0.3, 104.2@5 | 105(4400@5) | 2 mol/L Zn(CF3SO3)2 | [ |
| VO2(B)/GO | 0.3~1.7 | 423@0.5, 371@3 | 227(2750@15) | 3 mol/L Zn(CF3SO3)2 | [ |
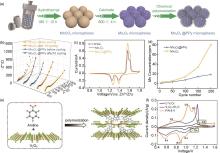
Fig. 5
(a) The flow chart of the formation process for mesoporous Mn2O3@PPy material; (b)—(d) Electrochemical and stability characterization of Mn2O3 with Mn2O3@PPy electrodes: (b) Electrochemical impedance spectroscopy before and after cycling (EIS), (c) The CV curves, (d) Manganese dissolution situation of the Mn2O3 and Mn2O3@PPy electrode after different cycles in 3 mol/L ZnSO4 electrolyte[144]; (e)—(f) Structural and property characterization of PANI-V: (e) Schematic illustration of superlattice synthesis, (f) Cyclic voltammograms (CV) of PANI-V in comparison to C-V2O5 and V2O5·nH2O[145]"
Table 3
The reported manganese/vanadium-based oxide/conductive polymer heterostructure cathodes and their electrochemical properties"
| Cathodes | Voltage/V | Capacity (mAh/g @A/g) | Cycling performance (cycles@A/g) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| V2O5@PEDOT | 0.2~1.4 | 370.5@0.5, 175@50 | 310.1(1000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| Od-V2O5@PEDOT | 0.3~1.6 | 449@0.2, 358@10 | 318(6000@10) | 3 mol/L Zn(CF3SO3)2 | [ |
| NH4V3O8@PEDOT | 0.4~1.6 | 356.8@0.05, 163.6@10 | 160.6(5000@10) | 3 mol/L ZnCF3SO3 | [ |
| VO@PEDOT | 0.3~1.4 | 390@0.3, 100@20 | 170(1000@5) | 3 mol/L ZnSO4 | [ |
| V2O5@PEDOT/CC | 0.2~1.6 | 360@0.1, 232@20 | 223.6(1000@5) | 2.5 mol/L Zn(CF3SO3)2 | [ |
| Na0.76V6O15@PEDOT | 0.3~1.5 | 355@0.05, 165@4 | 168(2600@4) | 3 mol/L ZnSO4+3 mol/L Zn(CF3SO3)2 | [ |
| (NH4)2V6O16·1.5H2O@PEDOT | 0.2~1.8 | 344@0.5, 155@20 | 209(1000@10) | 2.5 mol/L Zn(CF3SO3)2 | [ |
| V2O5@PPy | 0.3~1.6 | 441@0.1, 291@5 | 304(2000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| Mn2O3@PPy | 0.8~1.8 | 337.9@0.1, 77.6@3.2 | 73.5(3000@3) | 3 mol/L ZnSO4+0.5 mol/L MnSO4 | [ |
| MnO2/Mn2O3@PPy | 1.0~1.85 | 289.8@0.2, 199.8@3 | 253(1000@1) | 2 mol/L ZnSO4+0.2 mol/L MnSO4 | [ |
| Fe/α-MnO2@PPy | 0.8~1.9 | 270@0.1, 73@1 | 280(100@0.1) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| β-MnO2@PPy | 0.8~1.8 | 361.8@0.2, 69.9@1.5 | 361.8(160@0.2) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| MnO x @PPy | 0.4~1.9 | 302@0.15, 159.9@3 | 113.7(1000@6) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| V2O5·nH2O@PPy | 0.2~1.5 | 383@0.1, 281@2 | 203(2000@4) | 3 mol/L Zn(CF3SO3)2 | [ |
| V2O5@PPy | 0.3~1.5 | 374@0.2, 241@5 | 220(2000@10) | 3 mol/L Zn(CF3SO3)2 | [ |
| PANI@MnO2/CC | 0.8~1.8 | 286@0.5, 177@4 | 158(9000@4) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| MnO2@PANI | 1.0~1.8 | 280@0.2, 110@3 | 125(5000@2) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| MnVO@PANI | 0.3~1.6 | 462@0.1, 290@5 | 246.5(10000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| Mg/PANI/V2O5·nH2O | 0.2~1.6 | 424@0.5, 158@6 | 78(1000@10) | 3 mol/L Zn(CF3SO3)2 | [ |
| V2O5·H2O@PANI | 0.4~1.6 | 346@0.3, 186@5 | 223(800@3) | 3 mol/L Zn(TfO)2+6 mol/L LiTFSI | [ |
| V2O5-x @PANI | 0.4~1.6 | 283@1, 234@16 | 201(1000@1) | 2 mol/L ZnSO4 | [ |
| SP@PDA-d-δ-MnO2 | 1.0~1.8 | 483@0.2, 80.3@2 | 73.3(500@1) | 2 mol/L ZnSO4 | [ |
| VO-DP | 0.2~1.6 | 473@0.05, 144@10 | 117.5(15000@10) | 2 mol/L Zn(OTF)2 | [ |
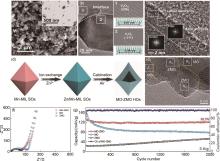
Fig. 6
(a)—(c) V3O7/V6O13 nanosheet structure characterization: (a) SEM and TEM images, (b)—(c) HRTEM images. The inset of (c) is the FFT patterns of the edge and central regions of the nanosheet[166]; (d)—(g) Structural and property characterization of MO-ZMO HOs: (d) Schematic illustration of the synthetic process, (e) HRTEM images, (f)—(g) EIS and cycling performance plots of MO-ZMO HO, MO HO and ZMO HOs electrodes, respectively[167]"
Table 4
Manganese/vanadium-based oxide/metal oxide heterostructure cathodes and their electrochemical storage zinc properties"
| Cathodes | Voltage/V | Capacity (mAh/g@A/g) | Cycling performance (cycles@A/g) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| VO2@V2O5 | 0.2~1.6 | 435.4@0.2, 167.5@10 | 126.8(1500@10) | 3 mol/L Zn(CF3SO3)2 | [ |
| VO2@V2O5 | 0.2~1.6 | 401@0.1, 178@5 | 167(2000@5) | 2 mol/L ZnSO4 | [ |
| V3O7@V6O13 | 0.2~1.4 | 445@0.1, 376@2 | 259(1000@1) | 3 mol/L Zn(CF3SO3)2 | [ |
| V2O5@NaV6O15 | 0.4~1.4 | 399@0.1, 275.8@1 | 164(2000@5) | 3 mol/L ZnSO4 | [ |
| V2O5@NaV6O15 | 0.2~1.8 | 390@0.3, 253.8@5 | 264(3000@5) | 2 mol/L Zn(CF3SO3)2 | [ |
| Al2O3@(NH4)2V4O9 | 0.2~1.6 | 269@0.5, 200@5 | 173(3000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| (NH4)2Co2V10O28·16H2O/ (NH4)2V10O25·8H2O | 0.2~1.8 | 367.7@0.1, 214@1 | 201.3(1000@1) | 3 mol/L ZnSO4 | [ |
| Zn3V3O8@ZnO@NC | 0.2~1.6 | 216.3@0.5, 183@3 | 120.5(2000@5) | 3 mol/L Zn(CF3SO3)2 | [ |
| ZnMn2O4@Mn2O3 | 0.8~1.9 | 82.6@0.5, 42.1@3.2 | 111.9(300@0.5) | 1 mol/L ZnSO4 | [ |
| ZnMn2O4@MnOOH | 0.8~1.85 | 336.7@0.1, 98.5@5 | 81.9(1000@1) | 2 mol/L ZnSO4+0.2 mol/L MnSO4 | [ |
| CeO2/MnO x @C | 0.8~1.8 | 365@0.05, 70@2 | 130(800@0.5) | 3 mol/L Zn(CF3SO3)2+0.1 mol/L MnSO4 | [ |
| Sn x MnO2@SnO2 | 0.8~1.8 | 316.1@0.3, 179.4@2 | 153.4(2000@2) | 2 mol/L ZnSO4+0.1 mol/L MnSO4 | [ |
| ZnMn2O4@Mn2O3 | 0.8~1.8 | 247.4@0.1, 120.2@5 | 108(2000@3) | 2 mol/L Zn(CF3SO3)2+0.1 mol/L MnSO4 | [ |
| V2O3/V3O5/Zn2VO4@NC | 0.2~1.5 | 358@0.2, 95.8@5 | 100.1(3000@5) | 2 mol/L ZnSO4 | [ |
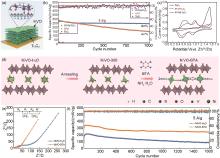
Fig. 7
(a)—(c) Structural and property characterization of HVO@Ti3C2: (a) Structure diagram of 2D heterostructure material, (b) The cycle performance of HVO@Ti3C2, HVO, and Ti3C2 electrodes at 5.0 A/g, (c) The first CV curve of Ti3C2, HVO-Ti3C2, and HVO@Ti3C2[180]; (d)—(f) Structural and property characterization of NiVO-BTA: (d) Schematic illustration of the preparation process, (e) Nyquist plots of NiVO-BTA and NiVO-H2O cathodes, the inset is the equivalent circuit, (f) The cycle performances of NiVO-BTA and NiVO-H2O at 5.0 A/g[181]"
