Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (7): 2161-2170.doi: 10.19799/j.cnki.2095-4239.2024.0117
• Special Issue on Low Temperature Batteries • Previous Articles Next Articles
Shuping WANG1( ), Xiankun YANG2,3(
), Xiankun YANG2,3( ), Changhao LI1, Ziqi ZENG2, Yifeng CHENG1, Jia XIE2
), Changhao LI1, Ziqi ZENG2, Yifeng CHENG1, Jia XIE2
Received:2024-02-05
Revised:2024-02-29
Online:2024-07-28
Published:2024-07-23
Contact:
Shuping WANG, Xiankun YANG
E-mail:wangshuping516@126.com;yxk0222@163.com
CLC Number:
Shuping WANG, Xiankun YANG, Changhao LI, Ziqi ZENG, Yifeng CHENG, Jia XIE. Diethyl ethylphosphonate-based flame-retardant wide-temperature-range electrolyte in lithium-ion batteries[J]. Energy Storage Science and Technology, 2024, 13(7): 2161-2170.

Fig. 1
(a) Initial charge/discharge curves of Li-Graphite cells in pure DEEP electrolyte with LiFSI:DEEP MR from 1∶8 to 1∶2; (b) Charge/discharge curves of Graphite electrodes in DEEP-EC electrolyte when LiFSI:DEEP:EC MR is 1∶2∶0 to 1:2:1; (c) The lowest unoccupied molecular orbital energy levels of DEEP and DEEP-Li+ clusters; (d) Initial charge-discharge curves and (e) cycling performance of Graphite electrodes in DEEP-EC-FEMC electrolyte with LiFSI∶DEEP∶EC∶FEMC MR from 1∶2∶0∶4 to 1∶2∶1∶4"
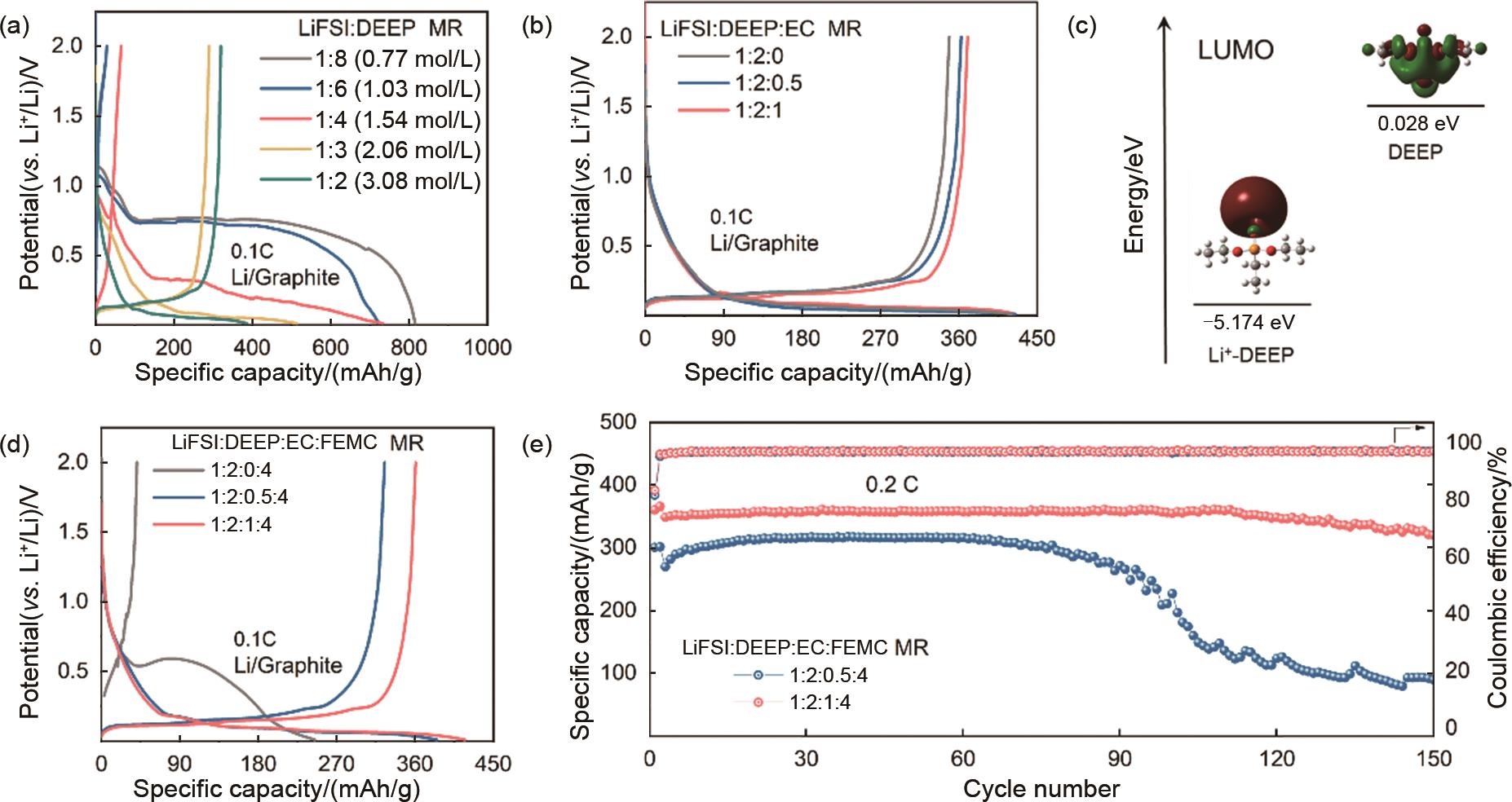

Fig. 5
(a) Flammability testing of RCE and L1P2E1F4 electrolytes; (b) Self-extinguishing time values (0.3 g electrolyte) and (c) differential scanning calorimetry analyses for RCE and L1P2E1F4 electrolytes; (d) Photographs of RCE and L1P2E1F4 electrolytes at 25 ℃ and -60 ℃; (e) Ionic conductivity of RCE and L1P2E1F4 electrolytes at different temperatures; (f) Fitting results of charge transfer process activation energy of RCE and L1P2E1F4 electrolytes in graphite/graphite symmetric cells"
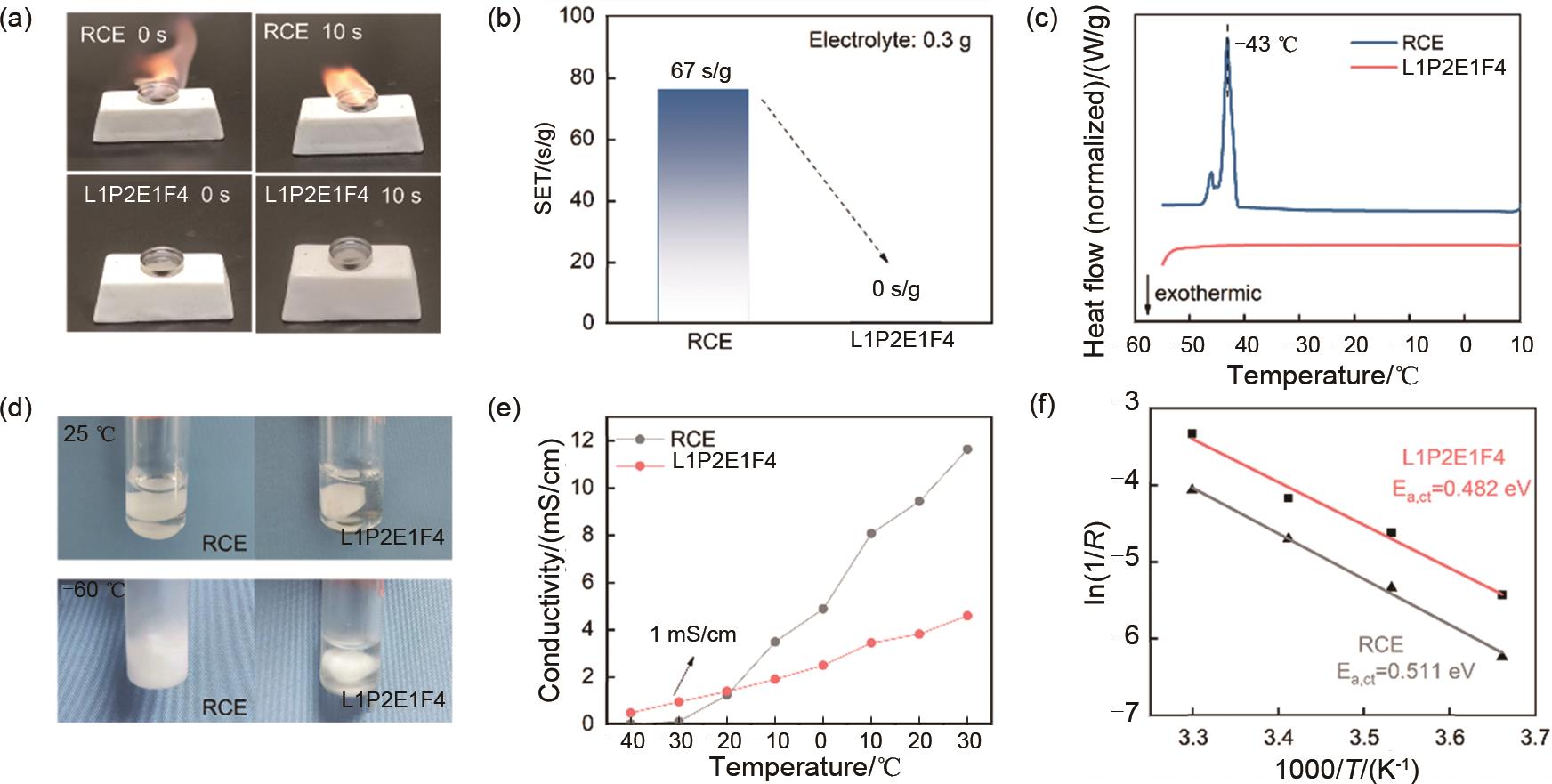
Fig. 6
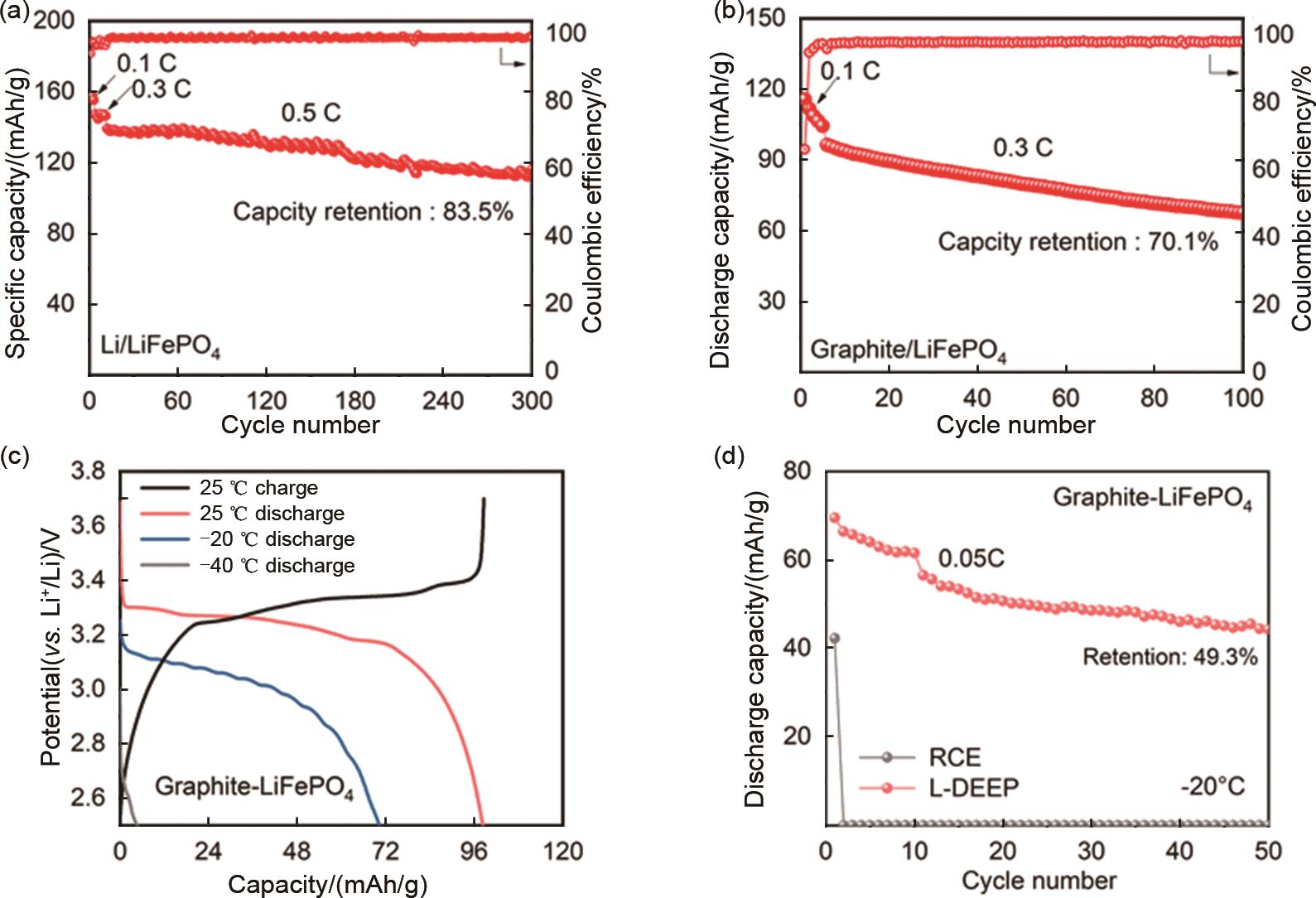
(a) Charge/discharge specific capacity of Li/Graphite assembled with RCE and L1P2F4E1 electrolytes at different multiplicities; (b) Electrochemical performance of Graphite/LiFePO4 battery (N/P=1∶1.1) in the designed electrolyte; cycling performance at 2.5—3.7 V with 0.2 C charging and 0.3 C discharging; (c) Charging at 25 ℃ and discharge curves at different temperatures; (d) Cycling performance of graphite/LiFePO4 batteries assembled with RCE and designed electrolyte at -20 ℃ and 0.05 C"
