Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (7): 2224-2242.doi: 10.19799/j.cnki.2095-4239.2024.0313
• Special Issue on Low Temperature Batteries • Previous Articles Next Articles
Yang LU( ), Shuaishuai YAN, Xiao MA, Zhi LIU, Weili ZHANG, Kai LIU(
), Shuaishuai YAN, Xiao MA, Zhi LIU, Weili ZHANG, Kai LIU( )
)
Received:2024-04-10
Revised:2024-04-23
Online:2024-07-28
Published:2024-07-23
Contact:
Kai LIU
E-mail:y-lu21@mails.tsinghua.edu.cn;liukai2019@tsinghua.edu.cn
CLC Number:
Yang LU, Shuaishuai YAN, Xiao MA, Zhi LIU, Weili ZHANG, Kai LIU. Low-temperature electrolytes and their application in lithium batteries[J]. Energy Storage Science and Technology, 2024, 13(7): 2224-2242.
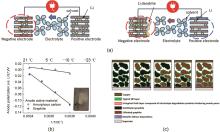
Fig. 3
Effect of low temperature on graphite anode: (a) Schematic diagram of lithium deposition on the surface of graphite anode at low temperature[8]; (b) Polarization in graphite/NMC and amorphous carbon/NCM cells at different temperatures[9]; (c) Schematic diagram of the aging layer derived from electrolyte decomposition prevents the Li+ diffusion[10]"
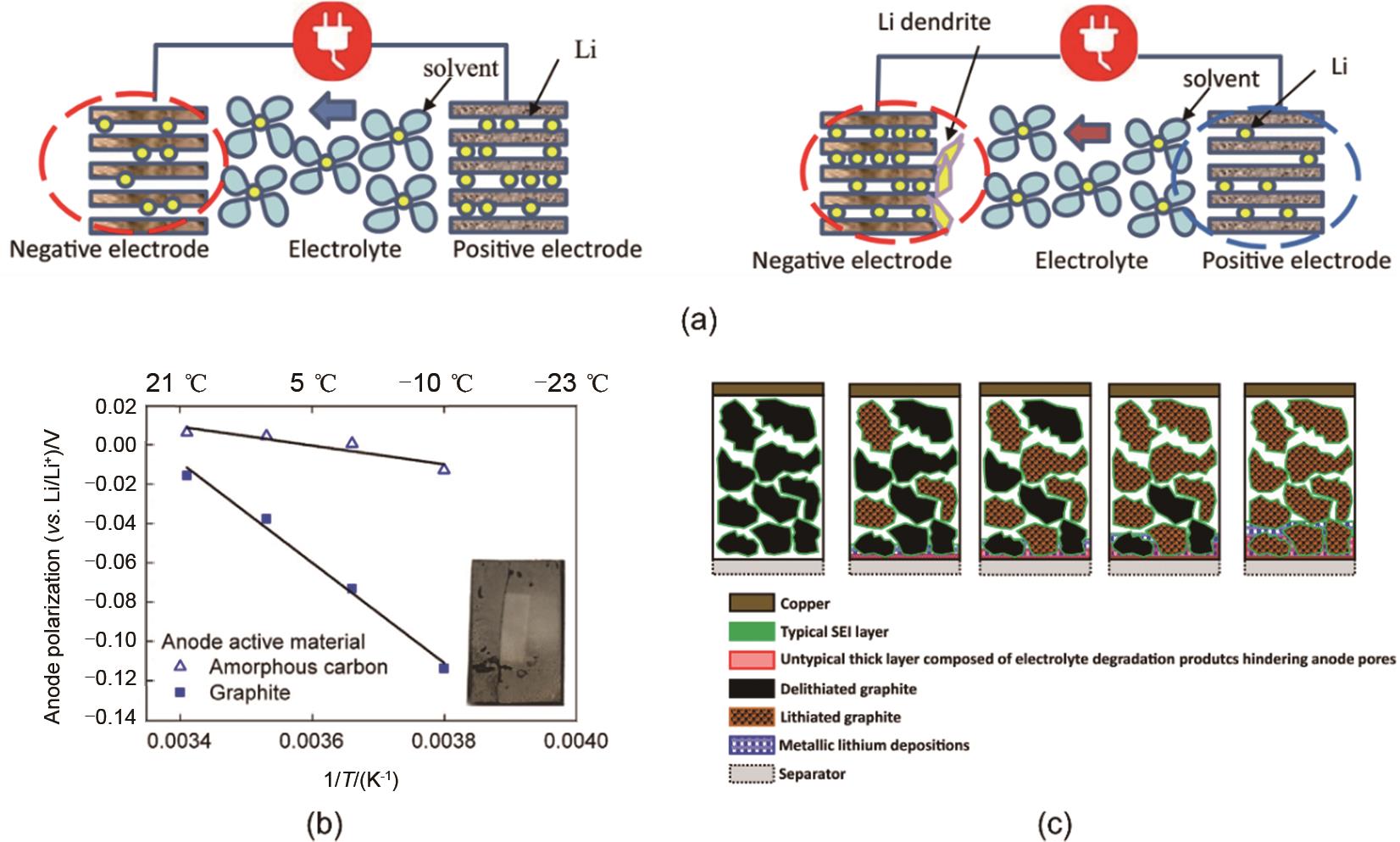
Fig. 4
Effect of Li+ desolvation on low temperature performance of lithium batteries: (a) Schematic diagram of Li+ migration process during discharging process; (b) ESI impedance results of different symmetrical batteries at low temperature; (c) Discharge capacity of reassembled graphite /NCA batteries in various electrolytes with passivated films formed in different electrolytes[15]"
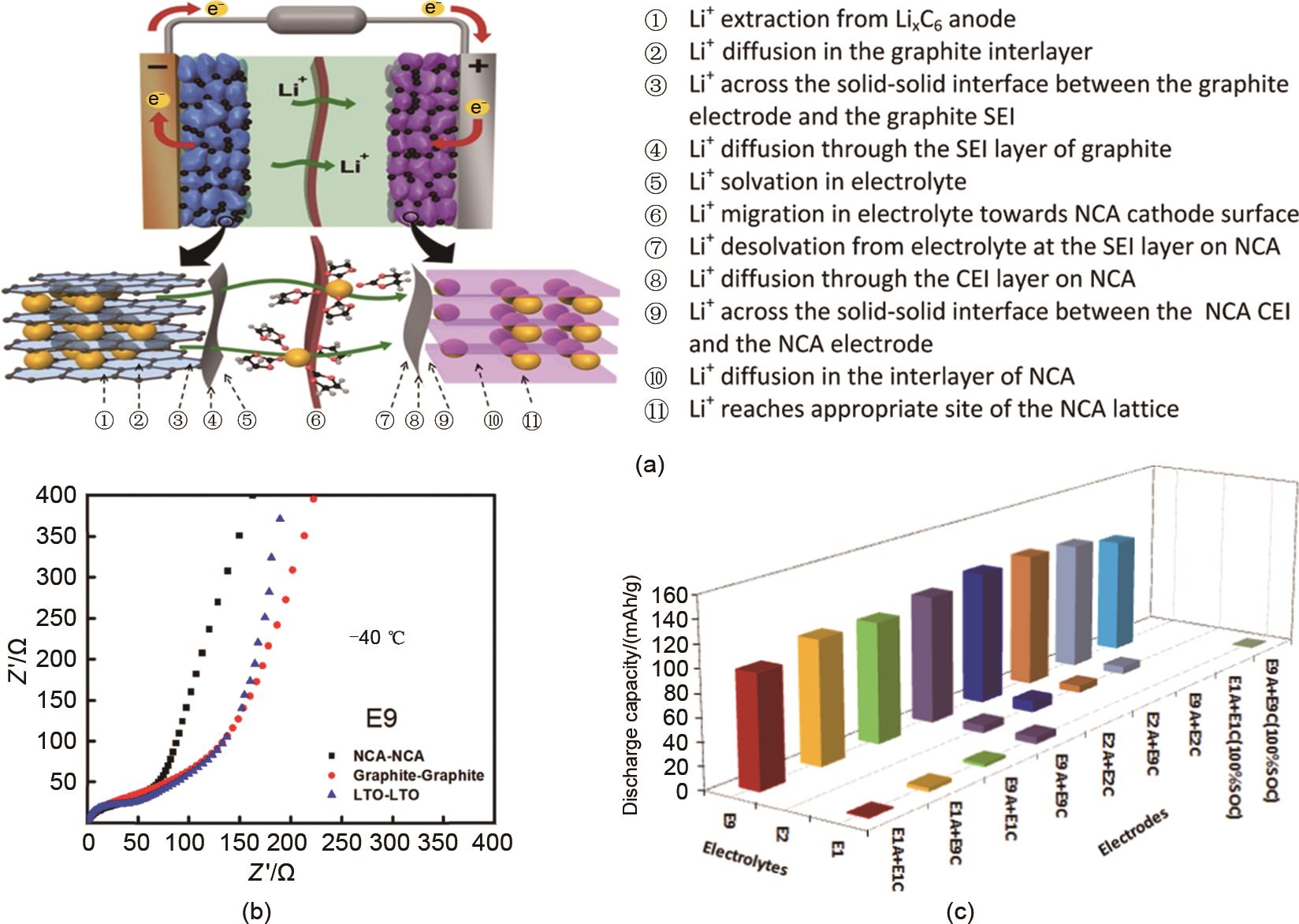

Fig. 5
Low-temperature discharge performance of lithium-ion batteries using carboxylate electrolyte: Discharge performance of lithium-ion batteries using different carboxylate electrolytes at (a) -40 ℃, (b) -50 ℃ and (c) -60 ℃; (d) Discharge energy of lithium-ion batteries using 1 mol/L LiPF6 EC-EMC-MP electrolyte at different temperatures[24]"
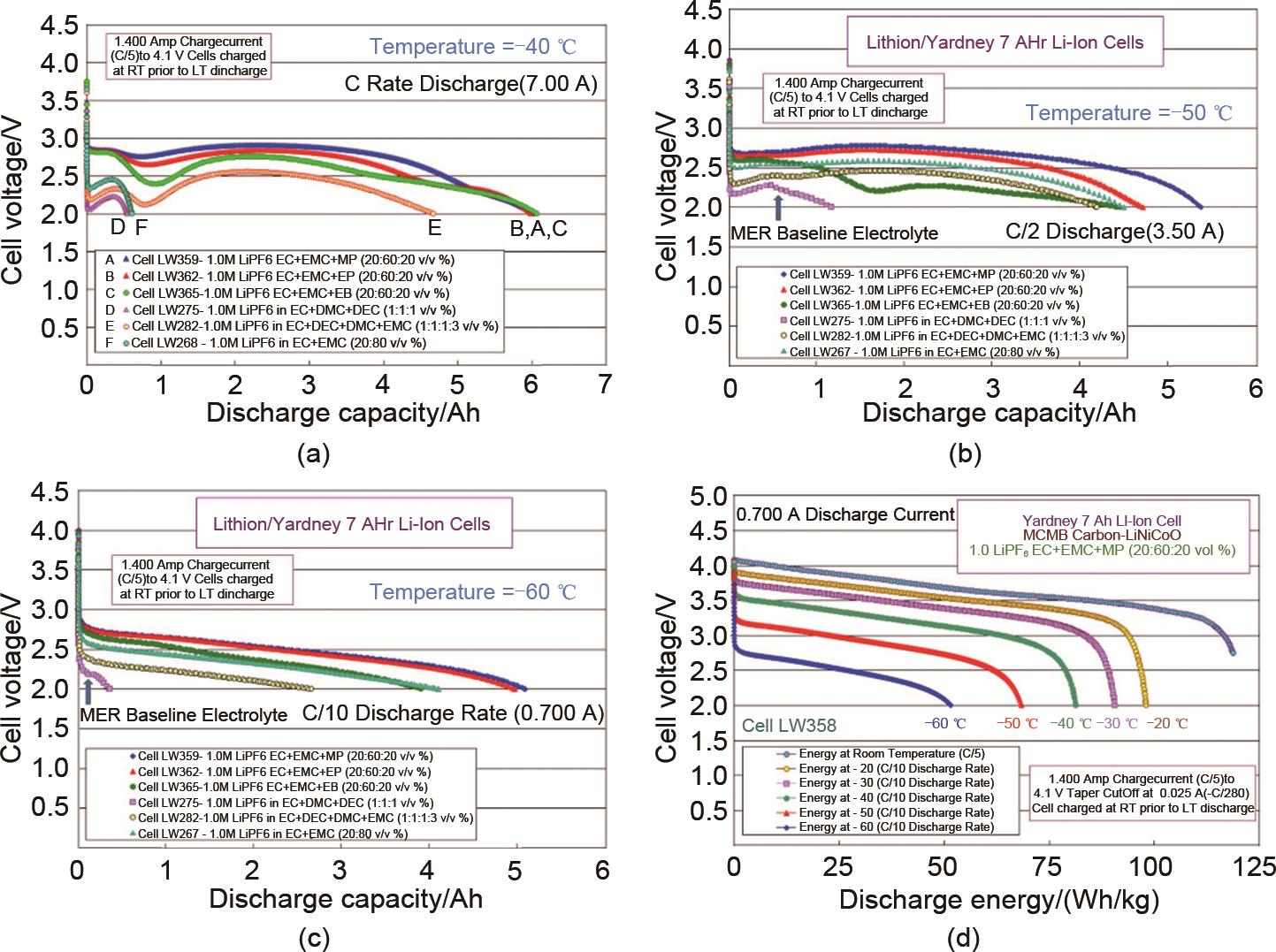
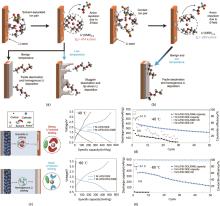
Fig. 7
Effects of solvation structure of (a) 1 mol/L LiFSI DOL/DME and (b) 1 mol/L LiFSI DEE on desolvation and lithium metal deposition at different temperatures; (c) The relationship between different solvation structures and lithium metal deposition; Cycling performance of Li/SPAN full cells using different electrolytes at (d) -40℃ and (e) -60℃[54]"