| 95 | SU Z H, WANG R H, HUANG J H, et al. Silver vanadate (Ag0.33V2O5) nanorods from Ag intercalated vanadium pentoxide for superior cathode of aqueous zinc-ion batteries[J]. Rare Metals, 2022, 41(8): 2844-2852. |
| 96 | YU X, HU F, GUO Z Q, et al. High-performance Cu0.95V2O5 nanoflowersas cathode materials for aqueous zinc-ion batteries[J]. Rare Metals, 2022, 41(1): 29-36. |
| 97 | GUAN C, HU F, YU X, et al. High performance of HNaV6O16·4H2O nanobelts for aqueous zinc-ion batteries with in situ phase transformation by Zn(CF3SO3)2 electrolyte[J]. Rare Metals, 2022, 41(2): 448-456. |
| 98 | LUO H, WANG B, WU F D, et al. Synergistic nanostructure and heterointerface design propelled ultra-efficient in situ self-transformation of zinc-ion battery cathodes with favorable kinetics[J]. Nano Energy, 2021, 81: 105601. |
| 99 | XU X L, CHEN Y, LI W R, et al. Achieving ultralong-cycle zinc-ion battery via synergistically electronic and structural regulation of a MnO2 nanocrystal-carbon hybrid framework[J]. Small, 2023, 19(10): e2207517. |
| 100 | LI C L, LI M, XU H T, et al. Constructing hollow nanotube-like amorphous vanadium oxide and carbon hybrid via in situ electrochemical induction for high-performance aqueous zinc-ion batteries[J]. Journal of Colloid and Interface Science, 2022, 623: 277-284. |
| 101 | WANG X W, ZHANG B, FENG J M, et al. Cu-MOF-derived and porous Cu0.26V2O5@C composite cathode for aqueous zinc-ion batteries[J]. Sustainable Materials and Technologies, 2020, 26: e00236. |
| 102 | ZHAO X, MAO L, CHENG Q H, et al. Dual-cation preintercalated and amorphous carbon confined vanadium oxides as a superior cathode for aqueous zinc-ion batteries[J]. Carbon, 2022, 186: 160-170. |
| 103 | YANG M, WANG Y Y, SUN Z W, et al. Anti-aggregation growth and hierarchical porous carbon encapsulation enables the C@VO2 cathode with superior storage capability for aqueous zinc-ion batteries[J]. Journal of Energy Chemistry, 2022, 67: 645-654. |
| 104 | LONG J, YANG Z H, YANG F H, et al. Electrospun core-shell Mn3O4/carbon fibers as high-performance cathode materials for aqueous zinc-ion batteries[J]. Electrochimica Acta, 2020, 344: 136155. |
| 105 | HUANG Y, LI Z X, JIN S Y, et al. Carbon nanohorns/nanotubes: An effective binary conductive additive in the cathode of high energy-density zinc-ion rechargeable batteries[J]. Carbon, 2020, 167: 431-438. |
| 106 | LONG J, GU J X, YANG Z H, et al. Highly porous, low band-gap NixMn3– xO4(0.55≤x≤1.2) spinel nanoparticles with in situ coated carbon as advanced cathode materials for zinc-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(30): 17854-17866. |
| 107 | LIU X D, WANG Z Q, NIU Y L, et al. Scalable synthesis of novel V2O3/carbon composite as advanced cathode material for aqueous zinc-ion batteries[J]. Ceramics International, 2022, 48(11): 15594-15602. |
| 108 | DING Y C, PENG Y Q, CHEN S H, et al. Hierarchical porous metallic V2O3@C for advanced aqueous zinc-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(47): 44109-44117. |
| 109 | WANG J X, LI G S, LIU X Q, et al. In-situ electrochemical oxidization of V2O3-C cathode for boosted zinc-ion storage performance[J]. Applied Surface Science, 2023, 616: 156481. |
| 110 | LUO P, ZHANG W W, CAI W Y, et al. Accelerated ion/electron transport kinetics and increased active sites via local internal electric fields in heterostructured VO2 - carbon cloth for enhanced zinc-ion storage[J]. Nano Research, 2023, 16(1): 503-512. |
| 111 | XIONG L, QU Z L, SHEN Z Y, et al. In situ construction of ball-in-ball structured porous vanadium pentoxide intertwined with carbon fibers induces superior electronic/ionic transport dynamics for aqueous zinc-ion batteries[J]. Journal of Colloid and Interface Science, 2022, 615: 184-195. |
| 112 | XU N, YAN C Y, HE W, et al. Flexible electrode material of V2O5 carbon fiber cloth for enhanced zinc ion storage performance in flexible zinc-ion battery[J]. Journal of Power Sources, 2022, 533: 231358. |
| 113 | LI C L, LI M, XU H T, et al. Hierarchical accordion-like manganese oxide@carbon hybrid with strong interaction heterointerface for high-performance aqueous zinc ion batteries[J]. Journal of Colloid and Interface Science, 2022, 628: 553-561. |
| 114 | ZHU Z X, LIN Z W, SUN Z W, et al. Deciphering H+/Zn2+ co-intercalation mechanism of MOF-derived 2D MnO/C cathode for long cycle life aqueous zinc-ion batteries[J]. Rare Metals, 2022, 41(11): 3729-3739. |
| 115 | YIN B S, ZHANG S W, KE K, et al. Binder-free V2O5/CNT paper electrode for high rate performance zinc ion battery[J]. Nanoscale, 2019, 11(42): 19723-19728. |
| 116 | BI S, WU Y, CAO A, et al. Free-standing three-dimensional carbon nanotubes/amorphous MnO2 cathodes for aqueous zinc-ion batteries with superior rate performance[J]. Materials Today Energy, 2020, 18: 100548. |
| 117 | REN G Y, LUO Z Q, DUAN Y Q, et al. Carbon nanotube@Mn3O4 composite as cathode for high-performance aqueous zinc ion battery[J]. Journal of Alloys and Compounds, 2022, 898: 162747. |
| 118 | SONG J L, WANG W J, FANG Y, et al. Freestanding CuV2O6/carbon nanotube composite films for flexible aqueous zinc-ion batteries[J]. Applied Surface Science, 2022, 578: 152053. |
| 1 | CHEN J H, NAVEED A, NULI Y N, et al. Designing an intrinsically safe organic electrolyte for rechargeable batteries[J]. Energy Storage Materials, 2020, 31: 382-400. |
| 2 | SHI W X. Renewable energy: Finding solutions for a greener tomorrow[J]. Reviews in Environmental Science and Bio/Technology, 2010, 9(1): 35-37. |
| 3 | FANG G Z, ZHOU J, PAN A Q, et al. Recent advances in aqueous zinc-ion batteries[J]. ACS Energy Letters, 2018, 3(10): 2480-2501. |