| 1 | ZHAO M, XU G J, LU D, et al. Formulating a non-flammable highly concentrated dual-salt electrolyte for wide temperature high-nickel lithium ion batteries[J]. Journal of the Electrochemical Society, 2021, 168(5): 050511. |
| 2 | YAO N, SUN S Y, CHEN X, et al. The anionic chemistry in regulating the reductive stability of electrolytes for lithium metal batteries[J]. Angewandte Chemie International Edition, 2022, 61(52): 2210859. |
| 3 | CHEN L, SHEN X H, CHEN H, et al. High-stable nonflammable electrolyte regulated by coordination-number rule for all-climate and safer lithium-ion batteries[J]. Energy Storage Materials, 2023, 55: 836-846. |
| 4 | GOND R, VAN EKEREN W, MOGENSEN R, et al. Non-flammable liquid electrolytes for safe batteries[J]. Materials Horizons, 2021, 8(11): 2913-2928. |
| 5 | JIANG L H, CHENG Y, WANG S P, et al. A nonflammable diethyl ethylphosphonate-based electrolyte improved by synergistic effect of lithium difluoro(oxalato)borate and fluoroethylene carbonate[J]. Journal of Power Sources, 2023, 570: 233051. |
| 6 | 许高洁, 王晓, 陆迪, 等. 锂离子电池高安全性阻燃电解液研究进展[J]. 储能科学与技术, 2018, 7(6): 1040-1059. |
| XU G J, WANG X, LU D, et al. Research progress of high safety flame retardant electrolytes for lithium-ion batteries[J]. Energy Storage Science and Technology, 2018, 7(6): 1040-1059. | |
| 7 | GU Y X, FANG S H, YANG L, et al. A safe electrolyte for high-performance lithium-ion batteries containing lithium difluoro(oxalato)borate, gamma-butyrolactone and non-flammable hydrofluoroether[J]. Electrochimica Acta, 2021, 394: 139120. |
| 8 | ZHANG K, AN Y L, WEI C L, et al. High-safety and dendrite-free lithium metal batteries enabled by building a stable interface in a nonflammable medium-concentration phosphate electrolyte[J]. ACS Applied Materials & Interfaces, 2021, 13(43): 50869-50877. |
