Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (7): 2131-2140.doi: 10.19799/j.cnki.2095-4239.2024.0338
• Special Issue on Low Temperature Batteries • Previous Articles Next Articles
Meilong WANG1( ), Yurui XUE1, Wenxi HU1, Keyu DU1, Ruitao SUN1, Bin ZHANG3(
), Yurui XUE1, Wenxi HU1, Keyu DU1, Ruitao SUN1, Bin ZHANG3( ), Ya YOU1,2(
), Ya YOU1,2( )
)
Received:2024-04-17
Revised:2024-05-16
Online:2024-07-28
Published:2024-07-23
Contact:
Bin ZHANG, Ya YOU
E-mail:wangmeilong@whut.edu.cn;10062904@libode.com.cn;yayou@whut.edu.cn
CLC Number:
Meilong WANG, Yurui XUE, Wenxi HU, Keyu DU, Ruitao SUN, Bin ZHANG, Ya YOU. Design and research of all-ether high-entropy electrolyte for low-temperature lithium iron phosphate batteries[J]. Energy Storage Science and Technology, 2024, 13(7): 2131-2140.
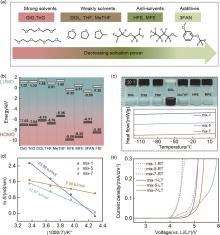
Fig. 1
(a) Solvation power of ether solvent molecules (decreasing from left to right); (b) Highest occupied molecular orbital (HOMO) energy level and lowest unoccupied molecular orbital (LUMO) energy level of ether solvent molecules and salt anions; (c) Optical images of ether-based electrolytes (1 mol/L LiFSI) at low temperature (-20 ℃) and the DSC curves for mix-1, mix-5, and mix-7 electrolytes; (d) Arrhenius plots of ionic conductivity for mix-1, mix-5, and mix-7 electrolytes between 25 ℃ and -20 ℃; (e) Electrochemical stability windows of mix-1, mix-5, and mix-7 electrolytes at room temperature (25 ℃) and low temperature (-20 ℃)"
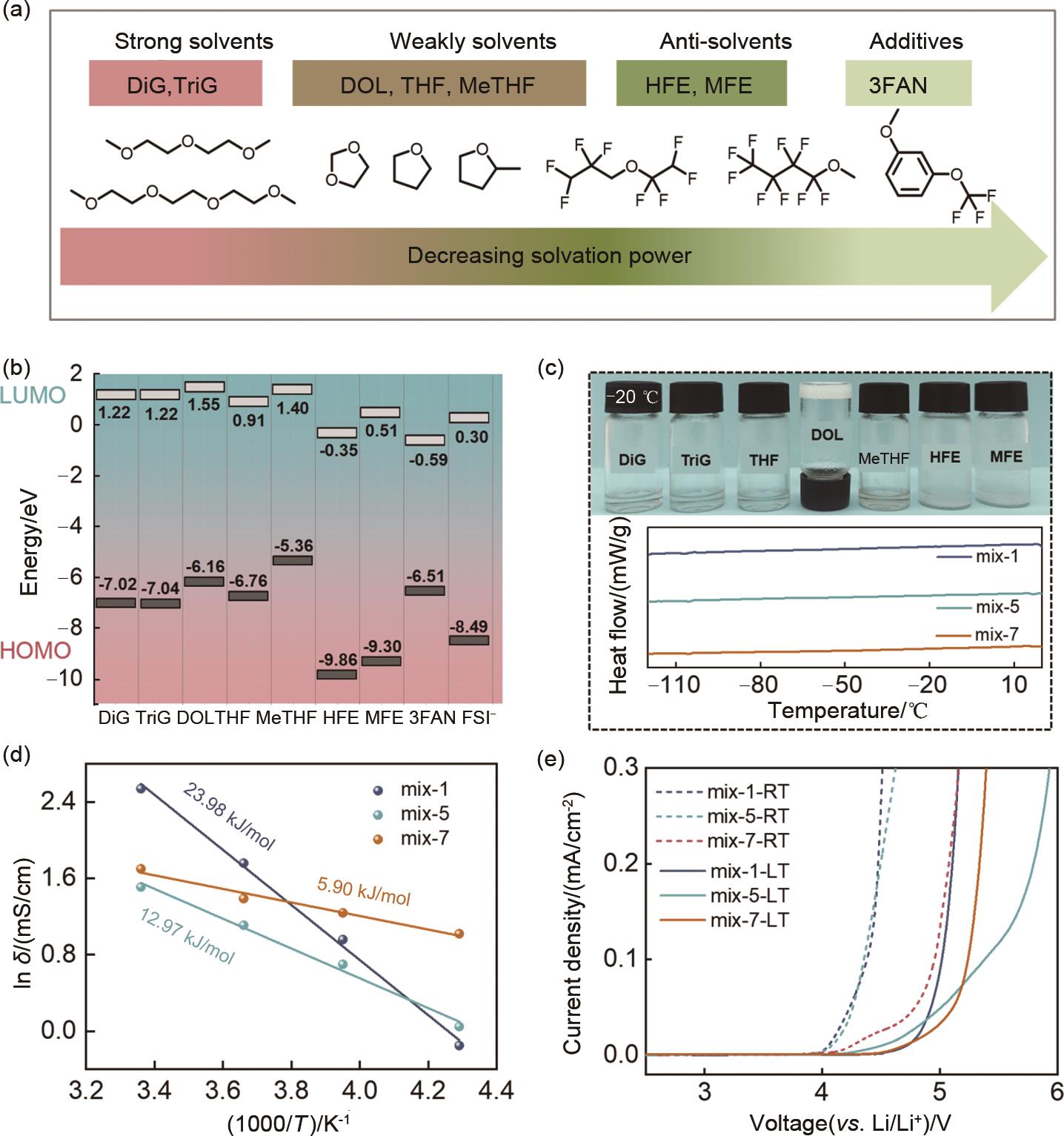

Fig. 2
(a) Infrared spectra of ether solvents and electrolytes; 2D 1H-1H COSY-NMR spectra of (b) mix-5 and (c) mix-7 electrolytes; Raman spectra of FSI- in (d) mix-1, (e) mix-5, and (f) mix-7 electrolytes; (g) 7Li NMR of mix-7 electrolyte and electrolyte without antisolvent and additives; (h) Schematic illustrations of intermolecular interactions in clusters of mix-7 electrolytes"
