Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (7): 2243-2258.doi: 10.19799/j.cnki.2095-4239.2024.0362
• Special Issue on Low Temperature Batteries • Previous Articles Next Articles
Yuhao WANG( ), Zhiyong LI, Xin GUO(
), Zhiyong LI, Xin GUO( )
)
Received:2024-04-24
Revised:2024-05-29
Online:2024-07-28
Published:2024-07-23
Contact:
Xin GUO
E-mail:yhao_wang@hust.edu.cn;xguo@hust.edu.cn
CLC Number:
Yuhao WANG, Zhiyong LI, Xin GUO. Applications and challenges of polymer-based electrolytes in low-temperature solid-state lithium batteries[J]. Energy Storage Science and Technology, 2024, 13(7): 2243-2258.
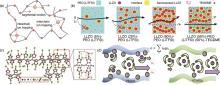
Fig. 1
Schematic of lithium ion conduction in polymer-based electrolytes. (a) Li-ion transport pathways in polymer electrolytes: In coordinating polymers Li-ion conduction occurs via intra-and interchain ion hopping, and segmental motion;(b) Li-ion pathways within LLZO (5%)-PEO (LiTFSI), LLZO (20%)-PEO (LiTFSI), LLZO (50%)-PEO (LiTFSI), and LLZO (50%)-PEO (LiTFSI) (50%)-TEGDME composite electrolytes[22];(c) Schematic diagram of coupling/decoupling between Li+ and oxygen atoms in C=O and C-O groups and transport path in PVEC-PE[23];(d) single ion conduction in PEGDA and PTHFDA gel polymer electrolytes[24]"
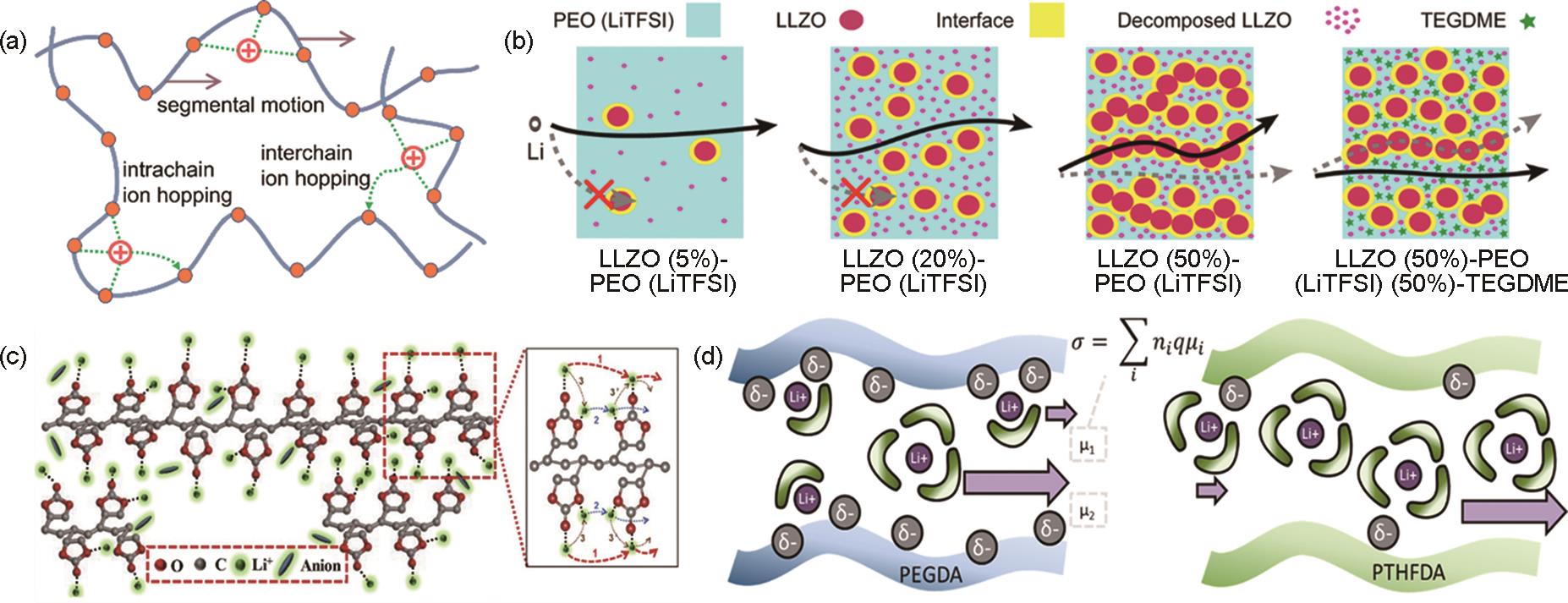
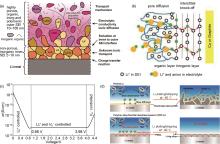
Fig. 2
Schematic diagram of SEI and involved processes. (a) structure of the solid-electrolyte interface[25]; (b) Li+ diffusion in porous organic SEI layers and diffusion in dense inorganic SEI layers[26]; (c) Li+ transport map in Li2CO3 as a function of the voltage of nearby electrode materials referenced to Li metal (zero voltage)[27]; (d)pristine Li metal electrode and polymer-alloy-fluoride interphase-based Li metal electrode (PAF-Li) working at a low temperature of -40 ℃[28]"
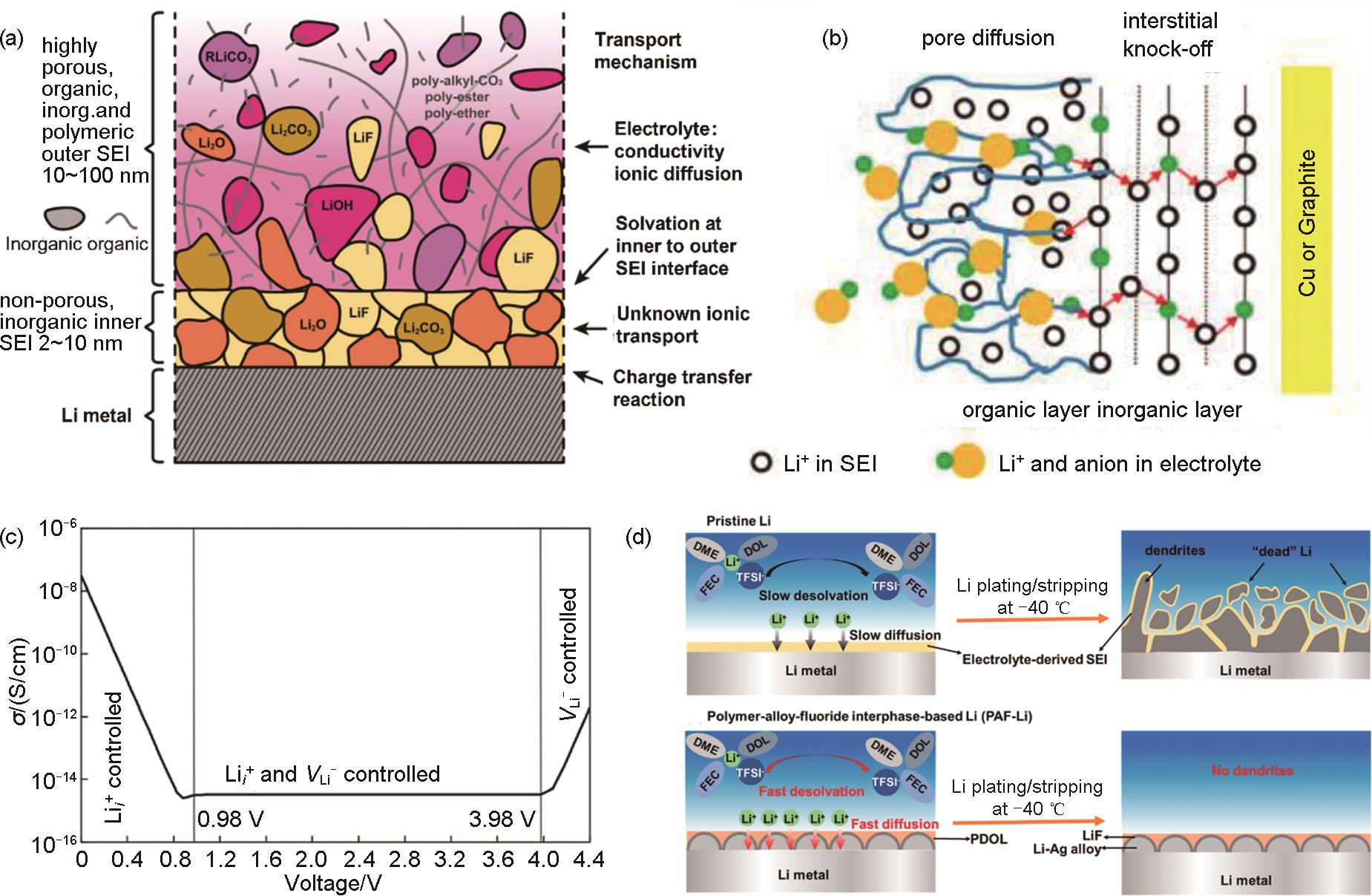

Fig. 3
Structure and ionic conductivity of polymer-based composite electrolytes and electrochemical properties of solid-state batteries. (a) Discharge capacity and coulombic efficiency of a Li/CPE-10% LLZTO/LFP battery at -20 ℃ and -10 ℃ under different current rates[30]; (b) possible Li+ conduction pathways in LCPE-60 composite electrolytes[31]; (c) The charge-discharge voltage profiles of Li|LCPE60|LFP at 0.1C and (-10± 2) ℃[31]; (d) Schematic diagram of ionic conduction in composite polymer electrolytes[32]; (e) Cycling performance obtained from LiFePO4|CPE|Protected Li cell at 0.2C and -20 ℃[32]"


Fig. 4
Formation process of gel polymer electrolyte and electrochemical performances of solid-state lithium batteries at low temperatures. (a) Gel network formation via dynamic condensation of PEG-CHOs with CTH[39]; (b) Cycling performance (0.2C) of solid-state batteries at different temperatures[39]; (c) The ionic conductivity gradient of the developed electrolytes at 23 ℃ on the ternary phase diagram[40]; (d) Charge-discharge curves of the Li|QSPE|NMC811 cell between -30 and 20 ℃[40]; (e) Li/GPE/LFP battery operation at -35 ℃[41]"

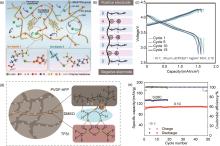
Fig. 5