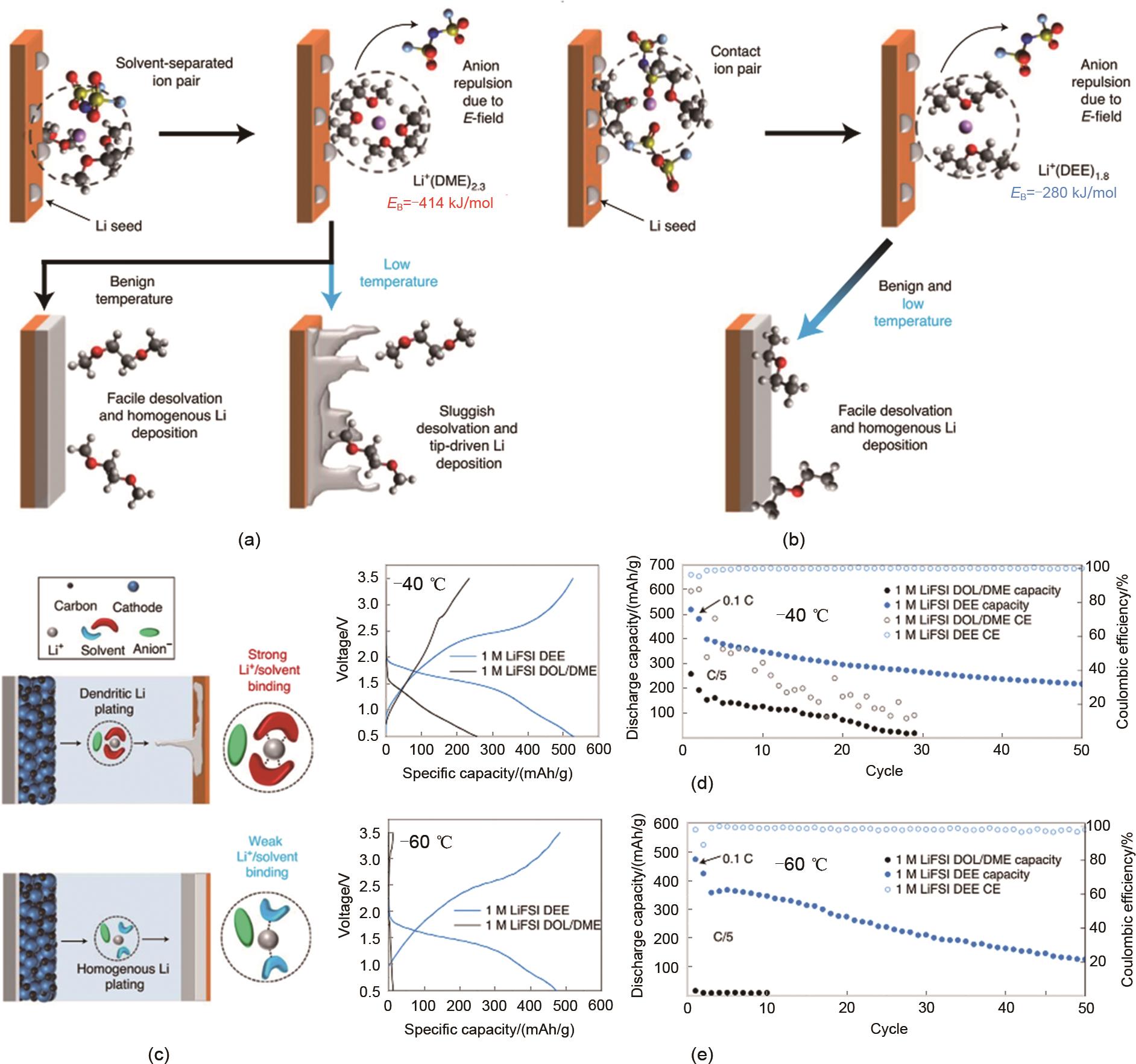
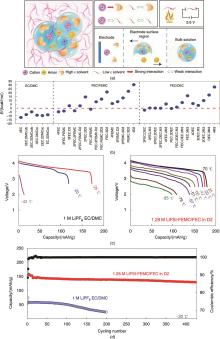
Fig. 9
(a) Schematic of solvation structure of localized high-concentration electrolyte; (b) Desolvation energy barriers of different electrolyte; Comparison of (c) discharge performance and (d) low-temperature cycling performance of localized high-concentration electrolyte and conventional electrolyte at different temperatures[61]"
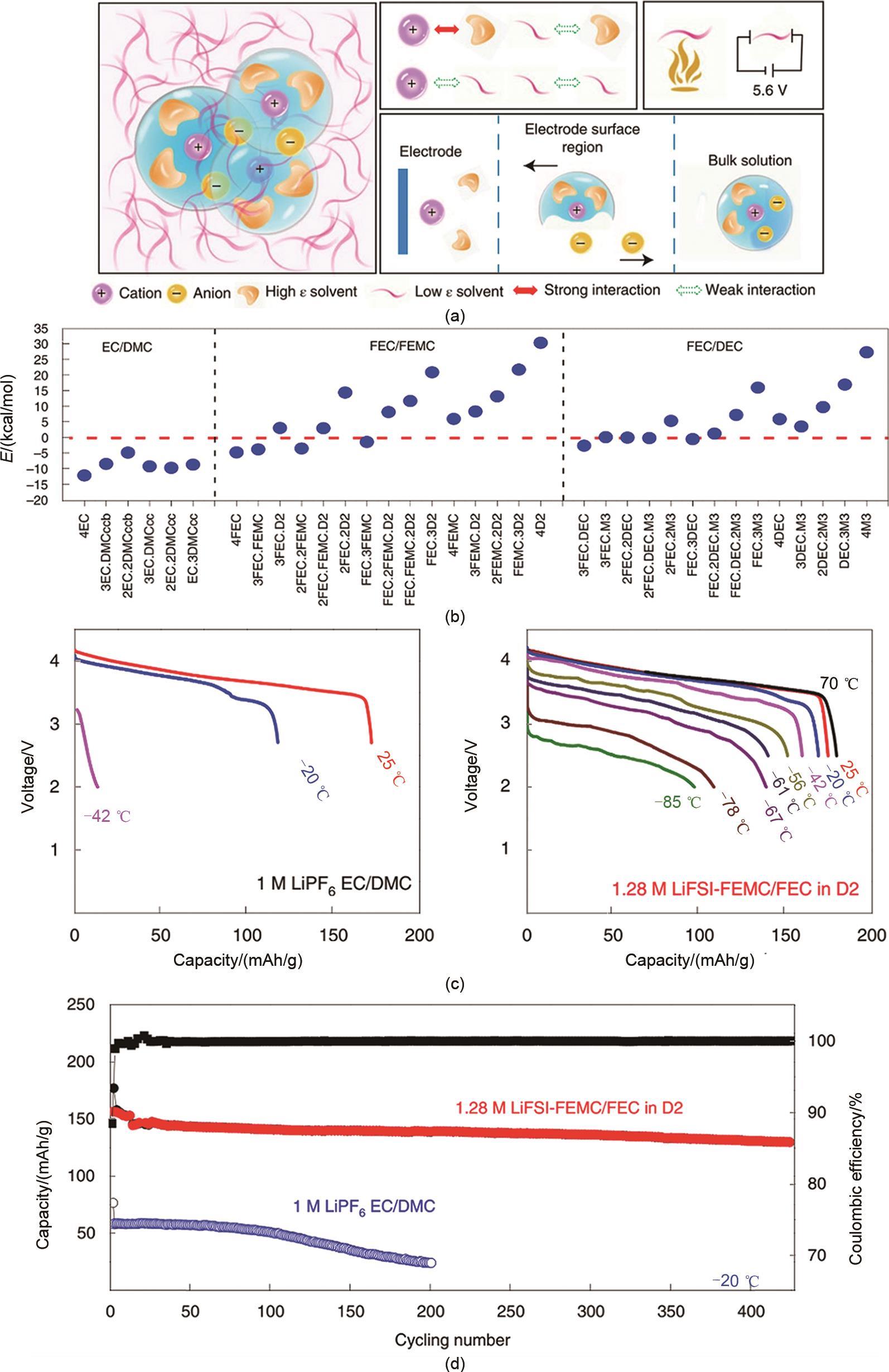

Fig. 10
(a) Schematic mechanism of the soft solvated structure; (b) Cycling performance of graphite/NMC cells with soft solvent electrolyte at different temperatures; (c) Mechanism of fast ion transport in small-size solvent electrolyte[62]; (d) Cycling performance of graphite/NMC cells with FAN-based electrolyte at different temperatures[63]"
| 1 | TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414: 359-367. |
| 2 | DON M. Building a better battery[J]. MRS Bulletin, 2020, 45(3): 246-247. |
| 3 | GUPTA A, MANTHIRAM A. Designing advanced lithium‐based batteries for low-temperature conditions[J]. Advanced Energy Materials, 2020, 10(38): 2001972. |
| 4 | LI Q, LIU G, CHENG H R, et al. Low-temperature electrolyte design for lithium-ion batteries: Prospect and challenges[J]. Chemistry, 2021, 27(64): 15842-15865. |
| 5 | HUBBLE D, BROWN D E, ZHAO Y Z, et al. Liquid electrolyte development for low-temperature lithium-ion batteries[J]. Energy & Environmental Science, 2022, 15(2): 550-578. |
| 6 | RODRIGUES M T F, BABU G, GULLAPALLI H, et al. A materials perspective on Li-ion batteries at extreme temperatures[J]. Nature Energy, 2017, 2(8): 17108. |
| 7 | WANG C Y, ZHANG G S, GE S H, et al. Lithium-ion battery structure that self-heats at low temperatures[J]. Nature, 2016, 529: 515-518. |
| 8 | ARAI J, NAKAHIGASHI R. Study of Li metal deposition in lithium ion battery during low-temperature cycle using in situ solid-State7Li nuclear magnetic resonance[J]. Journal of the Electrochemical Society, 2017, 164(13): A3403-A3409. |
| 9 | WALDMANN T, WILKA M, KASPER M, et al. Temperature dependent ageing mechanisms in Lithium-ion batteries - A Post-Mortem study[J]. Journal of Power Sources, 2014, 262: 129-135. |