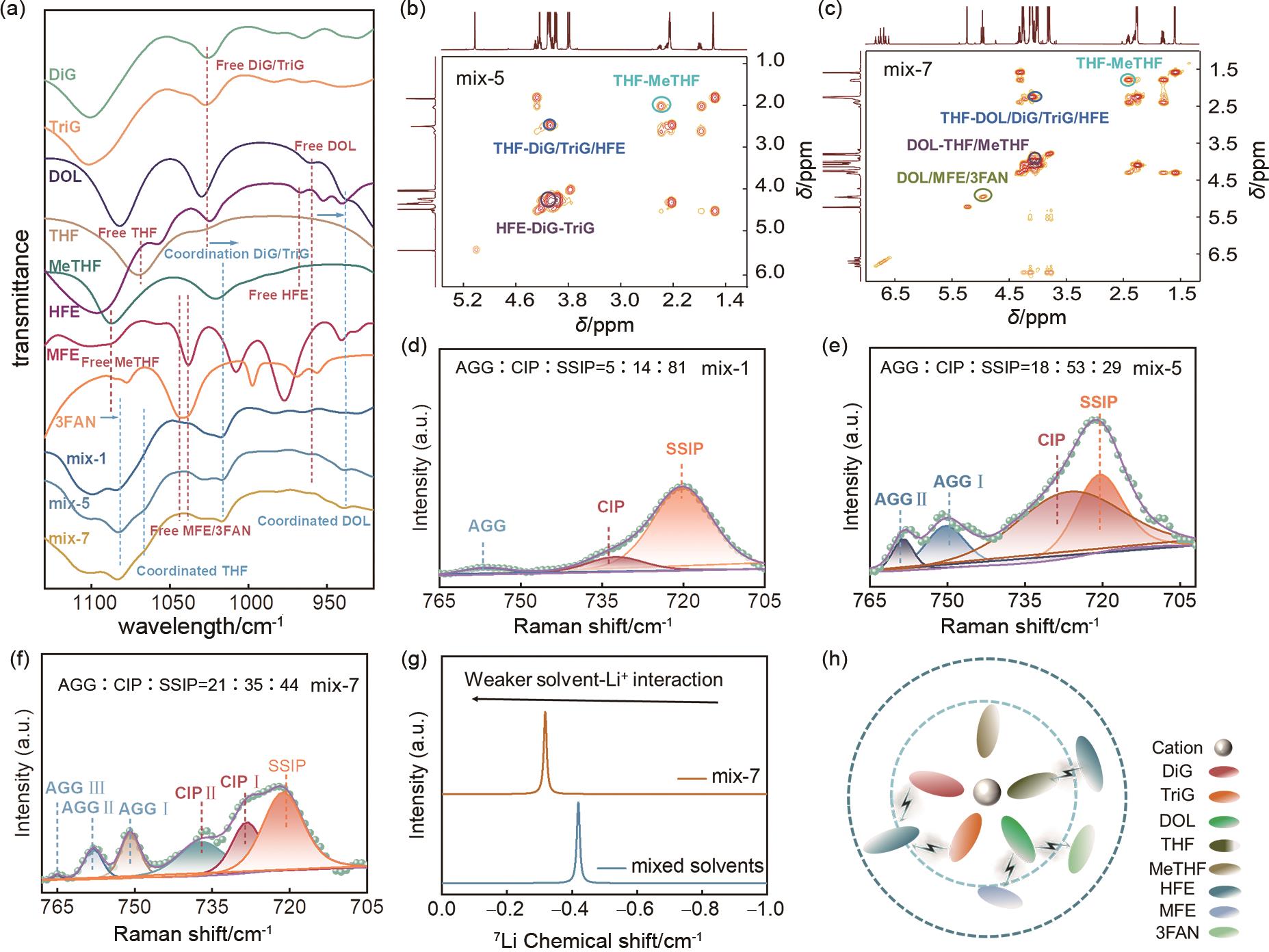
Fig. 3
(a) Linear cyclic voltammetry curves of mix-1, mix-5, and mix-7 electrolytes at room temperature (25 ℃) and low temperature (-20 ℃); (b) Tafel curves of mix-1, mix-5, and mix-7 electrolytes at low temperature (-20 ℃); (c) Potentiostatic polarization curves of mix-1, mix-5, and mix-7 electrolytes at low temperature (-20 ℃); Morphology of solid-electrolyte interphase (SME) on LFP electrodes after 10 cycles at low temperature (-20 ℃) using (d), (g) mix-1, (e), (h) mix-5, and (f), (i) mix-7 electrolytes"
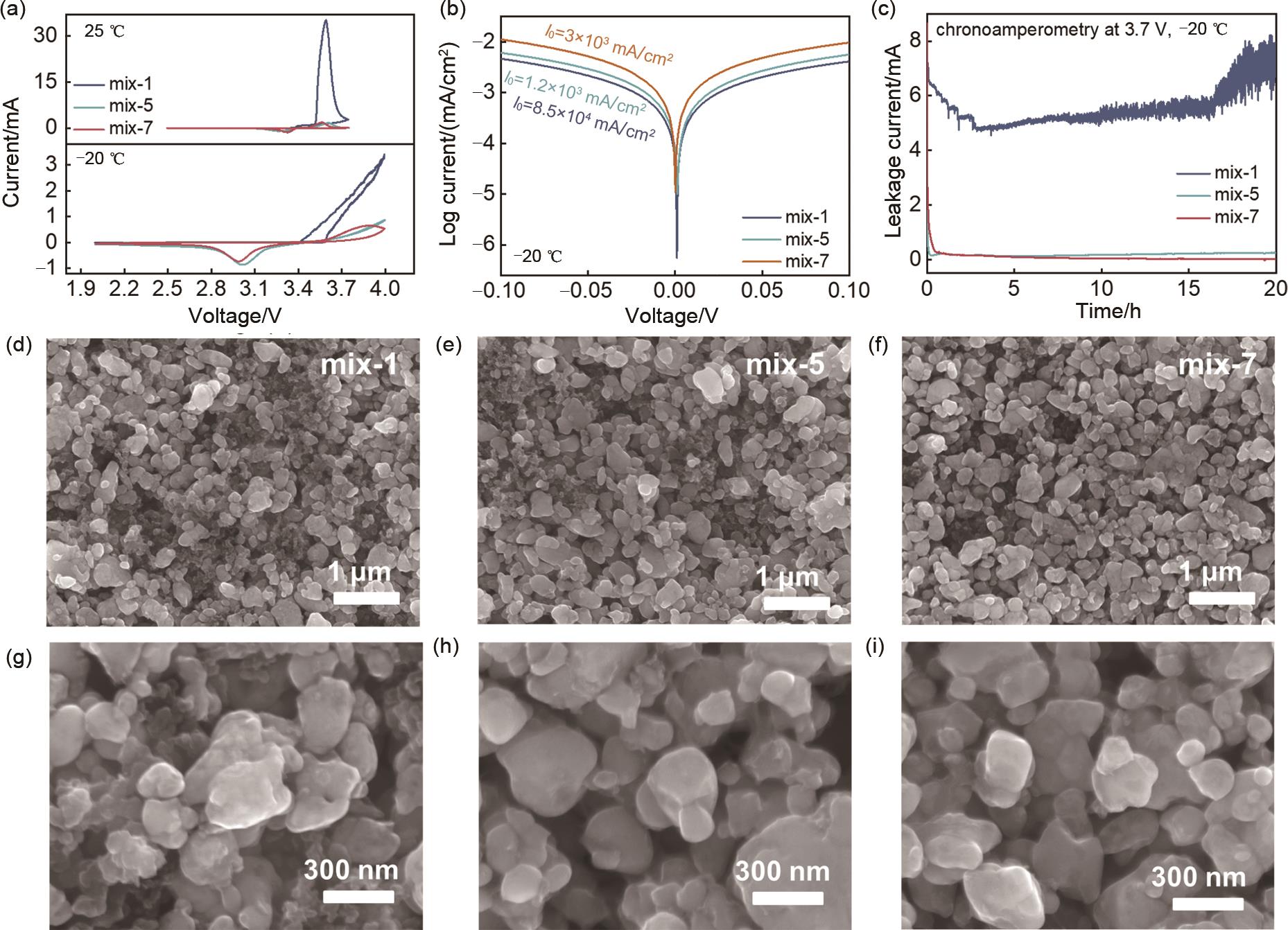

Fig. 4
XPS spectra of LFP positive electrode of mix-1 electrolyte after 10 cycles at low temperature (-20 ℃) at different argon (Ar+) etching depths: (a) C 1s, (b) Li 1s and (c) S 2p; XPS spectra of LFP positive electrode of mix-7 electrolyte after 10 cycles at low temperature (-20 ℃) at different argon (Ar+) etching depths: (d) C 1s, (e) Li 1s and (f) S 2p"
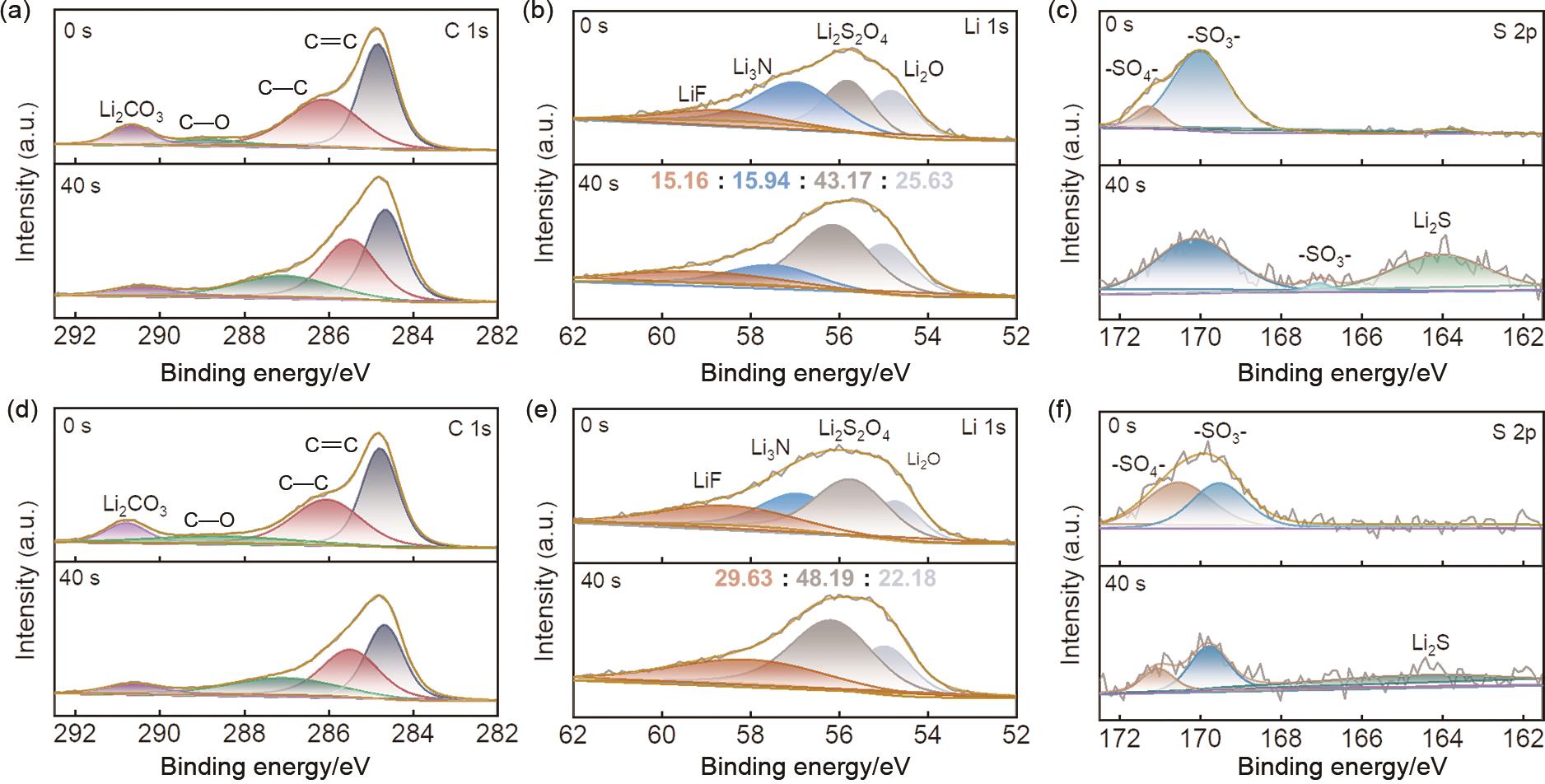

Fig. 5
(a) Rate performance of mix-1, mix-5, and mix-7 electrolytes at room temperature (25 ℃); (b) First cycle charge-discharge curves of LFP cells using mix-1, mix-5, and mix-7 electrolytes at low temperature (-20 ℃); (c) Long cycling performance of LFP/Li batteries; (d) Differential capacity versus voltage (dQ/dV) curves of mix-7 electrolyte at low temperature (-20 ℃); (e) First cycle charge -discharge curves of LCO cells using mix-A, mix-E, and mix-G electrolytes at low temperature (-20 ℃); (f) Long cycling performance of LCO/Li batteries"