Molecular modification and ionic conduction in polymer-based electrolytes and electrochemical performances of solid-state lithium batteries. (a) Schematic illustration of the ion-dipole interactions in the SIPC[49]; (b) Illustration of Li+ transport patterns during battery charging[50]; (c) The charge-discharge curves of Li|CPCE|NCA full cells at -20 ℃ under 0.1C[51]; (d) Li+ transport model in the S-LHCE[52]; (e) Cycling performance of LFP cell at -10 ℃ and 0.1C/0.05C[52]"
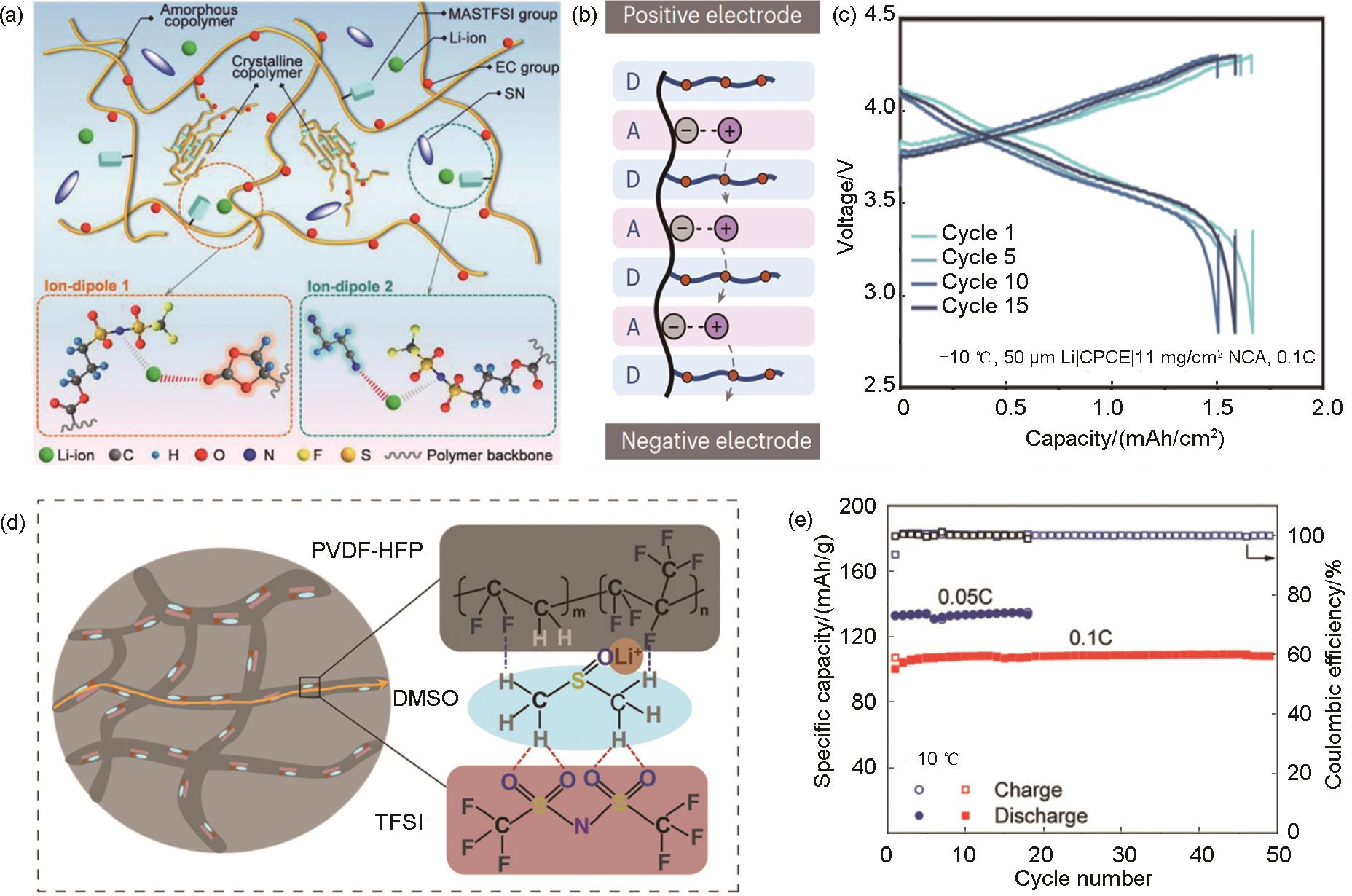
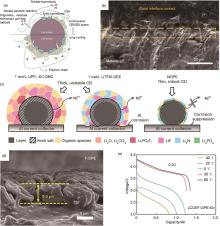
Fig. 6
Conformal interfaces formed by in situ polymerization and electrochemical properties of corresponding solid-state lithium batteries. (a) The unwanted impacts of oligomers, residual monomers and free radicals on electrode/electrolyte interfaces[61]; (b) In-situ-formed QSPE/Li interface[63]; (c) Schematic diagrams of corrosion behaviors of the NCM811 cathode|electrolyte interface and Al current collector in 1 mol/L LiPF6-EC:DMC, 1 mol/L LiTFSI-DEE and NGPE[64]; (d) Cross-sectional SEM images of Li deposited on Cu foil at 0.2 mA/cm2 current density[65]"
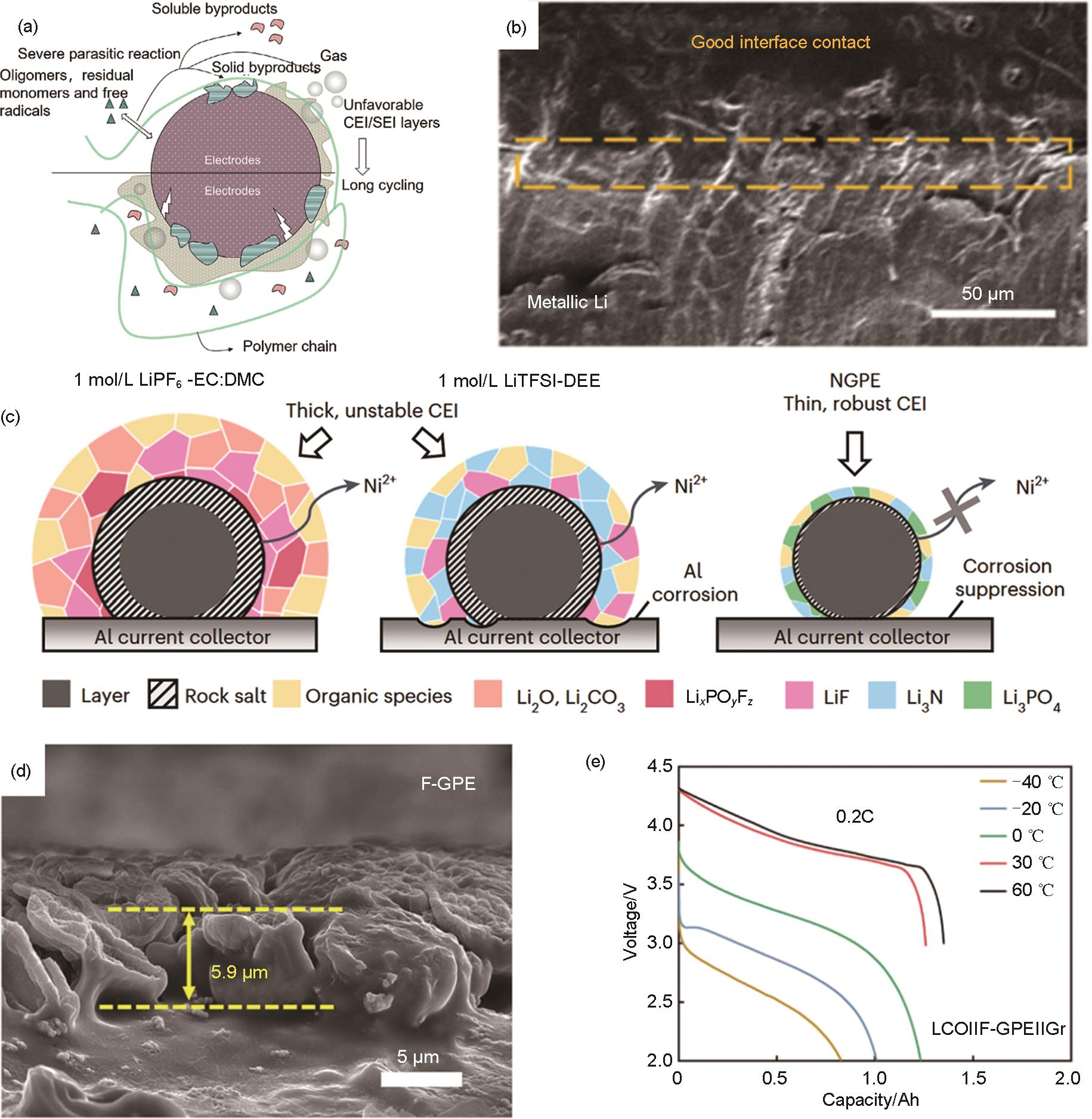

Fig. 7
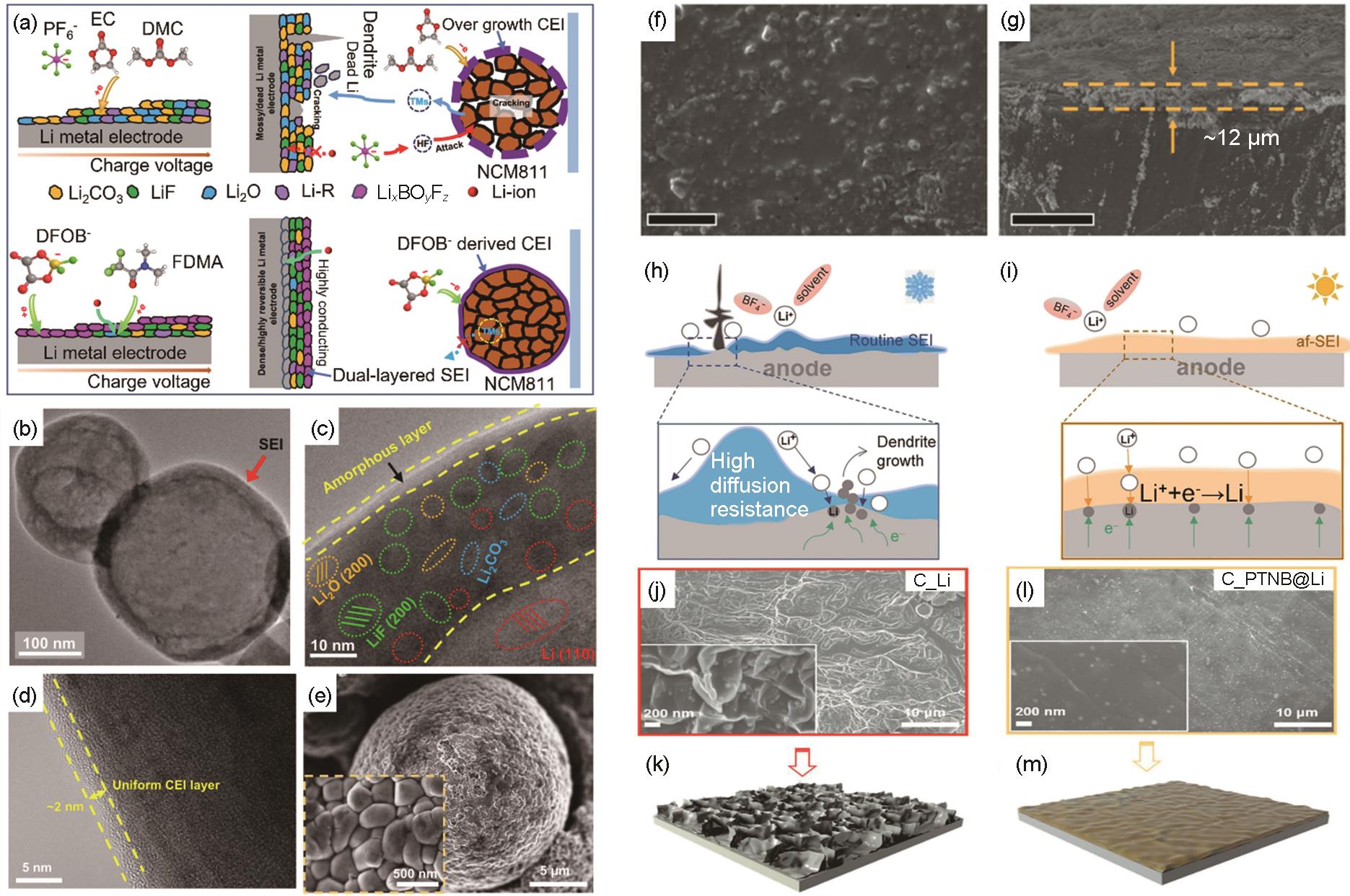
Design and formation of conductive SEI/CEIs. (a) Schematic diagram of the formation process of the solid electrolyte interface phase (SEI) formed on the Li metal electrode and the CEI on the positive electrode side in the Li||NCM811 cell[76]; (b), (c) Cryo-TEM images of deposited Li in the cell with the polymer electrolyte at different scales[76]; (d) TEM of NCM811 particles[76]; (e) SEM images of NCM811 particles[76]; (f) Top-view[77] and(g) cross-section SEM images of Li metal after 50 cycles in PDE[77]; Schematic diagrams of the charge and mass transport mediated by temperature-dependent SEI at (h) Low temperature, and [78] (i) ambient temperature[78]; morphology of (j), (k)recovered Li in Li||Li cell[79] and (l), (m) PTNB@Li electrode in PTNB@Li||PTNB@Li cell[79]"
| 1 | JAGUEMONT J, BOULON L, DUBÉ Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures[J]. Applied Energy, 2016, 164: 99-114. |
| 2 | WALDMANN T, HOGG B I, WOHLFAHRT-MEHRENS M. Li plating as unwanted side reaction in commercial Li-ion cells-A review[J]. Journal of Power Sources, 2018, 384: 107-124. |
| 3 | HOLOUBEK J, YU M Y, YU S C, et al. An all-fluorinated ester electrolyte for stable high-voltage Li metal batteries capable of ultra-low-temperature operation[J]. ACS Energy Letters, 2020, 5(5): 1438-1447. |
| 4 | ZHONG S E, YU Y S, YANG Y, et al. Molecular engineering on solvation structure of carbonate electrolyte toward durable sodium metal battery at -40 ℃[J]. Angewandte Chemie International Edition, 2023, 62(18): 2301169. |
| 5 | JANEK J, ZEIER W G. A solid future for battery development[J]. Nature Energy, 2016, 1(9): 16141. |
| 6 | HU Y S. Batteries: Getting solid[J]. Nature Energy, 2016, 1(4): 16042. |
| 7 | XIE H X, FU Q G, LI Z, et al. Ultraviolet-cured semi-interpenetrating network polymer electrolytes for high-performance quasi-solid-state lithium metal batteries[J]. Chemistry, 2021, 27(28): 7773-7780. |