| 10 | MATADI B P, GÉNIES S, DELAILLE A, et al. Irreversible capacity loss of Li-ion batteries cycled at low temperature due to an untypical layer hindering Li diffusion into graphite electrode[J]. Journal of the Electrochemical Society, 2017, 164(12): A2374-A2389. |
| 11 | JIANG L L, YAN C, YAO Y X, et al. Inhibiting solvent co-intercalation in a graphite anode by a localized high-concentration electrolyte in fast-charging batteries[J]. Angewandte Chemie (International Ed in English), 2021, 60(7): 3402-3406. |
| 12 | DONG X L, WANG Y G, XIA Y Y. Promoting rechargeable batteries operated at low temperature[J]. Accounts of Chemical Research, 2021, 54(20): 3883-3894. |
| 13 | ZHANG S S, XU K, JOW T R. Electrochemical impedance study on the low temperature of Li-ion batteries[J]. Electrochimica Acta, 2004, 49(7): 1057-1061. |
| 14 | ZHANG S S, XU K, JOW T R. The low temperature performance of Li-ion batteries[J]. Journal of Power Sources, 2003, 115(1): 137-140. |
| 15 | LI Q Y, LU D P, ZHENG J M, et al. Li+-desolvation dictating lithium-ion battery's low-temperature performances[J]. ACS Applied Materials & Interfaces, 2017, 9(49): 42761-42768. |
| 16 | LI Q Y, JIAO S H, LUO L L, et al. Wide-temperature electrolytes for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(22): 18826-18835. |
| 17 | XU K, VON CRESCE A, LEE U. Differentiating contributions to "ion transfer" barrier from interphasial resistance and Li+ desolvation at electrolyte/graphite interface[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2010, 26(13): 11538-11543. |
| 18 | PLICHTA E, HENDRICKSON M, THOMPSON R, et al. Development of low temperature Li-ion electrolytes for NASA and DoD applications[J]. Journal of Power Sources, 2001, 94(2): 160-162. |
| 19 | ZHANG S S, XU K, ALLEN J L, et al. Effect of propylene carbonate on the low temperature performance of Li-ion cells[J]. Journal of Power Sources, 2002, 110(1): 216-221. |
| 20 | SMART M C, RATNAKUMAR B V, SURAMPUDI S. Electrolytes for low‐temperature lithium batteries based on ternary mixtures of aliphatic carbonates[J]. Journal of the Electrochemical Society, 2019, 146(2): 486-492. |
| 21 | PLICHTA E, BEHL W. A low-temperature electrolyte for lithium and lithium-ion batteries[J]. Journal of Power Sources, 2000, 88(2): 192-196. |