| 9 | KIM G T, JEONG S S, JOOST M, et al. Use of natural binders and ionic liquid electrolytes for greener and safer lithium-ion batteries[J]. Journal of Power Sources, 2011, 196(4): 2187-2194. |
| 10 | SUN H, ZHU G Z, XU X T, et al. A safe and non-flammable sodium metal battery based on an ionic liquid electrolyte[J]. Nature Communications, 2019, 10: 3302. |
| 11 | FANG S H, WANG G J, QU L, et al. A novel mixture of diethylene glycol diethylether and non-flammable methyl-nonafluorobutyl ether as a safe electrolyte for lithium ion batteries[J]. Journal of Materials Chemistry A, 2015, 3(42): 21159-21166. |
| 12 | DONG L W, LIU Y P, WEN K C, et al. High-polarity fluoroalkyl ether electrolyte enables solvation-free Li+ transfer for high-rate lithium metal batteries[J]. Advanced Science, 2022, 9(5): e2104699. |
| 13 | WANG J L, YONG T Q, YANG J W, et al. Organosilicon functionalized glycerol carbonates as electrolytes for lithium-ion batteries[J]. RSC Advances, 2015, 5(23): 17660-17666. |
| 14 | YONG T Q, WANG J L, MAI Y J, et al. Organosilicon compounds containing nitrile and oligo(ethylene oxide) substituents as safe electrolytes for high-voltage lithium-ion batteries[J]. Journal of Power Sources, 2014, 254: 29-32. |
| 15 | 曾子琪, 艾新平, 杨汉西, 等. 有机磷酸酯阻燃电解液的研究进展[J]. 电化学, 2020, 26(5): 683-693. |
| ZENG Z Q, AI X P, YANG H X, et al. Research progress of high-safety phosphorus-based electrolyte[J]. Journal of Electrochemistry, 2020, 26(5): 683-693. | |
| 16 | WANG Q S, JIANG L H, YU Y, et al. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect[J]. Nano Energy, 2019, 55: 93-114. |
| 17 | TAN S J, TIAN Y F, ZHAO Y, et al. Noncoordinating flame-retardant functional electrolyte solvents for rechargeable lithium-ion batteries[J]. Journal of the American Chemical Society, 2022, 144(40): 18240-18245. |
| 18 | BOGLE X, VAZQUEZ R, GREENBAUM S, et al. Understanding Li+–solvent interaction in nonaqueous carbonate electrolytes with 17O NMR[J]. The Journal of Physical Chemistry Letters, 2013, 4(10): 1664-1668. |
| 19 | TAKADA K, YAMADA Y, YAMADA A. Optimized nonflammable concentrated electrolytes by introducing a low-dielectric diluent[J]. ACS Applied Materials & Interfaces, 2019, 11(39): 35770-35776. |