| 8 | ZHOU Q Y, XU B Y, CHIEN P H, et al. NASICON Li1.2Mg0.1Zr1.9(PO4)3 solid electrolyte for an all-solid-state Li-metal battery[J]. Small Methods, 2020, 4(12): 2000764. |
| 9 | 李泓. 全固态锂电池: 梦想照进现实[J]. 储能科学与技术, 2018, 7(2): 188-193. |
| LI H. All-solid lithium battery: Dreams may come[J]. Energy Storage Science and Technology, 2018, 7(2): 188-193. | |
| 10 | 邹文洪, 樊佑, 张焱焱, 等. 安全固态锂电池室温聚合物基电解质的研究进展[J]. 化工进展, 2021, 40(9): 5029-5044. |
| ZOU W H, FAN Y, ZHANG Y Y, et al. Research progress on room-temperature polymer-based electrolytes for safe solid-state lithium batteries[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5029-5044. | |
| 11 | XU B Y, LI X Y, YANG C, et al. Interfacial chemistry enables stable cycling of all-solid-state Li metal batteries at high current densities[J]. Journal of the American Chemical Society, 2021, 143(17): 6542-6550. |
| 12 | CABAÑERO MARTÍNEZ M A, BOARETTO N, NAYLOR A J, et al. Are polymer-based electrolytes ready for high-voltage lithium battery applications? An overview of degradation mechanisms and battery performance[J]. Advanced Energy Materials, 2022, 12(32): 2201264. |
| 13 | ZHOU Y M, ZHANG F R, HE P X, et al. Quasi-solid-state polymer plastic crystal electrolyte for subzero lithium-ion batteries[J]. Journal of Energy Chemistry, 2020, 46: 87-93. |
| 14 | MO S K, AN H W, LIU Q S, et al. Multistage bridge engineering for electrolyte and interface enables quasi-solid batteries to operate at -40 ℃[J]. Energy Storage Materials, 2024, 65: 103179. |
| 15 | LI Z, FU J L, ZHOU X Y, et al. Ionic conduction in polymer-based solid electrolytes[J]. Advanced Science, 2023, 10(10): e2201718. |
| 16 | LI Z Y, REN Y, GUO X. Polymer-based electrolytes for solid-state lithium batteries with a wide operating temperature range[J]. Materials Chemistry Frontiers, 2023, 7(24): 6305-6317. |
| 17 | YU T W, YANG X F, YANG R, et al. Progress and perspectives on typical inorganic solid-state electrolytes[J]. Journal of Alloys and Compounds, 2021, 885: 161013. |
| 18 | KARABELLI D, BIRKE K P, WEEBER M. A performance and cost overview of selected solid-state electrolytes: Race between polymer electrolytes and inorganic sulfide electrolytes[J]. Batteries, 2021, 7(1): 18. |
| 19 | YAO Z C, WANG Y T, WAN S, et al. Recent advances in designing solid-state electrolytes to reduce the working temperature of lithium batteries[J]. Materials Chemistry Frontiers, 2023, 7(23): 6061-6084. |
| 20 | RATNER M A, SHRIVER D F. Ion transport in solvent-free polymers[J]. Chemical Reviews, 1988, 88(1): 109-124. |
| 21 | XUE Z G, HE D, XIE X L. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2015, 3(38): 19218-19253. |
| 22 | ZHENG J, HU Y Y. New insights into the compositional dependence of Li-ion transport in polymer-ceramic composite electrolytes[J]. ACS Applied Materials & Interfaces, 2018, 10(4): 4113-4120. |
| 23 | LIN Z Y, GUO X W, WANG Z C, et al. A wide-temperature superior ionic conductive polymer electrolyte for lithium metal battery[J]. Nano Energy, 2020, 73: 104786. |