| 22 | XU K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries[J]. Chemical Reviews, 2004, 104(10): 4303-4417. |
| 23 | SAZHIN S V, KHIMCHENKO M Y, TRITENICHENKO Y N, et al. Performance of Li-ion cells with new electrolytes conceived for low-temperature applications[J]. Journal of Power Sources, 2000, 87(1): 112-117. |
| 24 | SMART M C, RATNAKUMAR B V, CHIN K B, et al. Lithium-ion electrolytes containing ester cosolvents for improved low temperature performance[J]. Journal of the Electrochemical Society, 2010, 157(12): A1361-A1374. |
| 25 | SMART M C, RATNAKUMAR B V, SURAMPUDI S. Use of organic esters as cosolvents in electrolytes for lithium-ion batteries with improved low temperature performance[J]. Journal of the Electrochemical Society, 2002, 149(4): A361. |
| 26 | HERREYRE S, HUCHET O, BARUSSEAU S, et al. New Li-ion electrolytes for low temperature applications[J]. Journal of Power Sources, 2001, 97: 576-580. |
| 27 | SHEN X H, LI P, LIU X W, et al. The underlying mechanism for reduction stability of organic electrolytes in lithium secondary batteries[J]. Chemical Science, 2021, 12(26): 9037-9041. |
| 28 | SMART M, RATNAKUMAR B, RYAN-MOWREY V, et al. Improved performance of lithium-ion cells with the use of fluorinated carbonate-based electrolytes[J]. Journal of Power Sources, 2003, 119: 359-367. |
| 29 | CHO Y G, KIM Y S, SUNG D G, et al. Nitrile-assistant eutectic electrolytes for cryogenic operation of lithium ion batteries at fast charges and discharges[J]. Energy & Environmental Science, 2014, 7(5): 1737-1743. |
| 30 | XU K. Electrolytes and interphases in Li-ion batteries and beyond[J]. Chemical Reviews, 2014, 114(23): 11503-11618. |
| 31 | ZHANG J G, XU W, XIAO J, et al. Lithium metal anodes with nonaqueous electrolytes[J]. Chemical Reviews, 2020, 120(24): 13312-13348. |
| 32 | WANG H S, YU Z A, KONG X, et al. Liquid electrolyte: The nexus of practical lithium metal batteries[J]. Joule, 2022, 6(3): 588-616. |
| 33 | ZHANG N, DENG T, ZHANG S Q, et al. Critical review on low-temperature Li-ion/metal batteries[J]. Advanced Materials, 2022, 34(15): e2107899. |
| 34 | XU J, WANG X, YUAN N Y, et al. Extending the low temperature operational limit of Li-ion battery to -80 ℃[J]. Energy Storage Materials, 2019, 23: 383-389. |