| 4 | YANG Z G, ZHANG J L, KINTNER-MEYER M C W, et al. Electrochemical energy storage for green grid[J]. Chemical Reviews, 2011, 111(5): 3577-3613. |
| 5 | CHEN R J, LUO R, HUANG Y X, et al. Advanced high energy density secondary batteries with multi-electron reaction materials[J]. Advanced Science, 2016, 3(10): 1600051. |
| 6 | XU C J, CHEN Y Y, SHI S, et al. Secondary batteries with multivalent ions for energy storage[J]. Scientific Reports, 2015, 5: 14120. |
| 7 | JEONG G, KIM Y U, KIM H, et al. Prospective materials and applications for Li secondary batteries[J]. Energy & Environmental Science, 2011, 4(6): 1986-2002. |
| 8 | FLAMME B, RODRIGUEZ GARCIA G, WEIL M, et al. Guidelines to design organic electrolytes for lithium-ion batteries: Environmental impact, physicochemical and electrochemical properties[J]. Green Chemistry, 2017, 19(8): 1828-1849. |
| 9 | KIM D J, YOO D J, OTLEY M T, et al. Rechargeable aluminium organic batteries[J]. Nature Energy, 2019, 4: 51-59. |
| 10 | DEMIR-CAKAN R, PALACIN M R, CROGUENNEC L. Rechargeable aqueous electrolyte batteries: From univalent to multivalent cation chemistry[J]. Journal of Materials Chemistry A, 2019, 7(36): 20519-20539. |
| 11 | PASTA M, WESSELLS C D, HUGGINS R A, et al. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage[J]. Nature Communications, 2012, 3: 1149. |
| 12 | 裴英伟, 张红, 王星辉. 可充电锌离子电池电解质的研究进展[J]. 储能科学与技术, 2022, 11(7): 2075-2082. |
| PEI Y W, ZHANG H, WANG X H. Recent advances in the electrolytes of rechargeable zinc-ion batteries[J]. Energy Storage Science and Technology, 2022, 11(7): 2075-2082. | |
| 119 | WAN F, HUANG S, CAO H M, et al. Freestanding potassium vanadate/carbon nanotube films for ultralong-life aqueous zinc-ion batteries[J]. ACS Nano, 2020, 14(6): 6752-6760. |
| 120 | KHAMSANGA S, NGUYEN M T, YONEZAWA T, et al. MnO2 heterostructure on carbon nanotubes as cathode material for aqueous zinc-ion batteries[J]. International Journal of Molecular Sciences, 2020, 21(13): 4689. |
| 121 | LI Y K, HUANG Z M, KALAMBATE P K, et al. V2O5 nanopaper as a cathode material with high capacity and long cycle life for rechargeable aqueous zinc-ion battery[J]. Nano Energy, 2019, 60: 752-759. |
| 122 | GAO F, MEI B, XU X Y, et al. Rational design of ZnMn2O4 nanoparticles on carbon nanotubes for high-rate and durable aqueous zinc-ion batteries[J]. Chemical Engineering Journal, 2022, 448: 137742. |
| 123 | LIU Y Z, CHI X W, HAN Q, et al. α-MnO2 nanofibers/carbon nanotubes hierarchically assembled microspheres: Approaching practical applications of high-performance aqueous Zn-ion batteries[J]. Journal of Power Sources, 2019, 443: 227244. |
| 124 | WANG Z Y, LI L N, ZHAO F, et al. Hierarchical amorphous vanadium oxide and carbon nanotubes microspheres with strong interface interaction for Superior performance aqueous Zinc-ion batteries[J]. Journal of Colloid and Interface Science, 2023, 645: 542. |
| 125 | YANG Y T, SHAO T, ZHANG Y, et al. Anionic S-doping of a ZnMn2O4/CNTs cathode material enhances its Zn2+ storage performance in aqueous zinc-ion batteries[J]. Journal of Power Sources, 2023, 564: 232863. |
| 126 | DAI X, WAN F, ZHANG L L, et al. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance[J]. Energy Storage Materials, 2019, 17: 143-150. |
| 127 | LIU Y Y, LI Q, MA K X, et al. Graphene oxide wrapped CuV2O6 nanobelts as high-capacity and long-life cathode materials of aqueous zinc-ion batteries[J]. ACS Nano, 2019, 13(10): 12081-12089. |
| 128 | WANG J J, WANG J G, LIU H Y, et al. A highly flexible and lightweight MnO2/graphene membrane for superior zinc-ion batteries[J]. Advanced Functional Materials, 2021, 31(7): 2007397. |
| 129 | DING S X, ZHANG M Z, QIN R Z, et al. Oxygen-deficient β-MnO2@Graphene oxide cathode for high-rate and long-life aqueous zinc ion batteries[J]. Nano-Micro Letters, 2021, 13(1): 173. |
| 130 | WANG C, ZENG Y X, XIAO X, et al. γ-MnO2 nanorods/graphene composite as efficient cathode for advanced rechargeable aqueous zinc-ion battery[J]. Journal of Energy Chemistry, 2020, 43: 182-187. |
| 13 | BIN D, WANG F, TAMIRAT A G, et al. Progress in aqueous rechargeable sodium-ion batteries[J]. Advanced Energy Materials, 2018, 8(17): 1703008. |
| 14 | WANG F, FAN X L, GAO T, et al. High-voltage aqueous magnesium ion batteries[J]. ACS Central Science, 2017, 3(10): 1121-1128. |
| 15 | GHEYTANI S, LIANG Y L, WU F L, et al. An aqueous Ca-ion battery[J]. Advanced Science, 2017, 4(12): 1700465. |
| 16 | GOODENOUGH J B, KIM Y. Challenges for rechargeable Li batteries[J]. Chemistry of Materials, 2010, 22(3): 587-603. |
| 17 | YANG C Y, CHEN J, QING T T, et al. 4.0V aqueous Li-ion batteries[J]. Joule, 2017, 1(1): 122-132. |
| 18 | SU D W, MCDONAGH A, QIAO S Z, et al. High-capacity aqueous potassium-ion batteries for large-scale energy storage[J]. Advanced Materials, 2017, 29(1)1604007 |
| 19 | BLANC L E, KUNDU D P, NAZAR L F. Scientific challenges for the implementation of Zn-ion batteries[J]. Joule, 2020, 4(4): 771-799. |
| 20 | CHEN L N, RUAN Y S, ZHANG G B, et al. Ultrastable and high-performance Zn/VO2 battery based on a reversible single-phase reaction[J]. Chemistry of Materials, 2019, 31(3): 699-706. |
| 21 | SONG M, TAN H, CHAO D L, et al. Recent advances in Zn-ion batteries[J]. Advanced Functional Materials, 2018, 28(41): 1802564. |
| 22 | LI C G, ZHANG X D, HE W, et al. Cathode materials for rechargeable zinc-ion batteries: From synthesis to mechanism and applications[J]. Journal of Power Sources, 2020, 449: 227596. |
| 23 | FANG G Z, ZHU C Y, CHEN M H, et al. Suppressing manganese dissolution in potassium manganate with rich oxygen defects engaged high-energy-density and durable aqueous zinc-ion battery[J]. Advanced Functional Materials, 2019, 29(15): 1808375. |