| 20 | LIU M C, ZENG Z Q, ZHONG W, et al. Non-flammable fluorobenzene-diluted highly concentrated electrolytes enable high-performance Li-metal and Li-ion batteries[J]. Journal of Colloid and Interface Science, 2022, 619: 399-406. |
| 21 | LIU M C, MA F F, GE Z C, et al. "In-N-out" design enabling high-content triethyl phosphate-based non-flammable and high-conductivity electrolytes for lithium-ion batteries[J]. Science China Chemistry, 2024, 67(2): 724-731. |
| 22 | ZENG Z Q, WU B B, XIAO L F, et al. Safer lithium ion batteries based on nonflammable electrolyte[J]. Journal of Power Sources, 2015, 279: 6-12. |
| 23 | WANG X M, YAMADA C, NAITO H, et al. High-concentration trimethyl phosphate-based nonflammable electrolytes with improved charge-discharge performance of a graphite anode for lithium-ion cells[J]. Journal of the Electrochemical Society, 2006, 153(1): A135. |
| 24 | WANG X F, HE W J, XUE H L, et al. A nonflammable phosphate-based localized high-concentration electrolyte for safe and high-voltage lithium metal batteries[J]. Sustainable Energy & Fuels, 2022, 6(5): 1281-1288. |
| 25 | ZENG Z Q, MURUGESAN V, HAN K S, et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries[J]. Nature Energy, 2018, 3: 674-681. |
| 26 | GAO Y T, LI W, OU B N, et al. A dilute fluorinated phosphate electrolyte enables 4.9V-class potassium ion full batteries[J]. Advanced Functional Materials, 2023, 33(47): 2305829. |
| 27 | LIU M C, ZENG Z Q, GU C K, et al. Ethylene carbonate regulated solvation of triethyl phosphate to enable high-conductivity, nonflammable, and graphite compatible electrolyte[J]. ACS Energy Letters, 2024, 9(1): 136-144. |
| 28 | FENG J K, MA P, YANG H X, et al. Understanding the interactions of phosphonate-based flame-retarding additives with graphitic anode for lithium ion batteries[J]. Electrochimica Acta, 2013, 114: 688-692. |
| 29 | HAN X P, SUN J. Design of a LiF-rich solid electrolyte interface layer through salt-additive chemistry for boosting fast-charging phosphorus-based lithium ion battery performance[J]. Chemical Communications, 2020, 56(45): 6047-6049. |