| 35 | THENUWARA A C, SHETTY P P, MCDOWELL M T. Distinct nanoscale interphases and morphology of lithium metal electrodes operating at low temperatures[J]. Nano Letters, 2019, 19(12): 8664-8672. |
| 36 | ZHAO Y M, HU Z L, ZHAO Z F, et al. Strong solvent and dual lithium salts enable fast-charging lithium-ion batteries operating from–78 to 60 ℃[J]. Journal of the American Chemical Society, 2023, 145(40): 22184-22193. |
| 37 | LI S Y, ZHAO W, ZHOU Z F, et al. Studies on electrochemical performances of novel electrolytes for wide-temperature-range lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2014, 6(7): 4920-4926. |
| 38 | DUDLEY J T, WILKINSON D P, THOMAS G, et al. Conductivity of electrolytes for rechargeable lithium batteries[J]. Journal of Power Sources, 1991, 35(1): 59-82. |
| 39 | ZHANG S S, XU K, JOW T R. A new approach toward improved low temperature performance of Li-ion battery[J]. Electrochemistry Communications, 2002, 4(11): 928-932. |
| 40 | ZHANG S S, XU K, JOW T R. Low-temperature performance of Li-ion cells with a LiBF4-based electrolyte[J]. Journal of Solid State Electrochemistry, 2003, 7(3): 147-151. |
| 41 | ZHANG S S, XU K, JOW T R. Enhanced performance of Li-ion cell with LiBF4-PC based electrolyte by addition of small amount of LiBOB[J]. Journal of Power Sources, 2006, 156(2): 629-633. |
| 42 | ZHANG S S. An unique lithium salt for the improved electrolyte of Li-ion battery[J]. Electrochemistry Communications, 2006, 8(9): 1423-1428. |
| 43 | XUE W J, HUANG M J, LI Y T, et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte[J]. Nature Energy, 2021, 6: 495-505. |
| 44 | MANDAL B K, PADHI A K, SHI Z, et al. New low temperature electrolytes with thermal runaway inhibition for lithium-ion rechargeable batteries[J]. Journal of Power Sources, 2006, 162(1): 690-695. |
| 45 | QIAO L X, OTEO U, MARTINEZ-IBAÑEZ M, et al. Stable non-corrosive sulfonimide salt for 4-V-class lithium metal batteries[J]. Nature Materials, 2022, 21: 455-462. |
| 46 | ZHENG J M, ENGELHARD M H, MEI D H, et al. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries[J]. Nature Energy, 2017, 2(3): 17012. |