| 1 | YUAN L X, WANG Z H, ZHANG W X, et al. Development and challenges of LiFePO4 cathode material for lithium-ion batteries[J]. Energy & Environmental Science, 2011, 4(2): 269-284. |
| 2 | YANG L, DENG W T, XU W, et al. Olivine LiMnxFe1- xPO4 cathode materials for lithium ion batteries: Restricted factors of rate performances[J]. Journal of Materials Chemistry A, 2021, 9(25): 14214-14232. |
| 3 | SAIFULLAH M, MOSTAFIZUR R, MD K, et al. Recent advances in lithium-ion battery materials for improved electrochemical performance: A review[J]. Results in Engineering, 2022, 15: 100472. |
| 4 | CHEN X L, GONG Y D, LI X, et al. Perspective on low-temperature electrolytes for LiFePO4-based lithium-ion batteries[J]. International Journal of Minerals, Metallurgy and Materials, 2023, 30(1): 1-13. |
| 5 | 李淼, 于永利, 吴剑扬, 等. 高能量密度磷酸铁锂正极设计[J]. 储能科学与技术, 2023, 12(7): 2045-2058. |
| LI M, YU Y L, WU J Y, et al. Design of high-energy-density LiFePO4 cathode materials[J]. Energy Storage Science and Technology, 2023, 12(7): 2045-2058. | |
| 6 | KE X, XIAO R, LIAO X F, et al. LiFePO4/C cathode material prepared with sphere mesoporous-FePO4 as precursors for lithium-ion batteries[J]. Journal of Electroanalytical Chemistry, 2018, 820: 18-23. |
| 7 | LI B K, XIAO J Q, ZHU X Y, et al. Enabling high-performance lithium iron phosphate cathodes through an interconnected carbon network for practical and high-energy lithium-ion batteries[J]. Journal of Colloid and Interface Science, 2024, 653: 942-948. |
| 8 | HSIEH C T, CHEN I L, CHEN W Y, et al. Synthesis of iron phosphate powders by chemical precipitation route for high-power lithium iron phosphate cathodes[J]. Electrochimica Acta, 2012, 83: 202-208. |