| 24 | FORD H O, PARK B, JIANG J Z, et al. Enhanced Li+ conduction within single-ion conducting polymer gel electrolytes via reduced cation–polymer interaction[J]. ACS Materials Letters, 2020, 2(3): 272-279. |
| 25 | HESS M. Non-linearity of the solid-electrolyte-interphase overpotential[J]. Electrochimica Acta, 2017, 244: 69-76. |
| 26 | SHI S Q, LU P, LIU Z Y, et al. Direct calculation of Li-ion transport in the solid electrolyte interphase[J]. Journal of the American Chemical Society, 2012, 134(37): 15476-15487. |
| 27 | SHI S Q, QI Y, LI H, et al. Defect thermodynamics and diffusion mechanisms in Li2CO3 and implications for the solid electrolyte interphase in Li-ion batteries[J]. The Journal of Physical Chemistry C, 2013, 117(17): 8579-8593. |
| 28 | LI Y J, MAO E Y, MIN Z W, et al. Hybrid polymer-alloy-fluoride interphase enabling fast ion transport kinetics for low-temperature lithium metal batteries[J]. ACS Nano, 2023, 17(19): 19459-19469. |
| 29 | LI Z, HUANG H M, ZHU J K, et al. Ionic conduction in composite polymer electrolytes: Case of PEO: Ga-LLZO composites[J]. ACS Applied Materials & Interfaces, 2019, 11(1): 784-791. |
| 30 | LI J, CAI Y J, CUI Y Y, et al. Fabrication of asymmetric bilayer solid-state electrolyte with boosted ion transport enabled by charge-rich space charge layer for -20~70 ℃ lithium metal battery[J]. Nano Energy, 2022, 95: 107027. |
| 31 | ZHANG X Y, FU C K, CHENG S C, et al. Novel PEO-based composite electrolyte for low-temperature all-solid-state lithium metal batteries enabled by interfacial cation-assistance[J]. Energy Storage Materials, 2023, 56: 121-131. |
| 32 | HUANG X Y, HUANG S, WANG T Y, et al. Polyether-b-amide based solid electrolytes with well-adhered interface and fast kinetics for ultralow temperature solid-state lithium metal batteries[J]. Advanced Functional Materials, 2023, 33(27): 2300683. |
| 33 | ALARCO P J, ABU-LEBDEH Y, ABOUIMRANE A, et al. The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors[J]. Nature Materials, 2004, 3: 476-481. |
| 34 | FU C K, MA Y L, ZUO P J, et al. In-situ thermal polymerization boosts succinonitrile-based composite solid-state electrolyte for high performance Li-metal battery[J]. Journal of Power Sources, 2021, 496: 229861. |
| 35 | MINDEMARK J, LACEY M J, BOWDEN T, et al. Beyond PEO—Alternative host materials for Li+-conducting solid polymer electrolytes[J]. Progress in Polymer Science, 2018, 81: 114-143. |
| 36 | LEE M J, HAN J, LEE K, et al. Elastomeric electrolytes for high-energy solid-state lithium batteries[J]. Nature, 2022, 601: 217-222. |
| 37 | FU C K, ZHANG X, CUI C, et al. Molecular bridges stabilize lithium metal anode and solid-state electrolyte interface[J]. Chemical Engineering Journal, 2022, 432: 134271. |
| 38 | ZHOU X Y, ZHOU Y F, YU L, et al. Gel polymer electrolytes for rechargeable batteries toward wide-temperature applications[J]. Chemical Society Reviews, 2024, 53(10): 5291-5337. |
| 39 | TANG J Q, ZHAI B B, LIU J F, et al. A robust, freeze-resistant and highly ion conductive ionogel electrolyte towards lithium metal batteries workable at -30 ℃[J]. Physical Chemistry Chemical Physics: PCCP, 2021, 23(11): 6775-6782. |