| 24 | HUANG J H, WANG Z, HOU M Y, et al. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery[J]. Nature Communications, 2018, 9: 2906. |
| 25 | JIANG B Z, XU C J, WU C L, et al. Manganese sesquioxide as cathode material for multivalent zinc ion battery with high capacity and long cycle life[J]. Electrochimica Acta, 2017, 229: 422-428. |
| 131 | HUANG Y, LIU J W, HUANG Q Y, et al. Flexible high energy density zinc-ion batteries enabled by binder-free MnO2/reduced graphene oxide electrode[J]. NPJ Flexible Electronics, 2018, 2: 21. |
| 132 | WU Y Z, WANG M C, TAO Y, et al. Electrochemically derived graphene-like carbon film as a superb substrate for high-performance aqueous Zn-ion batteries[J]. Advanced Functional Materials, 2020, 30(5): 1907120. |
| 133 | ZHANG Y, DENG S J, PAN G X, et al. Introducing oxygen defects into phosphate ions intercalated manganese dioxide/vertical multilayer graphene arrays to boost flexible zinc ion storage[J]. Small Methods, 2020, 4(6): 1900828. |
| 134 | CHEN L L, YANG Z H, QIN H G, et al. Advanced electrochemical performance of ZnMn2O4/N-doped graphene hybrid as cathode material for zinc ion battery[J]. Journal of Power Sources, 2019, 425: 162-169. |
| 135 | CHEN L L, YANG Z H, QIN H G, et al. Graphene-wrapped hollow ZnMn2O4 microspheres for high-performance cathode materials of aqueous zinc ion batteries[J]. Electrochimica Acta, 2019, 317: 155-163. |
| 136 | ZHANG W Y, LIANG S Q, FANG G Z, et al. Ultra-high mass-loading cathode for aqueous zinc-ion battery based on graphene-wrapped aluminum vanadate nanobelts[J]. Nano-Micro Letters, 2019, 11(1): 69. |
| 137 | CAI Y S, LIU F, LUO Z G, et al. Pilotaxitic Na1.1V3O7.9 nanoribbons/graphene as high-performance sodium ion battery and aqueous zinc ion battery cathode[J]. Energy Storage Materials, 2018, 13: 168-174. |
| 138 | LUO H, WANG B, WANG C L, et al. Synergistic deficiency and heterojunction engineering boosted VO2 redox kinetics for aqueous zinc-ion batteries with superior comprehensive performance[J]. Energy Storage Materials, 2020, 33: 390-398. |
| 139 | YANG H Y, WANG Y, WANG P P, et al. Three-in-one organic-inorganic heterostructures: From scalable ball-milling synthesis to freestanding cathodes with high areal capacity for aqueous zinc-ion batteries[J]. Chemical Engineering Journal, 2023, 457: 141140. |
| 140 | ZHANG Y B, QIN J D, BATMUNKH M, et al. Scalable spray drying production of amorphous V2O5-EGO 2D heterostructured xerogels for high-rate and high-capacity aqueous zinc ion batteries[J]. Small, 2022, 18(10): 2105761. |
| 141 | ZHANG X, TANG Y C, HE P G, et al. Edge-rich vertical graphene nanosheets templating V2O5 for highly durable zinc ion battery[J]. Carbon, 2021, 172: 207-213. |
| 142 | RAO L, ZHOU Z, LIU H B, et al. In-situ electrochemical conversion of Na5V12O32@graphene for enhanced cycle stability in aqueous zinc ion batteries[J]. Journal of Colloid and Interface Science, 2023, 629: 473-481. |
| 26 | ZHANG N, JIA M, DONG Y, et al. Hydrated layered vanadium oxide as a highly reversible cathode for rechargeable aqueous zinc batteries[J]. Advanced Functional Materials, 2019, 29(10): 1807331. |
| 27 | LI Y, YANG W, YANG W, et al. High-performance zinc-ion batteries enabled by electrochemically induced transformation of vanadium oxide cathodes[J]. Journal of Energy Chemistry, 2021, 60: 233-240. |
| 28 | QI Z C, XIONG T, CHEN T, et al. Harnessing oxygen vacancy in V2O5 as high performing aqueous zinc-ion battery cathode[J]. Journal of Alloys and Compounds, 2021, 870: 159403. |
| 29 | MA L T, CHEN S M, LONG C B, et al. Achieving high-voltage and high-capacity aqueous rechargeable zinc ion battery by incorporating two-species redox reaction[J]. Advanced Energy Materials, 2019, 9(45): 1902446. |
| 30 | ZHANG L Y, CHEN L, ZHOU X F, et al. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: The zinc/zinc hexacyanoferrate system[J]. Advanced Energy Materials, 2015, 5(2): 1400930. |
| 31 | TRÓCOLI R, LA MANTIA F. An aqueous zinc-ion battery based on copper hexacyanoferrate[J]. ChemSusChem, 2015, 8(3): 481-485. |
| 32 | ZHAO Y L, ZHU Y H, ZHANG X B. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries[J]. InfoMat, 2020, 2(2): 237-260. |
| 33 | WU Y, FEE J, TOBIN Z, et al. Amorphous manganese oxides: An approach for reversible aqueous zinc-ion batteries[J]. ACS Applied Energy Materials, 2020, 3(2): 1627-1633. |
| 34 | JURAN T R, YOUNG J, SMEU M. Density functional theory modeling of MnO2 polymorphs as cathodes for multivalent ion batteries[J]. The Journal of Physical Chemistry C, 2018, 122(16): 8788-8795. |
| 35 | CHENG F, CHEN J, GOU X , et al. High-power alkaline Zn-MnO2 batteries using γ-MnO2 nanowires/nanotubes and electrolytic zinc powder[J]. Advanced Materials, 2005, 17(22): 2753-2756. |
| 36 | LIU S D, KANG L, KIM J M, et al. Recent advances in vanadium-based aqueous rechargeable zinc-ion batteries[J]. Advanced Energy Materials, 2020, 10(25): 2000477. |
| 37 | WAN F, NIU Z Q. Design strategies for vanadium-based aqueous zinc-ion batteries[J]. Angewandte Chemie International Edition, 2019, 58(46): 16358-16367. |
| 38 | XUE T, FAN H J. From aqueous Zn-ion battery to Zn-MnO2 flow battery: A brief story[J]. Journal of Energy Chemistry, 2021, 54: 194-201. |
| 39 | CHEN L L, YANG Z H, CUI F, et al. Ultrathin MnO2 nanoflakes grown on N-doped hollow carbon spheres for high-performance aqueous zinc ion batteries[J]. Materials Chemistry Frontiers, 2020, 4(1): 213-221. |
| 40 | SONG Q Y, ZHOU S H, WANG S Y, et al. Insights into the oxygen vacancies in transition metal oxides for aqueous Zinc-Ion batteries[J]. Chemical Engineering Journal, 2023, 461: 142033. |