| 9 | SCANLAN K, MANTHIRAM A. Revealing the electrochemical kinetics of electrolytes in nanosized LiFePO4 electrodes[J]. Journal of the Electrochemical Society, 2023, 170(10): 100515. |
| 10 | APACHITEI G, HEYMER R, LAIN M, et al. Scale-up of lithium iron phosphate cathodes with high active materials contents for lithium ion cells[J]. Batteries, 2023, 9(10): 518-. |
| 11 | GAO X S, ZHENG C Y, SHAO Y Q, et al. Lithium iron phosphate enhances the performance of high-areal-capacity sulfur composite cathodes[J]. ACS Applied Materials & Interfaces, 2023, 15(15): 19011-19020. |
| 12 | HU Q, HE Y F, REN D S, et al. Targeted masking enables stable cycling of LiNi0.6Co0.2Mn0.2O2 at 4.6V[J]. Nano Energy, 2022, 96: 107123. |
| 13 | RUI X, JIN Y J, FENG X Y, et al. A comparative study on the low-temperature performance of LiFePO4/C and Li3V2(PO4)3/C cathodes for lithium-ion batteries[J]. Journal of Power Sources, 2011, 196(4): 2109-2114. |
| 14 | YANG S C, HE R, ZHANG Z J, et al. CHAIN: Cyber hierarchy and interactional network enabling digital solution for battery full-lifespan management[J]. Matter, 2020, 3(1): 27-41. |
| 15 | SONG Y N, ZAVALIJ P Y, CHERNOVA N A, et al. Synthesis, crystal structure, and electrochemical and magnetic study of new iron (III) hydroxyl-phosphates, isostructural with lipscombite[J]. Chemistry of Materials, 2005, 17(5): 1139-1147. |
| 16 | HUBBLE D, BROWN D E, ZHAO Y Z, et al. Liquid electrolyte development for low-temperature lithium-ion batteries[J]. Energy & Environmental Science, 2022, 15(2): 550-578. |
| 17 | THENUWARA A C, SHETTY P P, KONDEKAR N, et al. Efficient low-temperature cycling of lithium metal anodes by tailoring the solid electrolyte interphase[J]. ACS Energy Letters, 2020, 5(7): 2411-2420. |