| 47 | JONES J P, SMART M C, KRAUSE F C, et al. The effect of electrolyte additives upon lithium plating during low temperature charging of graphite-LiNiCoAlO2 lithium-ion three electrode cells[J]. Journal of the Electrochemical Society, 2020, 167(2): 020536. |
| 48 | THENUWARA A C, SHETTY P P, KONDEKAR N, et al. Efficient low-temperature cycling of lithium metal anodes by tailoring the solid-electrolyte interphase[J]. ACS Energy Letters, 2020, 5(7): 2411-2420. |
| 49 | SMART M C, LUCHT B L, DALAVI S, et al. The effect of additives upon the performance of MCMB/LiNixCo1– xO2 Li-ion cells containing methyl butyrate-based wide operating temperature range electrolytes[J]. Journal of the Electrochemical Society, 2012, 159(6): A739-A751. |
| 50 | YANG B W, ZHANG H, YU L, et al. Lithium difluorophosphate as an additive to improve the low temperature performance of LiNi0.5Co0.2Mn0.3O2/graphite cells[J]. Electrochimica Acta, 2016, 221: 107-114. |
| 51 | LIAO B, LI H Y, XU M Q, et al. Designing low impedance interface films simultaneously on anode and cathode for high energy batteries[J]. Advanced Energy Materials, 2018, 8(22): 1800802. |
| 52 | LIU B, LI Q Y, ENGELHARD M H, et al. Constructing robust electrode/electrolyte interphases to enable wide temperature applications of lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(24): 21496-21505. |
| 53 | YAMADA Y, SAGANE F, IRIYAMA Y, et al. Kinetics of lithium-ion transfer at the interface between Li0.35La0.55TiO3 and binary electrolytes[J]. The Journal of Physical Chemistry C, 2009, 113(32): 14528-14532. |
| 54 | HOLOUBEK J, LIU H D, WU Z H, et al. Tailoring electrolyte solvation for Li metal batteries cycled at ultra-low temperature[J]. Nature Energy, 2021, 6: 303-313. |
| 55 | HOU R L, GUO S H, ZHOU H S. Atomic insights into advances and issues in low-temperature electrolytes[J]. Advanced Energy Materials, 2023, 13(14): 2300053. |
| 56 | YAO Y X, CHEN X, YAN C, et al. Regulating interfacial chemistry in lithium-ion batteries by a weakly solvating electrolyte[J]. Angewandte Chemie (International Ed in English), 2021, 60(8): 4090-4097. |
| 57 | MA T, NI Y X, WANG Q R, et al. Optimize lithium deposition at low temperature by weakly solvating power solvent[J]. Angewandte Chemie (International Ed in English), 2022, 61(39): e202207927. |