| 30 | YAMADA Y, FURUKAWA K, SODEYAMA K, et al. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries[J]. Journal of the American Chemical Society, 2014, 136(13): 5039-5046. |
| 31 | GIFFIN G A. The role of concentration in electrolyte solutions for non-aqueous lithium-based batteries[J]. Nature Communications, 2022, 13: 5250. |
| 32 | SMART M C, RATNAKUMAR B V. Effects of electrolyte composition on lithium plating in lithium-ion cells[J]. Journal of the Electrochemical Society, 2011, 158(4): A379-A389. |
| 33 | MING J, CAO Z, LI Q, et al. Molecular-scale interfacial model for predicting electrode performance in rechargeable batteries[J]. ACS Energy Letters, 2019, 4(7): 1584-1593. |
| 34 | SHI C Y, HUANG X J, GU J H, et al. Structural regulation chemistry of lithium-ion solvation in nonflammable phosphate-based electrolytes for high interfacial compatibility with graphite anode[J]. Journal of Energy Chemistry, 2023, 87: 501-508. |
| 35 | LIU X W, SHEN X H, LUO L B, et al. Designing advanced electrolytes for lithium secondary batteries based on the coordination number rule[J]. ACS Energy Letters, 2021, 6(12): 4282-4290. |
| 36 | LIU X W, SHEN X H, LI H, et al. Ethylene carbonate-free propylene carbonate-based electrolytes with excellent electrochemical compatibility for Li-ion batteries through engineering electrolyte solvation structure[J]. Advanced Energy Materials, 2021, 11(19): 2003905. |
| 37 | ZHU B Y, SHI X T, ZHENG T L, et al. Usefulness of uselessness: Teamwork of wide temperature electrolyte enables LFP/Li cells from -40 ℃ to 140 ℃[J]. Electrochimica Acta, 2022, 425: 140698. |
| 38 | FAN X L, JI X, CHEN L, et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents[J]. Nature Energy, 2019, 4: 882-890. |
| 39 | FAN X L, CHEN L, BORODIN O, et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries[J]. Nature Nanotechnology, 2018, 13(8): 715-722. |
| 40 | LI W, GAO J, TIAN H Y, et al. SnF2-catalyzed formation of polymerized dioxolane as solid electrolyte and its thermal decomposition behavior[J]. Angewandte Chemie, 2022, 134(6): e202114805. |
| 41 | WENG S T, ZHANG X, YANG G J, et al. Temperature-dependent interphase formation and Li+ transport in lithium metal batteries[J]. Nature Communications, 2023, 14: 4474. |