| 18 | WANG Z C, HAN R, HUANG D, et al. Co-intercalation-free ether-based weakly solvating electrolytes enable fast-charging and wide-temperature lithium-ion batteries[J]. ACS Nano, 2023, 17(18): 18103-18113. |
| 19 | LIU J X, NGUYEN D, WANG J Q, et al. Reevaluate low-concentration ether-based electrolytes for lithium metal batteries[J]. Nano Energy, 2024, 124: 109492. |
| 20 | LIU J, IHUAENYI S, KUPHAL R, et al. A Comparison of carbonate-based and ether-based electrolyte systems for lithium metal batteries[J]. Journal of The Electrochemical Society, 2023, 170(1): 010535. |
| 21 | JIANG Z, YANG T, LI C, et al. Synergistic additives enabling stable cycling of ether electrolyte in 4.4 V Ni-rich/Li metal batteries[J]. Advanced Functional Materials, 2023, 33(51): 2306868. |
| 22 | YIN L M, WANG M L, XIE C, et al. High-voltage cyclic ether-based electrolytes for low-temperature sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2023: 15(7): 9517-9523. |
| 23 | LI A M, BORODIN O, POLLARD T P, et al. Methylation enables the use of fluorine-free ether electrolytes in high-voltage lithium metal batteries[J]. Nature Chemistry, 2024. |
| 24 | ZHANG G Z, CHANG J, WANG L G, et al. A monofluoride ether-based electrolyte solution for fast-charging and low-temperature non-aqueous lithium metal batteries[J]. Nature Communications, 2023, 14: 1081. |
| 25 | FAN X L, CHEN L, JI X, et al. Highly fluorinated interphases enable high-voltage Li-metal batteries[J]. Chem, 2018, 4(1): 174-185. |
| 26 | PAL U, RAKOV D, LU B Y, et al. Interphase control for high performance lithium metal batteries using ether aided ionic liquid electrolyte[J]. Energy & Environmental Science, 2022, 15(5): 1907-1919. |
| 27 | 李萌, 邱景义, 张松通, 等. 新型锂盐氟代磺酰亚胺锂电解液对锂离子电池性能的影响[J]. 储能科学与技术, 2017, 6(1): 101-107. |