| 58 | YAMADA Y, WANG J H, KO S, et al. Advances and issues in developing salt-concentrated battery electrolytes[J]. Nature Energy, 2019, 4: 269-280. |
| 59 | REN X D, ZOU L F, CAO X, et al. Enabling high-voltage lithium-metal batteries under practical conditions[J]. Joule, 2019, 3(7): 1662-1676. |
| 60 | CHEN S R, ZHENG J M, MEI D H, et al. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes[J]. Advanced Materials, 2018, 30(21): e1706102. |
| 61 | FAN X L, JI X, CHEN L, et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents[J]. Nature Energy, 2019, 4: 882-890. |
| 62 | XU J J, ZHANG J X, POLLARD T P, et al. Electrolyte design for Li-ion batteries under extreme operating conditions[J]. Nature, 2023, 614: 694-700. |
| 63 | LU D, LI R H, RAHMAN M M, et al. Ligand-channel-enabled ultrafast Li-ion conduction[J]. Nature, 2024, 627: 101-107. |
| 64 | ZHANG W L, LU Y, WAN L, et al. Engineering a passivating electric double layer for high performance lithium metal batteries[J]. Nature Communications, 2022, 13: 2029. |
| 65 | WANG H W, ZHANG J K, ZHANG H D, et al. Regulating interfacial structure enables high-voltage dilute ether electrolytes[J]. Cell Reports Physical Science, 2022, 3: 100919. |
| [1] | Hong ZHOU, Zhulin XIN, Hao FU, Qiang ZHANG, Feng WEI. Analysis of the key materials employed in solid-state lithium batteries based on patent data mining [J]. Energy Storage Science and Technology, 2024, 13(7): 2386-2398. |
| [2] | Changhao LI, Shuping WANG, Xiankun YANG, Ziqi ZENG, Xinyue ZHOU, Jia XIE. Nonaqueous electrolyte in low-temperature lithium-ion battery [J]. Energy Storage Science and Technology, 2024, 13(7): 2286-2299. |
| [3] | Zongxun LI, Qiuqiu LYU, Haoyu ZHAO, Jianyu HE, Yang LIU, Zaihong SUN, Kaihua SUN, Tenglong ZHU. Research of GDC barrier layer applications by hydrothermal insitu growth in industrial-sized SOFC [J]. Energy Storage Science and Technology, 2024, 13(7): 2407-2413. |
| [4] | Sen JIANG, Long CHEN, Chuangchao SUN, Jinze WANG, Ruhong LI, Xiulin FAN. Low-temperature lithium battery electrolytes: Progress and perspectives [J]. Energy Storage Science and Technology, 2024, 13(7): 2270-2285. |
| [5] | Shijie LIAO, Ying WEI, Yunhui HUANG, Renzong HU, Henghui XU. 1,3-Difluorobenzene diluent-stabilizing electrode interface for high-performance low-temperature lithium metal batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2124-2130. |