| 40 | YU J, LIN X D, LIU J P, et al. In situ fabricated quasi-solid polymer electrolyte for high-energy-density lithium metal battery capable of subzero operation[J]. Advanced Energy Materials, 2022, 12(2): 2102932. |
| 41 | GREEN M, KAYDANIK K, OROZCO M, et al. Closo-borate gel polymer electrolyte with remarkable electrochemical stability and a wide operating temperature window[J]. Advanced Science, 2022, 9(16): 2106032. |
| 42 | LI S P, LORANDI F, WANG H, et al. Functional polymers for lithium metal batteries[J]. Progress in Polymer Science, 2021, 122: 101453. |
| 43 | WANG A L, XU H, ZHOU Q, et al. A new all-solid-state hyperbranched star polymer electrolyte for lithium ion batteries: Synthesis and electrochemical properties[J]. Electrochimica Acta, 2016, 212: 372-379. |
| 44 | HOMANN G, STOLZ L, NEUHAUS K, et al. Effective optimization of high voltage solid-state lithium batteries by using poly(ethylene oxide)-based polymer electrolyte with semi-interpenetrating network[J]. Advanced Functional Materials, 2020, 30(46): 2006289. |
| 45 | JIANG Y, YAN X M, MA Z F, et al. Development of the PEO based solid polymer electrolytes for all-solid state lithium ion batteries[J]. Polymers, 2018, 10(11): 1237. |
| 46 | WU Y X, LI Y, WANG Y, et al. Advances and prospects of PVDF based polymer electrolytes[J]. Journal of Energy Chemistry, 2022, 64: 62-84. |
| 47 | NGUYEN A G, PARK C J. Insights into tailoring composite solid polymer electrolytes for solid-state lithium batteries[J]. Journal of Membrane Science, 2023, 675: 121552. |
| 48 | XI G, XIAO M, WANG S J, et al. Polymer-based solid electrolytes: Material selection, design, and application[J]. Advanced Functional Materials, 2021, 31(9): 2007598. |
| 49 | WEN K H, XIN C Z, GUAN S D, et al. Ion-dipole interaction regulation enables high-performance single-ion polymer conductors for solid-state batteries[J]. Advanced Materials, 2022, 34(32): e2202143. |
| 50 | HAN S T, WEN P, WANG H J, et al. Sequencing polymers to enable solid-state lithium batteries[J]. Nature Materials, 2023, 22: 1515-1522. |
| 51 | WANG A X, GENG S X, ZHAO Z F, et al. In situ cross-linked plastic crystal electrolytes for wide-temperature and high-energy-density lithium metal batteries[J]. Advanced Functional Materials, 2023, 33(17): 2201861. |
| 52 | XU S J, XU R G, YU T, et al. Decoupling of ion pairing and ion conduction in ultrahigh-concentration electrolytes enables wide-temperature solid-state batteries[J]. Energy & Environmental Science, 2022, 15(8): 3379-3387. |
| 53 | PAN J, ZHAO P, WANG N N, et al. Research progress in stable interfacial constructions between composite polymer electrolytes and electrodes[J]. Energy & Environmental Science, 2022, 15(7): 2753-2775. |
| 54 | XU L, TANG S, CHENG Y, et al. Interfaces in solid-state lithium batteries[J]. Joule, 2018, 2(10): 1991-2015. |
| 55 | LI C, WANG Z Y, HE Z J, et al. An advance review of solid-state battery: Challenges, progress and prospects[J]. Sustainable Materials and Technologies, 2021, 29: e00297. |