| LI M, QIU J Y, ZHANG S T, et al. The effect of lithium bis(fluorosulfonyl)imide salt on the performance of Li-ion battery[J]. Energy Storage Science and Technology, 2017, 6(1): 101-107. | |
| 28 | HEHRE W J, DITCHFIELD R, POPLE J A. Self—Consistent molecular orbital methods. XII. further extensions of Gaussian—Type basis sets for use in molecular orbital studies of organic molecules[J]. Journal of Chemical Physics, 1972, 56(5): 2257-2261. |
| 29 | DITCHFIELD R, HEHRE W J, POPLE J A. Self-consistent molecular-orbital methods. IX. an extended gaussian-type basis for molecular-orbital studies of organic molecules[J]. The Journal of Chemical Physics, 1971, 54(2): 724-728. |
| 30 | LI Y, WU F, LI Y, et al. Ether-based electrolytes for sodium ion batteries[J]. Chemical Society Reviews, 2022, 51(11): 4484-4536. |
| 31 | SEO D M, BORODIN O, HAN S, et al. Electrolyte solvation and ionic association II. acetonitrile-lithium salt mixtures: Highly dissociated salts[J]. Journal of The Electrochemical Society, 2012, 159(9): A1489. |
| 32 | KIM S C, WANG J Y, XU R, et al. High-entropy electrolytes for practical lithium metal batteries[J]. Nature Energy, 2023, 8: 814-826. |
| 33 | JIANG L L, YAN C, YAO Y X, et al. Inhibiting solvent co-intercalation in a graphite anode by a localized high-concentration electrolyte in fast-charging batteries[J]. Angewandte Chemie (International Ed in English), 2021, 60(7): 3402-3406. |
| 34 | LI Y, LIU M, WANG K, et al. Single-solvent-based electrolyte enabling a high-voltage lithium-metal battery with long cycle life[J]. Advanced Energy Materials, 2023, 13(30): 2300918. |
| 35 | YIN Y C, YANG J T, LUO J D, et al. A LaCl3-based lithium superionic conductor compatible with lithium metal[J]. Nature, 2023, 616: 77-83. |