| [6] | Xiang LI, Dezhong LIU, Kai YUAN, Dapeng CHEN. Solid-state electrolyte for low-temperature lithium metal batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2327-2347. |
| [7] | Meilong WANG, Yurui XUE, Wenxi HU, Keyu DU, Ruitao SUN, Bin ZHANG, Ya YOU. Design and research of all-ether high-entropy electrolyte for low-temperature lithium iron phosphate batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2131-2140. |
| [8] | Haotian WANG, Yonggang WANG, Xiaoli DONG. Advances in low-temperature organic batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2259-2269. |
| [9] | Guozheng MA, Jinwei CHEN, Xingyu XIONG, Zhenzhong YANG, Gang ZHOU, Rengzong HU. High-rate lithium storage performance of SnSb-Li4Ti5O12 composite anode for Li-ion batteries at low-temperature [J]. Energy Storage Science and Technology, 2024, 13(7): 2107-2115. |
| [10] | Wentao WANG, Yifan WEI, Kun HUANG, Guowei LV, Siyao ZHANG, Xinya TANG, Zeyan CHEN, Qingyuan LIN, Zhipeng MU, Kunhua WANG, Hua CAI, Jun CHEN. Testing standards and developmental advances for low-temperature Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2300-2307. |
| [11] | Zheng LI, Zhenzhong YANG, Qiong WANG, Renzong HU. Patent intelligence analysis of the research progress in low-temperature electrolytes for Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2317-2326. |
| [12] | Guangyu CHENG, Xinwei LIU, Shuo LIU, Haitao GU, Ke WANG. Controlling electrolyte solvent components to enhance cycle life of LCO/C low-temperature 18650 batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2171-2180. |
| [13] | Fei ZHAO, Yinghua CHEN, Zheng MA, Qian LI, Jun MING. Advances in low-temperature electrolytes for potassium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2308-2316. |
| [14] | Lifeng WANG, Naiqing REN, Hai YANG, Yu YAO, Yan YU. Advances in low-temperature electrolytes for sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2206-2223. |
| [15] | Yuhao WANG, Zhiyong LI, Xin GUO. Applications and challenges of polymer-based electrolytes in low-temperature solid-state lithium batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2243-2258. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