| 56 | ZHU X Q, WANG K, XU Y N, et al. Strategies to boost ionic conductivity and interface compatibility of inorganic-organic solid composite electrolytes[J]. Energy Storage Materials, 2021, 36: 291-308. |
| 57 | RAJ V, AETUKURI N P B, NANDA J. Solid state lithium metal batteries-Issues and challenges at the lithium-solid electrolyte interface[J]. Current Opinion in Solid State and Materials Science, 2022, 26(4): 100999. |
| 58 | TU Q S, BARROSO-LUQUE L, SHI T, et al. Electrodeposition and mechanical stability at lithium-solid electrolyte interface during plating in solid-state batteries[J]. Cell Reports Physical Science, 2020, 1: 100106. |
| 59 | LYU Z L, ZHOU Q, ZHANG S, et al. Cyano-reinforced in situ polymer electrolyte enabling long-life cycling for high-voltage lithium metal batteries[J]. Energy Storage Materials, 2021, 37: 215-223. |
| 60 | 李卓, 郭新. 面向高比能固态电池的聚合物基电解质固化技术[J]. 储能科学与技术, 2024, 13(1): 212-230. |
| LI Z, GUO X. Solidification of polymer-based electrolytes for energy-density solid-state batteries[J]. Energy Storage Science and Technology, 2024, 13(1): 212-230. | |
| 61 | ZHANG S H, XIE B, ZHUANG X C, et al. Great challenges and new paradigm of the in situ polymerization technology inside lithium batteries[J]. Advanced Functional Materials, 2024, 34(17): 2314063. |
| 62 | DONG T T, XU G J, XIE B, et al. An electrode-crosstalk-suppressing smart polymer electrolyte for high safety lithium-ion batteries[J]. Advanced Materials, 2024: e2400737. |
| 63 | REN W H, ZHANG Y F, LV R X, et al. In-situ formation of quasi-solid polymer electrolyte for improved lithium metal battery performances at low temperatures[J]. Journal of Power Sources, 2022, 542: 231773. |
| 64 | MENG Y F, ZHOU D, LIU R L, et al. Designing phosphazene-derivative electrolyte matrices to enable high-voltage lithium metal batteries for extreme working conditions[J]. Nature Energy, 2023, 8: 1023-1033. |
| 65 | HE H, WANG Y, LI M, et al. In situ cross-linked fluorinated gel polymer electrolyte based on PEGDA-enabled lithium-ion batteries with a wide temperature operating range[J]. Chemical Engineering Journal, 2023, 467: 143311. |
| 66 | WU J R, WANG X S, LIU Q, et al. A synergistic exploitation to produce high-voltage quasi-solid-state lithium metal batteries[J]. Nature Communications, 2021, 12: 5746. |
| 67 | TAN S, SHADIKE Z, LI J Z, et al. Additive engineering for robust interphases to stabilize high-Ni layered structures at ultra-high voltage of 4.8 V[J]. Nature Energy, 2022, 7: 484-494. |
| 68 | ZHANG S H, SUN F, DU X F, et al. In situ-polymerized lithium salt as a polymer electrolyte for high-safety lithium metal batteries[J]. Energy & Environmental Science, 2023, 16(6): 2591-2602. |
| 69 | MA C, CUI W F, LIU X Z, et al. In situ preparation of gel polymer electrolyte for lithium batteries: Progress and perspectives[J]. InfoMat, 2022, 4(2): e12232. |
| 70 | WU H, TANG B, DU X F, et al. LiDFOB initiated in situ polymerization of novel eutectic solution enables room-temperature solid lithium metal batteries[J]. Advanced Science, 2020, 7(23): 2003370. |