| [1] | Changhao LI, Shuping WANG, Xiankun YANG, Ziqi ZENG, Xinyue ZHOU, Jia XIE. Nonaqueous electrolyte in low-temperature lithium-ion battery [J]. Energy Storage Science and Technology, 2024, 13(7): 2286-2299. |
| [2] | Yang LU, Shuaishuai YAN, Xiao MA, Zhi LIU, Weili ZHANG, Kai LIU. Low-temperature electrolytes and their application in lithium batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2224-2242. |
| [3] | Wentao WANG, Yifan WEI, Kun HUANG, Guowei LV, Siyao ZHANG, Xinya TANG, Zeyan CHEN, Qingyuan LIN, Zhipeng MU, Kunhua WANG, Hua CAI, Jun CHEN. Testing standards and developmental advances for low-temperature Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2300-2307. |
| [4] | Fei ZHAO, Yinghua CHEN, Zheng MA, Qian LI, Jun MING. Advances in low-temperature electrolytes for potassium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2308-2316. |
| [5] | Zeheng LI, Lei XU, Yuxing YAO, Chong YAN, Ximin ZHAI, Xuechun HAO, Aibing CHEN, Jiaqi HUANG, Xiaofei BIE, Huanli SUN, Lizhen FAN, Qiang ZHANG. A review of electrolyte reducing lithium plating in low-temperature lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2192-2205. |
| [6] | Pengfei XIAO, Lin MEI, Libao CHEN. Multicomponent-coated graphite composite anodes for low-temperature electrochemical energy storage [J]. Energy Storage Science and Technology, 2024, 13(7): 2116-2123. |
| [7] | Chen LI, Huilin ZHANG, Jianping ZHANG. Estimated state of health for retired lithium batteries using kernel function and hyperparameter optimization [J]. Energy Storage Science and Technology, 2024, 13(6): 2010-2021. |