| 42 | XU J J, ZHANG J X, POLLARD T P, et al. Electrolyte design for Li-ion batteries under extreme operating conditions[J]. Nature, 2023, 614: 694-700. |
| 43 | LYONS M E G, BRANDON M P. The significance of electrochemical impedance spectra recorded during active oxygen evolution for oxide covered Ni, Co and Fe electrodes in alkaline solution[J]. Journal of Electroanalytical Chemistry, 2009, 631(1/2): 62-70. |
| [1] | Guohe CHEN, Peizhao LYU, Menghan LI, Zhonghao RAO. Research progress on thermal runaway propagation characteristics of lithium-ion batteries and its inhibiting strategies [J]. Energy Storage Science and Technology, 2024, 13(7): 2470-2482. |
| [2] | Chengxin LIU, Ziheng LI, Zeyu CHEN, Pengxiang LI, Qingyi TAO. Characterization study on overheat-induced thermal runaway for lithium-ion battery in energy storage [J]. Energy Storage Science and Technology, 2024, 13(7): 2425-2431. |
| [3] | Shijie LIAO, Ying WEI, Yunhui HUANG, Renzong HU, Henghui XU. 1,3-Difluorobenzene diluent-stabilizing electrode interface for high-performance low-temperature lithium metal batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2124-2130. |
| [4] | Meilong WANG, Yurui XUE, Wenxi HU, Keyu DU, Ruitao SUN, Bin ZHANG, Ya YOU. Design and research of all-ether high-entropy electrolyte for low-temperature lithium iron phosphate batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2131-2140. |
| [5] | Xiongwen XU, Ying MO, Wang ZHOU, Huandong YAO, Juan HONG, Hua LEI, Jian TU, Jilei LIU. Effect of hard carbon kinetic properties on low-temperature performance of Na-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2141-2150. |
| [6] | Guozheng MA, Jinwei CHEN, Xingyu XIONG, Zhenzhong YANG, Gang ZHOU, Rengzong HU. High-rate lithium storage performance of SnSb-Li4Ti5O12 composite anode for Li-ion batteries at low-temperature [J]. Energy Storage Science and Technology, 2024, 13(7): 2107-2115. |
| [7] | Wentao WANG, Yifan WEI, Kun HUANG, Guowei LV, Siyao ZHANG, Xinya TANG, Zeyan CHEN, Qingyuan LIN, Zhipeng MU, Kunhua WANG, Hua CAI, Jun CHEN. Testing standards and developmental advances for low-temperature Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2300-2307. |