| 71 | LI Z, XIE H X, ZHANG X Y, et al. In situ thermally polymerized solid composite electrolytes with a broad electrochemical window for all-solid-state lithium metal batteries[J]. Journal of Materials Chemistry A, 2020, 8(7): 3892-3900. |
| 72 | HOLOUBEK J, YIN Y J, LI M Q, et al. Exploiting mechanistic solvation kinetics for dual-graphite batteries with high power output at extremely low temperature[J]. Angewandte Chemie International Edition, 2019, 58(52): 18892-18897. |
| 73 | ZHANG S, XU K, JOW T. Low-temperature performance of Li-ion cells with a LiBF4-based electrolyte[J]. Journal of Solid State Electrochemistry, 2003, 7(3): 147-151. |
| 74 | STOLZ L, GABERŠČEK M, WINTER M, et al. Different efforts but similar insights in battery R&D: Electrochemical impedance spectroscopy vs galvanostatic (constant current) technique[J]. Chemistry of Materials, 2022, 34(23): 10272-10278. |
| 75 | CHI S S, LIU Y C, ZHAO N, et al. Solid polymer electrolyte soft interface layer with 3D lithium anode for all-solid-state lithium batteries[J]. Energy Storage Materials, 2019, 17: 309-316. |
| 76 | LI Z, YU R, WENG S T, et al. Tailoring polymer electrolyte ionic conductivity for production of low-temperature operating quasi-all-solid-state lithium metal batteries[J]. Nature Communications, 2023, 14: 482. |
| 77 | XIANG J W, ZHANG Y, ZHANG B, et al. A flame-retardant polymer electrolyte for high performance lithium metal batteries with an expanded operation temperature[J]. Energy & Environmental Science, 2021, 14(6): 3510-3521. |
| 78 | DUAN J Y, CHEN J X, WANG F F, et al. Ambiently fostering solid electrolyte interphase for low-temperature lithium metal batteries[J]. Journal of Energy Chemistry, 2023, 87: 473-478. |
| 79 | CHEN Z, KIM G T, KIM J K, et al. Highly stable quasi-solid-state lithium metal batteries: Reinforced Li1.3Al0.3Ti1.7(PO4)3/Li interface by a protection interlayer[J]. Advanced Energy Materials, 2021, 11(30): 2101339. |
| 80 | XIANG J W, YUAN L X, SHEN Y, et al. Improved rechargeability of lithium metal anode via controlling lithium-ion flux[J]. Advanced Energy Materials, 2018, 8(36): 1802352. |
| 81 | LIN D C, LIU Y Y, CUI Y. Reviving the lithium metal anode for high-energy batteries[J]. Nature Nanotechnology, 2017, 12: 194-206. |
| 82 | PASTA M, ARMSTRONG D, BROWN Z L, et al. 2020 roadmap on solid-state batteries[J]. Journal of Physics: Energy, 2020, 2(3): 032008. |
| 83 | SUNG J, KIM S Y, HARUTYUNYAN A, et al. Ultra-thin lithium silicide interlayer for solid-state lithium-metal batteries[J]. Advanced Materials, 2023, 35(22): 2210835. |
| 84 | HAN X, WU T T, GU L H, et al. Li-MOF-based ions regulator enabling fast-charging and dendrite-free lithium metal anode[J]. Chinese Chemical Letters, 2023, 34(2): 107594. |
| 85 | LI T, CHEN Z Y, BAI F W, et al. Diluted low concentration electrolyte for interphase stabilization of high-voltage LiNi0.5Mn1.5O4 cathode[J]. Journal of Energy Chemistry, 2023, 81: 404-409. |
| [1] | Changhao LI, Shuping WANG, Xiankun YANG, Ziqi ZENG, Xinyue ZHOU, Jia XIE. Nonaqueous electrolyte in low-temperature lithium-ion battery [J]. Energy Storage Science and Technology, 2024, 13(7): 2286-2299. |