| 143 | YUAN Z Y, XIAO F Y, FANG Y X, et al. Defect engineering on VO2(B) nanoleaves/graphene oxide for the high performance of cathodes of zinc-ion batteries with a wide temperature range[J]. Journal of Power Sources, 2023, 559: 232688. |
| 144 | CAI K X, LUO S H, QIAN L X, et al. Three-dimensional porous composite Mn2O3@PPy as cathode material for zinc ion battery with high energy density[J]. Journal of Power Sources, 2023, 564: 232854. |
| 145 | LI W J, HAN C, GU Q F, et al. Electron delocalization and dissolution-restraint in vanadium oxide superlattices to boost electrochemical performance of aqueous zinc-ion batteries[J]. Advanced Energy Materials, 2020, 10(48): 2001852. |
| 146 | HUANG A X, ZHOU W J, WANG A R, et al. Self-initiated coating of polypyrrole on MnO2/Mn2O3 nanocomposite for high-performance aqueous zinc-ion batteries[J]. Applied Surface Science, 2021, 545: 149041. |
| 147 | LIU S C, ZHU H, ZHANG B H, et al. Tuning the kinetics of zinc-ion insertion/extraction in V2O5 by in situ polyaniline intercalation enables improved aqueous zinc-ion storage performance[J]. Advanced Materials, 2020, 32(26): 2001113. |
| 148 | FENG Z Y, SUN J J, LIU Y Y, et al. Polypyrrole-intercalation tuning lamellar structure of V2O5·nH2O boosts fast zinc-ion kinetics for aqueous zinc-ion battery[J]. Journal of Power Sources, 2022, 536: 231489. |
| 149 | WANG W J, HE D X, FANG Y, et al. Pillaring of a conductive polymer in layered V2O5 boosting ultra-fast Zn2+/H+ storage in aqueous media[J]. Electrochimica Acta, 2022, 416: 140270. |
| 150 | KIM J, LEE S H, PARK C, et al. Controlling vanadate nanofiber interlayer via intercalation with conducting polymers: Cathode material design for rechargeable aqueous zinc ion batteries[J]. Advanced Functional Materials, 2021, 31(26): 2100005. |
| 151 | LI S L, WEI X J, WU C H, et al. Constructing three-dimensional structured V2O5/conductive polymer composite with fast ion/electron transfer kinetics for aqueous zinc-ion battery[J]. ACS Applied Energy Materials, 2021, 4(4): 4208-4216. |
| 152 | DU Y H, WANG X Y, SUN J C. Tunable oxygen vacancy concentration in vanadium oxide as mass-produced cathode for aqueous zinc-ion batteries[J]. Nano Research, 2021, 14(3): 754-761. |
| 153 | BIN D, HUO W C, YUAN Y B, et al. Organic-inorganic-induced polymer intercalation into layered composites for aqueous zinc-ion battery[J]. Chem, 2020, 6(4): 968-984. |
| 154 | XU D M, WANG H W, LI F Y, et al. Conformal conducting polymer shells on V2O5 nanosheet arrays as a high-rate and stable zinc-ion battery cathode[J]. Advanced Materials Interfaces, 2019, 6(2): 1801506. |
| 41 | CHEN X Y, WANG L B, LI H, et al. Porous V2O5 nanofibers as cathode materials for rechargeable aqueous zinc-ion batteries[J]. Journal of Energy Chemistry, 2019, 38: 20-25. |
| 42 | WANG X, LI Y G, WANG S, et al. 2D amorphous V2O5/graphene heterostructures for high-safety aqueous Zn-ion batteries with unprecedented capacity and ultrahigh rate capability[J]. Advanced Energy Materials, 2020, 10(22): 2000081. |
| 43 | DENG S Z, TIE Z W, YUE F, et al. Rational design of ZnMn2 O4 quantum dots in a carbon framework for durable aqueous zinc-ion batteries[J]. Angewandte Chemie (International Ed in English), 2022, 61(12): e202115877. |
| 44 | WANG B, DAI S M, ZHU Z H, et al. A two-dimensional conductive polymer/V2O5 composite with rapid zinc-ion storage kinetics for high-power aqueous zinc-ion batteries[J]. Nanoscale, 2022, 14(33): 12013-12021. |
| 45 | VENKATKARTHICK R, RODTHONGKUM N, ZHANG X Y, et al. Vanadium-based oxide on two-dimensional vanadium carbide MXene (V2Ox@V2CTx) as cathode for rechargeable aqueous zinc-ion batteries[J]. ACS Applied Energy Materials, 2020, 3(5): 4677-4689. |
| 46 | ZHANG N, CHENG F Y, LIU Y C, et al. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery[J]. Journal of the American Chemical Society, 2016, 138(39): 12894-12901. |
| 47 | PAN Z H, YANG J, YANG J, et al. Stitching of Zn3(OH)2V2O7·2H2O 2D nanosheets by 1D carbon nanotubes boosts ultrahigh rate for wearable quasi-solid-state zinc-ion batteries[J]. ACS Nano, 2020, 14(1): 842-853. |
| 48 | JIA X X, LIU C F, NEALE Z G, et al. Active materials for aqueous zinc ion batteries: Synthesis, crystal structure, morphology, and electrochemistry[J]. Chemical Reviews, 2020, 120(15): 7795-7866. |
| 49 | CHEN X D, ZHANG H, LIU J H, et al. Vanadium-based cathodes for aqueous zinc-ion batteries: Mechanism, design strategies and challenges[J]. Energy Storage Materials, 2022, 50: 21-46. |
| 50 | ZHU Q N, WANG Z Y, WANG J W, et al. Challenges and strategies for ultrafast aqueous zinc-ion batteries[J]. Rare Metals, 2021, 40(2): 309-328. |
| 51 | ZHOU T, ZHU L M, XIE L L, et al. Cathode materials for aqueous zinc-ion batteries: A mini review[J]. Journal of Colloid and Interface Science, 2022, 605: 828-850. |
| 52 | ZHOU Y Z, CHEN F D, ARANDIYAN H, et al. Oxide-based cathode materials for rechargeable zinc ion batteries: Progresses and challenges[J]. Journal of Energy Chemistry, 2021, 57: 516-542. |
| 155 | BI W C, GAO G, WU G M, et al. Sodium vanadate/PEDOT nanocables rich with oxygen vacancies for high energy conversion efficiency zinc ion batteries[J]. Energy Storage Materials, 2021, 40: 209-218. |
| 156 | ZHANG Y, HUANG R X, WANG X Y, et al. Facile large-scale preparation of vanadium pentoxide-polypyrrole composite for aqueous zinc-ion batteries[J]. Journal of Alloys and Compounds, 2022, 907: 164434. |
| 157 | XU J W, GAO Q L, XIA Y M, et al. High-performance reversible aqueous zinc-ion battery based on iron-doped alpha-manganese dioxide coated by polypyrrole[J]. Journal of Colloid and Interface Science, 2021, 598: 419-429. |