| [8] | Weiqi LIN, Qiaoyu LU, Yuhong CHEN, Linyuan QIU, Yurong JI, Lianyu GUAN, Xiang DING. Advances in cathode materials for low-temperature sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2348-2360. |
| [9] | Guangyu CHENG, Xinwei LIU, Shuo LIU, Haitao GU, Ke WANG. Controlling electrolyte solvent components to enhance cycle life of LCO/C low-temperature 18650 batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2171-2180. |
| [10] | Guobin ZHONG, Xin YAO, Yongchao LIU, Qian HOU, Hongfa XIANG. Challenges and prospects of high-safety composite separators for lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(6): 1794-1806. |
| [11] | Ziwei TANG, Yupu SHI, Yuchan ZHANG, Yibo ZHOU, Huiling DU. Prediction of lithium-ion battery capacity degradation trajectory based on Informer [J]. Energy Storage Science and Technology, 2024, 13(5): 1658-1666. |
| [12] | Nana FENG, Ming YANG, Zhouli HUI, Ruijie WANG, Hongyang NING. Prediction of the remaining useful life of lithium batteries based on Antlion optimization Gaussian process regression [J]. Energy Storage Science and Technology, 2024, 13(5): 1643-1652. |
| [13] | Yuanyuan JIANG, Fangfang TU, Fangping ZHANG, Yinglai WANG, Jiawen CAI, Donghui YANG, Yanhong LI, Jiayuan XIANG, Xinhui XIA, Jipeng FU. Study on technology and mechanism of prelithiation for high-performance lithium iron phosphate battery [J]. Energy Storage Science and Technology, 2024, 13(5): 1435-1442. |
| [14] | Gaoqi LIAN, Min YE, Qiao WANG, Yan LI, Yuchuan MA, Yiding SUN, Penghui DU. State-of-charge estimation of lithium-ion batteries in rapid temperature-varying environments based on improved battery model and optimized adaptive cubature Kalman filter [J]. Energy Storage Science and Technology, 2024, 13(5): 1667-1676. |
| [15] | Junjie LU, Dan PENG, Wenjing NI, Yuan YANG, Jinglun WANG. Research progress on electrolyte for Li/CF x battery [J]. Energy Storage Science and Technology, 2024, 13(5): 1487-1495. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