| [8] | Chenwei LI, Shiguo XU, Haifeng YU, Songmin YU, Hao JIANG. Synthesis of Mg-doped LiFe0.5Mn0.5PO4/C cathode materials for Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(6): 1767-1774. |
| [9] | Qi SUN, Hao PENG, Qingguo MENG, Dekai KONG, Rui FENG. Thermal adaptability of energy storage battery pack in extreme conditions [J]. Energy Storage Science and Technology, 2024, 13(6): 2039-2043. |
| [10] | Yuchao ZHANG, Fengjiao ZHANG, Wei LOU, Feixiang ZAN, Linling WANG, Anxu SHENG, Xiaohui WU, Jing CHEN. Transformation process of valuable metals in the recycling of spent lithium-ion batteries and the potential environmental impact [J]. Energy Storage Science and Technology, 2024, 13(6): 1861-1870. |
| [11] | Runyuan LI, Fu'ao GUO, Gangchao ZHAO. Early warning method for fire safety of containerized lithium-ion battery energy storage systems [J]. Energy Storage Science and Technology, 2024, 13(5): 1595-1602. |
| [12] | Yuanhui TANG, Boxing YUAN, Jie LI, Yunlong ZHANG. Study on the safety of cylindrical lithium-ion batteries under nail penetration conditions [J]. Energy Storage Science and Technology, 2024, 13(4): 1326-1334. |
| [13] | Bingjin LI, Xiaoxia HAN, Wenjie ZHANG, Weiguo ZENG, Jinde WU. Review of the remaining useful life prediction methods for lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(4): 1266-1276. |
| [14] | Jiamu YANG, Yuxin CHEN, Cheng LIAN, Zhi XU, Honglai LIU. Flow field analysis and structural optimization of coating die with electrode slurry [J]. Energy Storage Science and Technology, 2024, 13(4): 1109-1117. |
| [15] | Mingming SUN. Patent analysis of organic-inorganic composite solid-state electrolytes for lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(3): 1096-1105. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