| 158 | LIAO X B, PAN C L, PAN Y S, et al. Synthesis of three-dimensional β-MnO2/PPy composite for high-performance cathode in zinc-ion batteries[J]. Journal of Alloys and Compounds, 2021, 888: 161619. |
| 159 | LI Z X, HUANG Y, ZHANG J Y, et al. One-step synthesis of MnOx/PPy nanocomposite as a high-performance cathode for a rechargeable zinc-ion battery and insight into its energy storage mechanism[J]. Nanoscale, 2020, 12(6): 4150-4158. |
| 160 | XUE Q, LI L, HUANG Y X, et al. Polypyrrole-modified Prussian blue cathode material for potassium ion batteries via in situ polymerization coating[J]. ACS Applied Materials & Interfaces, 2019, 11(25): 22339-22345. |
| 161 | RUAN P C, XU X L, GAO X L, et al. Achieving long-cycle-life Zn-ion batteries through interfacial engineering of MnO2-polyaniline hybrid networks[J]. Sustainable Materials and Technologies, 2021, 28: e00254. |
| 162 | ZHANG Y, DU Y H, SONG B X, et al. Manganese-ions and polyaniline co-intercalation into vanadium oxide for stable zinc-ion batteries[J]. Journal of Power Sources, 2022, 545: 231920. |
| 163 | YAN X T, FENG X C, HAO B Y, et al. Enhancing the kinetics of vanadium oxides via conducting polymer and metal ions co-intercalation for high-performance aqueous zinc-ions batteries[J]. Journal of Colloid and Interface Science, 2022, 628(Pt B): 204-213. |
| 164 | HAN Y, WU Q, LI S Q, et al. Consecutive core-shell SP@PDA-d-δ-MnO2 cathode material for aqueous zinc-ion batteries[J]. Journal of Alloys and Compounds, 2023, 938: 168555. |
| 165 | ZHANG M Y, ZHANG X Q, DONG Q, et al. Organic molecular intercalated V3O7·H2O with high operating voltage for long cycle life aqueous Zn-ion batteries[J]. Advanced Functional Materials, 2023, 33(31): 2213187. |
| 166 | WANG W J, LIU D X, JIANG Y Q, et al. Mechanism enhancement of V3O7/V6O13 heterostructures to achieve high-performance aqueous Zn-Ion batteries[J]. Chemical Engineering Journal, 2023, 463: 142309. |
| 53 | SELVAKUMARAN D, PAN A Q, LIANG S Q, et al. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(31): 18209-18236. |
| 54 | MING F W, LIANG H F, LEI Y J, et al. Layered MgxV2O5·nH2O as cathode material for high-performance aqueous zinc ion batteries[J]. ACS Energy Letters, 2018, 3(10): 2602-2609. |
| 55 | JING F Y, PEI J, ZHOU Y M, et al. High-performance reversible aqueous Zinc-Ion battery based on Zn2+ pre-intercalation alpha-manganese dioxide nanowires/carbon nanotubes[J]. Journal of Colloid and Interface Science, 2022, 609: 557-565. |
| 56 | VOLKOV F S, ELISEEVA S N, KAMENSKII M A, et al. Vanadium oxide-poly(3, 4-ethylenedioxythiophene) nanocomposite as high-performance cathode for aqueous Zn-ion batteries: The structural and electrochemical characterization[J]. Nanomaterials, 2022, 12(21): 3896. |
| 57 | LI K, LIANG M Y, WANG H, et al. 3D MXene architectures for efficient energy storage and conversion[J]. Advanced Functional Materials, 2020, 30(47): 2000842. |
| 58 | LIM J, KASIRI G, SAHU R, et al. Irreversible structural changes of copper hexacyanoferrate used as a cathode in Zn-ion batteries[J]. Chemistry, 2020, 26(22): 4917-4922. |
| 59 | KUNDU D, ADAMS B D, DUFFORT V, et al. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode[J]. Nature Energy, 2016, 1(10): 16119. |
| 60 | ZHANG M W, LIANG R L, OR T, et al. Recent progress on high-performance cathode materials for zinc-ion batteries[J]. Small Structures, 2021, 2(2): 2000064. |
| 61 | LIU N, LI B, HE Z X, et al. Recent advances and perspectives on vanadium- and manganese-based cathode materials for aqueous zinc ion batteries[J]. Journal of Energy Chemistry, 2021, 59: 134-159. |
| 62 | LI G J, SUN L, ZHANG S L, et al. Developing cathode materials for aqueous zinc ion batteries: Challenges and practical prospects[J]. Advanced Functional Materials, 2024, 34(5): 2301291. |
| 63 | ZUO S Y, XU X J, JI S M, et al. Cathodes for aqueous Zn-ion batteries: Materials, mechanisms, and kinetics[J]. Chemistry, 2021, 27(3): 830-860. |
| 64 | WANG M S, ZHANG J, ZHANG L Z, et al. Graphene-like vanadium oxygen hydrate (VOH) nanosheets intercalated and exfoliated by polyaniline (PANI) for aqueous zinc-ion batteries (ZIBs)[J]. ACS Applied Materials & Interfaces, 2020, 12(28): 31564-31574. |
| 167 | ZENG Y X, WANG Y, JIN Q, et al. Rationally designed Mn2O3-ZnMn2O4 hollow heterostructures from metal-organic frameworks for stable Zn-ion storage[J]. Angewandte Chemie (International Ed in English), 2021, 60(49): 25793-25798. |
| 168 | WANG M L, NIE K Q, WU H B, et al. Carbon nanotubes intertwined porous vanadium oxide heterostructured microfibers as high-performance cathodes for aqueous zinc-ion batteries[J]. Applied Surface Science, 2023, 612: 155791. |
| 169 | TONG Y, ZHAO Y, LUO M, et al. MOF-derived heterostructured C@VO2@V2O5 for stable aqueous zinc-ion batteries cathode[J]. Journal of Alloys and Compounds, 2023, 932: 167681. |
| 170 | QIN M L, LIU W M, SHAN L T, et al. Construction of V2O5/NaV6O15 biphase composites as aqueous zinc-ion battery cathode[J]. Journal of Electroanalytical Chemistry, 2019, 847: 113246. |
| 171 | FAN L L, LI Z H, KANG W M, et al. Highly stable aqueous rechargeable Zn-ion battery: The synergistic effect between NaV6O15 and V2O5 in skin-core heterostructured nanowires cathode[J]. Journal of Energy Chemistry, 2021, 55: 25-33. |
| 172 | ZHANG L, HOU L H, SHENG R, et al. Constructing an Al2O3/(NH4)2V4O9 heterostructure as a cathode material for high performance aqueous rechargeable zinc ion batteries[J]. CrystEngComm, 2022, 24(47): 8248-8255. |
| 173 | DENG W N, XU Y X, ZHANG X C, et al. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O heterostructure as cathode for high-performance aqueous Zn-ion batteries[J]. Journal of Alloys and Compounds, 2022, 903: 163824. |