| [2] | Shijie LIAO, Ying WEI, Yunhui HUANG, Renzong HU, Henghui XU. 1,3-Difluorobenzene diluent-stabilizing electrode interface for high-performance low-temperature lithium metal batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2124-2130. |
| [3] | Xiang LI, Dezhong LIU, Kai YUAN, Dapeng CHEN. Solid-state electrolyte for low-temperature lithium metal batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2327-2347. |
| [4] | Jiaqi HUANG, Jieming XIONG, Enzhong TAN, Xinyu SUN, Liwei CHENG, Hua WANG. Revisiting the Na metal half-cell at low-temperature [J]. Energy Storage Science and Technology, 2024, 13(7): 2151-2160. |
| [5] | Xiongwen XU, Ying MO, Wang ZHOU, Huandong YAO, Juan HONG, Hua LEI, Jian TU, Jilei LIU. Effect of hard carbon kinetic properties on low-temperature performance of Na-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2141-2150. |
| [6] | Weiqi LIN, Qiaoyu LU, Yuhong CHEN, Linyuan QIU, Yurong JI, Lianyu GUAN, Xiang DING. Advances in cathode materials for low-temperature sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2348-2360. |
| [7] | Zheng LI, Zhenzhong YANG, Qiong WANG, Renzong HU. Patent intelligence analysis of the research progress in low-temperature electrolytes for Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2317-2326. |
| [8] | Lifeng WANG, Naiqing REN, Hai YANG, Yu YAO, Yan YU. Advances in low-temperature electrolytes for sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2206-2223. |
| [9] | Zeheng LI, Lei XU, Yuxing YAO, Chong YAN, Ximin ZHAI, Xuechun HAO, Aibing CHEN, Jiaqi HUANG, Xiaofei BIE, Huanli SUN, Lizhen FAN, Qiang ZHANG. A review of electrolyte reducing lithium plating in low-temperature lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2192-2205. |
| [10] | Shuping WANG, Xiankun YANG, Changhao LI, Ziqi ZENG, Yifeng CHENG, Jia XIE. Diethyl ethylphosphonate-based flame-retardant wide-temperature-range electrolyte in lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2161-2170. |
| [11] | Lulu NIE, Lige YUAN. Application and optimization of intelligent electronic control system in low temperature battery management [J]. Energy Storage Science and Technology, 2024, 13(7): 2432-2434. |
| [12] | Hui FANG. Functional safety guarantee strategy for low temperature lithium battery energy storage system under network analysis mode [J]. Energy Storage Science and Technology, 2024, 13(7): 2447-2449. |
| [13] | Junjie LU, Dan PENG, Wenjing NI, Yuan YANG, Jinglun WANG. Research progress on electrolyte for Li/CF x battery [J]. Energy Storage Science and Technology, 2024, 13(5): 1487-1495. |
| [14] | Qianqian ZHANG. Application of phase change storage technology in food cold chain logistics [J]. Energy Storage Science and Technology, 2024, 13(2): 480-482. |
| [15] | Xuejiao DAI, Jie YAN, Guan WANG, Haotian DONG, Danfeng JIANG, Zewei WEI, Fanxing MENG, Songtao LIU, Haitao ZHANG. Research progress of key materials for niobium-based low temperature batteries [J]. Energy Storage Science and Technology, 2024, 13(1): 311-324. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