| 174 | SUN R, GUO X C, DONG S Y, et al. Zn3V3O8@ZnO@NC heterostructure for stable zinc ion storage from assembling nanodisks into cross-stacked architecture[J]. Journal of Power Sources, 2023, 567: 232946. |
| 175 | YANG S N, ZHANG M S, WU X W, et al. The excellent electrochemical performances of ZnMn2O4/Mn2O3: The composite cathode material for potential aqueous zinc ion batteries[J]. Journal of Electroanalytical Chemistry, 2019, 832: 69-74. |
| 176 | JIA H F, LI Y X, ALI U, et al. In-situ formation of ultrafine ZnMn2O4-MnOOH composite nanoparticles embedded into porous carbon nanospheres for stable aqueous zinc-ion batteries[J]. Applied Surface Science, 2022, 592: 153279. |
| 177 | WANG K N, QIN M R, WANG C T, et al. CeO2/MnOx@C hollow cathode derived from self-assembly of Ce-Mn-MOFs for high-performance aqueous zinc-ion batteries[J]. Journal of Colloid and Interface Science, 2023, 629: 733-743. |
| 178 | WANG S, MA W B, SANG Z Y, et al. Dual-modification of manganese oxide by heterostructure and cation pre-intercalation for high-rate and stable zinc-ion storage[J]. Journal of Energy Chemistry, 2022, 67: 82-91. |
| 65 | LUO H, WANG B, WANG F, et al. Anodic oxidation strategy toward structure-optimized V2O3 cathode via electrolyte regulation for Zn-ion storage[J]. ACS Nano, 2020, 14(6): 7328-7337. |
| 66 | ZHOU W J, CHEN J Z, HE C L, et al. Hybridizing δ-type NaxV2O5·nH2O with graphene towards high-performance aqueous zinc-ion batteries[J]. Electrochimica Acta, 2019, 321: 134689. |
| 67 | ZHOU T, HAN Q, XIE L L, et al. Recent developments and challenges of vanadium oxides (VxOy) cathodes for aqueous zinc-ion batteries[J]. The Chemical Record, 2022, 22(4): e202100275. |
| 68 | ZHONG W, ZHANG J H, LI Z M, et al. Issues and strategies of cathode materials for mild aqueous static zinc-ion batteries[J]. Green Chemical Engineering, 2023, 4(3): 264-284. |
| 69 | CHEN M, ZHANG S C, ZOU Z G, et al. Review of vanadium-based oxide cathodes as aqueous zinc-ion batteries[J]. Rare Metals, 2023, 42(9): 2868-2905. |
| 70 | WANG C Y, WANG M Q, HE Z C, et al. Rechargeable aqueous zinc-manganese dioxide/graphene batteries with high rate capability and large capacity[J]. ACS Applied Energy Materials, 2020, 3(2): 1742-1748. |
| 71 | ZANG X L, WANG X S, LIU H L, et al. Enhanced ion conduction via epitaxially polymerized two-dimensional conducting polymer for high-performance cathode in zinc-ion batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(8): 9347-9354. |
| 72 | TAN THONG P, SADHASIVAM T, KIM N I, et al. Highly conductive current collector for enhancing conductivity and power supply of flexible thin-film Zn-MnO2 battery[J]. Energy, 2021, 221: 119856. |
| 73 | 婷婷, 林其杭, 刘长洋, 等. 水系锌离子电池二氧化锰正极改性研究进展[J]. 储能科学与技术, 2023, 12(3): 754-767. |
| TING T, LIN Q H, LIU C Y, et al. Research progress in modification of manganese dioxide as cathode materials for aqueous zinc-ion batteries[J]. Energy Storage Science and Technology, 2023, 12(3): 754-767. | |
| 74 | ZHOU T, XIE L L, HAN Q, et al. Investigation of Na6V10O28 as a promising rechargeable aqueous zinc-ion batteries cathode[J]. Chemical Engineering Journal, 2022, 445: 136789. |
| 75 | LIU X R, SHEN X X, CHEN T T, et al. The spinel MnFe2O4 grown in biomass-derived porous carbons materials for high-performance cathode materials of aqueous zinc-ion batteries[J]. Journal of Alloys and Compounds, 2022, 904: 164002. |
| 76 | LAN B X, PENG Z, CHEN L N, et al. Metallic silver doped vanadium pentoxide cathode for aqueous rechargeable zinc ion batteries[J]. Journal of Alloys and Compounds, 2019, 787: 9-16. |
| 77 | ZHANG Y B, LI Z H, GONG L, et al. Rational construction of Ag@MIL-88B(V)-derived hierarchical porous Ag-V2O5 heterostructures with enhanced diffusion kinetics and cycling stability for aqueous zinc-ion batteries[J]. Journal of Energy Chemistry, 2023, 77: 561-571. |
| 78 | ZHENG J Q, LIU C F, TIAN M, et al. Fast and reversible zinc ion intercalation in Al-ion modified hydrated vanadate[J]. Nano Energy, 2020, 70: 104519. |
| 79 | XIA J J, ZHOU Y R, ZHANG J, et al. Triggering high capacity and superior reversibility of manganese oxides cathode via magnesium modulation for Zn//MnO2 batteries[J]. Small, 2023, 19(37): e2301906. |
| 80 | XIA C, GUO J, LI P, et al. Highly stable aqueous zinc-ion storage using a layered calcium vanadium oxide bronze cathode[J]. Angewandte Chemie (International Ed in English), 2018, 57(15): 3943-3948. |
| 81 | WU F F, WANG Y W, RUAN P C, et al. Fe-doping enabled a stable vanadium oxide cathode with rapid Zn diffusion channel for aqueous zinc-ion batteries[J]. Materials Today Energy, 2021, 21: 100842. |
| 82 | RAN Y, REN J, KONG Y L, et al. Electrochemical zinc and hydrogen co-intercalation in Li3(V6O16): A high-capacity aqueous zinc-ion battery cathode[J]. Electrochimica Acta, 2022, 412: 140120. |
| 83 | TANG B Y, ZHOU J, FANG G Z, et al. Engineering the interplanar spacing of ammonium vanadates as a high-performance aqueous zinc-ion battery cathode[J]. Journal of Materials Chemistry A, 2019, 7(3): 940-945. |
| 84 | LI J W, MCCOLL K, LU X K, et al. Multi-scale investigations of δ-Ni0.25V2O5·nH2O cathode materials in aqueous zinc-ion batteries[J]. Advanced Energy Materials, 2020, 10(15): 2000058. |
| 85 | HE P, ZHANG G B, LIAO X B, et al. Sodium ion stabilized vanadium oxide nanowire cathode for high-performance zinc-ion batteries[J]. Advanced Energy Materials, 2018, 8(10): 1702463. |
| 86 | WANG L L, HUANG K W, CHEN J T, et al. Ultralong cycle stability of aqueous zinc-ion batteries with zinc vanadium oxide cathodes[J]. Science Advances, 2019, 5(10): eaax4279. |
| 87 | LONG F N, XIANG Y H, YANG S N, et al. Layered manganese dioxide nanoflowers with Cu2+ and Bi3+ intercalation as high-performance cathode for aqueous zinc-ion battery[J]. Journal of Colloid and Interface Science, 2022, 616: 101-109. |
| 88 | LI D, WANG Z R, XIA Y M, et al. Copper-doped manganese tetroxide composites with excellent electrochemical performance for aqueous zinc-ion batteries[J]. Journal of Electroanalytical Chemistry, 2021, 888: 115214. |
| 89 | FENG Z Y, ZHANG Y F, SUN J J, et al. Dual ions enable vanadium oxide hydration with superior Zn2+ storage for aqueous zinc-ion batteries[J]. Chemical Engineering Journal, 2022, 433: 133795. |
| 179 | WU X T, YIN C S, ZHANG M F, et al. The intercalation cathode of MOFs-driven vanadium-based composite embedded in N-doped carbon for aqueous zinc ion batteries[J]. Chemical Engineering Journal, 2023, 452: 139573. |
| 180 | XIAO B Q, CHEN J, HU C F, et al. 2D dynamic heterogeneous interface coupling endowing extra Zn2+ storage[J]. Advanced Functional Materials, 2023, 33(9): 2211679. |
| 181 | GUO J D, LIU J X, MA W B, et al. Vanadium oxide intercalated with conductive metal-organic frameworks with dual energy-storage mechanism for high capacity and high-rate capability Zn ion storage[J]. Advanced Functional Materials, 2023, 33(41): 2302659. |
| 90 | LV T T, ZHU G Y, DONG S Y, et al. Co-intercalation of dual charge carriers in metal-ion-confining layered vanadium oxide nanobelts for aqueous zinc-ion batteries[J]. Angewandte Chemie International Edition, 2023, 62(5): 2216089. |
| 91 | WANG K N, WANG J W, CHEN P M, et al. Structural transformation by crystal engineering endows aqueous zinc-ion batteries with ultra-long cyclability[J]. Small, 2023, 19(29): e2300585. |
| 92 | HE H, PAN F C, LIANG X W, et al. Unveiling the effect of structural water on Zn-ion storage of polyoxovanadate for high-rate and long-life aqueous zinc ion battery[J]. Chemical Engineering Journal, 2023, 462: 142221. |
| 93 | XIE Z W, LIU S F, WU C H, et al. Homo-interface and gradient N-doping cooperation to boost the rate capability of porous NaV8O20·nH2O nanoflake cathode in Zn-ion batteries[J]. Energy Storage Materials, 2023, 60: 102823. |
| 94 | HE W D, MENG C, AI Z Z, et al. Achieving fast ion diffusion in aqueous zinc-ion batteries by cathode reconstruction design[J]. Chemical Engineering Journal, 2023, 454: 140260. |
| [1] | Yang LENG, Shuo HUANG, Kaixuan GUI, Wenqi YAN, Qi LIU. Study on polyanionic COFs-based composite separators for stabilizing aqueous zinc-ion battery anodes [J]. Energy Storage Science and Technology, 2025, 14(5): 1900-1909. |
| [2] | Xinyi NI, Xiaomeng XU, Luowei CAO, Le LI, Xuejia YAO, Guodong JIA. Simulation of ultrasonic guided wave propagation characteristics in multilayer heterogeneous absorber tubes with non-homogeneous salt films [J]. Energy Storage Science and Technology, 2025, 14(3): 1168-1176. |
| [3] | Lishuai ZHANG, Yifei ZHANG, Yiyang MA, Sibo ZHAO, Hongquan LIU, Shengting SHI, Yanjun ZHONG. Research progress on sodium-ion battery cathode materials based on iron-based prussian blue analogues [J]. Energy Storage Science and Technology, 2025, 14(2): 525-543. |
| [4] | Jie LU, Xian DU, Yupu SHI, Zhuo LI, Na CAO, Xuntao DU, Huiling DU. PANI-coated vanadium compound as high-stable aqueous zinc-ion batteries cathode material [J]. Energy Storage Science and Technology, 2025, 14(1): 42-53. |
| [5] | Weiqi LIN, Qiaoyu LU, Yuhong CHEN, Linyuan QIU, Yurong JI, Lianyu GUAN, Xiang DING. Advances in cathode materials for low-temperature sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2348-2360. |
| [6] | Xiuli GUO, Xiaolong ZHOU, Caineng ZOU, Yongbing TANG. Research progress and perspectives of aqueous dual-ions batteries [J]. Energy Storage Science and Technology, 2024, 13(2): 462-479. |
| [7] | Shuyuan CHEN, Chen CHENG, Xiao XIA, Huanxin JU, Liang ZHANG. Research progress in the X-ray spectroscopy investigation of cathode materials for high-energy-density secondary batteries [J]. Energy Storage Science and Technology, 2024, 13(1): 113-129. |
| [8] | Jintao LI, Yue MU, Jing WANG, Jingyi QIU, Hai MING. Investigation of the structural evolution and interface behavior in cathode materials for Li-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(5): 1636-1654. |
| [9] | Yuwen ZHAO, Huan YANG, Junpeng GUO, Yi ZHANG, Qi SUN, Zhijia ZHANG. Application of magnetic metal elements in sodium ion batteries [J]. Energy Storage Science and Technology, 2023, 12(5): 1332-1347. |
| [10] | Shangzhuo LI, Yutong LONG, Zhaomeng LIU, Xuanwen GAO, Wenbin LUO. Advances toward polyanionic cathode materials for potassium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(5): 1348-1363. |
| [11] | Qingfei MENG, Rui YANG, Chenglong JIN, Yuliang CAO, Wenjie LI, Zhou ZHOU, Jiliang WU. Preparation and performance of high-capacity Cr8O21 as a cathode material for lithium batteries [J]. Energy Storage Science and Technology, 2023, 12(10): 3049-3055. |
| [12] | Mengyang ZU, Meng ZHANG, Zikun LI, Ling HUANG. Cycle performance and degradation mechanism of Ni-Rich NCA, NCM, and NCMA [J]. Energy Storage Science and Technology, 2023, 12(1): 51-60. |
| [13] | Shaocong WANG, Wei LI, Ruiqin HUANG, Yifei GUO, Zheng LIU. Progress of the Jahn-Teller effect suppression method for manganese-based sodium-ion battery cathode materials [J]. Energy Storage Science and Technology, 2023, 12(1): 139-149. |
| [14] | Kai ZHANG, Youlong XU. Research progress and development trend of sodium manganate cathode materials for sodium ion batteries [J]. Energy Storage Science and Technology, 2023, 12(1): 86-110. |
| [15] | Chang SUN, Zerong DENG, Ningbo JIANG, Lulu ZHANG, Hui FANG, Xuelin YANG. Recent research progress of sodium vanadium fluorophosphate as cathode material for sodium-ion batteries [J]. Energy Storage Science and Technology, 2022, 11(4): 1184-1200. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
